Abstract
Previous studies in mice with multiple gestational exposures to perfluorooctanoic acid (PFOA) demonstrate numerous dose dependent growth and developmental effects which appeared to worsen if offspring exposed in utero nursed from PFOA-exposed dams. To evaluate the disposition of PFOA in the pregnant and lactating dam and her offspring, time-pregnant CD-1 mice received a single 0, 0.1, 1, or 5 mg PFOA/kg BW dose (n = 25/dose group) by gavage on gestation day 17. Maternal and pup fluids and tissues were collected over time. Pups exhibited significantly higher serum PFOA concentrations than their respective dams, and their body burden increased after birth until at least postnatal day 8, regardless of dose. The distribution of milk:serum PFOA varied by dose and time, but was typically in excess of 0.20. These data suggest that milk is a substantial PFOA exposure route in mice and should be considered in risk assessment modeling designs for this compound.
Keywords: PFOA, Serum, Amniotic fluid, Urine, Milk, Mammary gland, Dosimetry, Disposition
1. Introduction
Perfluorooctanoic acid (PFOA) is a member of the perfluoroalkyl acid (PFAA) family of man-made, fluorinated organic compounds used in a number of consumer goods and industrial surfactants due to their grease and water-repellant properties. The use of PFAAs in many common applications, such as stain repellants for clothing, carpeting, and upholstery, and the stability of the carbon–fluorine bond have made them ubiquitous in the environment. The predominant route of exposure in North American and European consumers is likely oral intake, including drinking water, while inhalation and dermal absorption comprise routes of lesser exposure [1–5].
PFAAs are persistent, readily absorbed, not known to be metabolized, and are poorly eliminated, with half-lives in humans ranging from roughly 4 to 8 years [2–4]. In fact, the arithmetic and geometric mean half-lives of serum elimination, respectively, were 5.4 years [95% confidence interval (CI), 3.9–6.9] and 4.8 years (95% CI, 4.0–5.8) for PFOS; 8.5 years (95% CI, 6.4–10.6) and 7.3 years (95% CI, 5.8–9.2) for PFHS; 3.8 years (95% CI, 3.1–4.4) and 3.5 years (95% CI, 3.0–4.1) for PFOA [4].
These characteristics led to increased concern for the potential health risks of PFAAs and a program to reduce product and emission content of PFOA and related chemicals was recently initiated [1]. PFAAs are continually detected worldwide in both human and wildlife samples [3,6–9]. A recent analysis of American Red Cross blood donors indicated a reduction of 60% in blood perfluorooctane sulfonate (PFOS) and 25% in blood PFOA levels between the years 2000 and 2006 [10]. However, while the production of and potential for human and wildlife exposures to certain PFAAs has been reduced in the US in recent years, it is not clear that perfluorinated compounds produced in other countries will not continue to replace them in the US marketplace or in the contribution to worldwide exposure.
Much of the recent health effects research on PFOA in mice, commonly associated with gestational exposures of 0.01–5 mg PFOA/kg BW, has focused on developmental toxicities such as decreased maternal weight gain, reduced neonatal survival and body weight, as well as later life effects such as pubertal delays, mammary gland abnormalities, and excessive weight gain [2,11–16]. Early postnatal adverse health observations prompted studies examining the effect of PFOA on maternal lactation and health effects of the nursing offspring. White et al. [14] described reduced epithelial differentiation on postnatal day (PND) 10 in mammary glands of CD-1 mouse dams exposed to 5 mg PFOA/kg BW from GD1–17, as well as delays in epithelial involution and alterations in milk protein gene expression on PND20. In addition, female offspring of exposed dams displayed stunted mammary epithelial branching and growth on PND10 and PND20. In a cross-foster study utilizing CD-1 mice, Wolf et al. [16] reported that although in utero exposure to 5 mg PFOA/kg BW from GD1–17 in the absence of lactational exposure was sufficient to induce postnatal body weight deficits and developmental delays, pup survival from birth to weaning was affected only in those both in utero and lactationally exposed. Furthermore, recent studies [15] have shown that unexposed neonates lactation-ally exposed to PFOA quickly developed mammary gland growth deficits and that control dams nursing in utero-exposed pups (dams exposed via pup grooming) demonstrated slowed differentiation of their own mammary glands that was evident in whole mount preparations of the tissue by the 5th day of lactation. These results support a role for impaired lactational development and possibly a significant lactational transfer of PFOA in the observation of early growth effects.
The concern for potential prenatal and neonatal exposures in humans has been raised further by the detection of PFAAs in human breast milk and cord blood and the development-related outcomes associated with these observations. So et al. [17] indicated a range of 47–210 ng/l (0.047–0.21 ng/ml) PFOA in 19 samples of breast milk from Chinese women. PFOA was detected in only 1 of 12 human milk samples collected from 1996 to 2004 in Sweden at a concentration of 492 pg/ml (0.492 ng/ml) [18], and a mean of 43.8 pg/ml (0.044 ng/ml) was reported for 45 U.S. breast milk samples collected in 2004 [19]. Two studies recently determined a negative association between PFOA and growth indices in children with median cord serum levels of 1.6 ng/ml PFOA in the U.S. [20] and 5.6 ng/ml PFOA in Denmark [21]. While only one sample was found to contain PFOA in the Karrman et al. [18] study, these researchers reported a significant milk to serum correlation (r2 = 0.7–0.8, p < 0.05) for other PFAAs detected. Furthermore, Tao et al. [19] suggested that there may be preferential partitioning of PFOA to milk compared to other PFAAs and also indicated that women who were nursing for the first time exhibited 49% higher concentrations of PFOA in breast milk than women who had nursed previously, although inter-individual variation, daily milk output and milk protein concentration were not taken into consideration. The only study that has evaluated the distribution coefficient of PFCs between blood and milk in animal models was a pharmacokinetic study of placental and lactational transport of PFOA in rats [22]. Although female rats are known to have a serum PFOA half-life of only a few hours [23], unlike mice which have a half-life of about 15 days [13], the study [22] indicated concentrations in rat milk approximately 10 times less than that of maternal plasma and that the milk concentrations were generally of the same magnitude as the concentrations in pup plasma.
The increasing amount of research confirming the developmental toxicity of PFAAs in animal studies, coupled with their detection in human cord blood and milk, supports the need for examining the disposition of PFAAs during pregnancy/lactation in an appropriate animal model in order to fully establish the association between prenatal/neonatal exposure and offspring effects. While other studies have examined the pharmacokinetics of PFAAs in limited contexts, little data currently exist on the disposition of PFOA in pregnant and lactating mice or their offspring. We recently developed an analytical method for the analysis of PFOA in mouse serum, urine, milk, mammary tissue, amniotic fluid, and pups [24]. Utilizing these methods, we report here data on the distribution of PFOA in various matrices of pregnant and lactating CD-1 mice, as well as the serum concentration and total body load of their offspring, following a single exposure of PFOA on GD17. These data will allow us to reduce the uncertainties in risk assessment for this particular PFAA.
2. Materials and methods
2.1. Chemicals
PFOA (ammonium salt; >98% pure), used in animal exposures, was purchased from Fluka Chemical (Steinheim, Switzerland). PFOA was completely dissolved by agitation in deionized tap water, in which PFOA was below the level of detection (LOD—0.5 ng/l for water), and prepared fresh just prior to use.
2.2. Animals
All animal studies were conducted as approved by the National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee. Confirmed timed pregnant female CD-1 mice (n = 100) were purchased from Charles River Laboratories (Raleigh, NC). Pregnant mice were received at the U.S. EPA's Laboratory Animal Care Facility on gestation day (GD) 14 (day of sperm-positive designated as GD0). Upon arrival, mice (approximately 12-week-old) were weighed and randomly distributed among PFOA treatment groups. They were housed individually in polypropylene cages with Alpha-dri (Shepherd Specialty Papers, Kalamazoo, MI) bedding and nesting materials. They were provided pelleted chow (LabDiet 5001, PMI Nutrition International LLC, Brentwood, MO) and tap water ad libitum (both contained PFOA at concentrations below the LOD). Animal facilities were controlled for temperature (20–24 °C) and relative humidity (40–60%), and kept under a 14:10-h light–dark cycle. Mice (n = 25/dose group) received either water vehicle or a single dose (0.1, 1.0 or 5.0 mg/kg) of PFOA (in water; 10 μl/g) by oral gavage on GD17.
2.3. Animal assessments and sample collection
Live dam body weights were recorded on GD17, GD18 (prior to parturition), PND1 (day after parturition), and PNDs 2, 4, 8, 11, and 18. On GD18, 24 h after the PFOA exposure, five dams in each dose group were sacrificed and trunk blood, urine, amniotic fluid (fluid immediately surrounding each fetus), and the 4th and 5th mammary gland were collected. Liver weight, total number of fetuses (live, dead, or resorbed), and fetal weights were determined. One entire fetus from each litter was euthanized by decapitation and quick frozen on dry ice in a 15 ml screw-cap vial. Remaining fetuses were quickly euthanized and discarded. The dam mammary gland, urine, and amniotic fluid were kept on ice, and then frozen until assayed. The trunk blood was allowed to clot; serum was collected after centrifugation and frozen until assayed. All samples were kept frozen at −80 °C.
A similar routine was followed on PND1 (48 h after exposure, n = 5 dams/dose group). Weights of the dam, pups, dam liver, and the number of live pups in each litter were recorded. A single pup from each litter was weighed, euthanized, and quick frozen in a collection vial (including all blood). Blood from all remaining pups in each litter was pooled into a single vial, allowed to clot, and separated to serum by centrifugation. Dam and pup serum, dam urine and mammary tissue were frozen until assayed. All remaining litters, in all dose groups, were equalized to 10 pups each on PND1. Biological samples, including a single pup and pup serum, as described for PND1, were also collected on PNDs 4, 8 and 18 (n = 5 dams/dose group), at the same time of day.
Milk collection was attempted, following administration of oxytocin (1 U/ml, i.p., 20 min prior to milking) on both GD18 and PND1, but was unsuccessful. Milk was collected on PNDs 2, 8, 11, and 18 following a 2 h separation of the pups from the dam and an oxytocin stimulus. The milk was vacuum aspirated using low, pulsatile pressure, into a pre-weighed microcentrifuge tube. Collected milk was weighed and frozen until analyzed. Biological samples including urine, dam and pup serum, amniotic fluid, mammary tissue, whole pup, and milk were analyzed for PFOA using the methods described briefly below and in our companion paper [24].
2.4. PFOA sample analyses
Briefly, the analysis of PFOA was performed using a Waters Acquity™ ultra-performance liquid chromatography system interfaced with a Waters Quattro Premier XE triple quadrupole mass spectrometer (UPLC–MS/MS) (Waters, Milford, MA). Either 25 or 50 μl of serum and amniotic fluid (50 μl used for controls), 20 μl aliquots of urine and milk, and 300 μl of pup or mammary tissue homogenates were utilized as starting material for these analyses. Samples were extracted, purified, and concentrated or diluted exactly as described by Reiner et al. [24]. Ten to forty microliters of the prepared sample, depending on the concentration of the original exposure, was injected and run on the UPLC–MS/MS [24]. Refer to Reiner et al. [24] for method performance and quality control steps that were performed to insure the precision and accuracy of the methods used. The limit of quantitation (LOQ) for these experiments was 5 ng/ml (serum), 1 ng/ml (amniotic fluid, urine, milk), and 1 ng/g (whole pups, mammary tissue).
2.5. Urinary creatinine measures
Creatinine concentrations were measured as a basis to evaluate PFOA in mouse urine. The QuantiChrom creatinine assay (BioAssay Systems, Hayward, CA) exhibited an LOD of 0.10 ng/ml and was linear up to 300 ng/ml. Thirty microliters of each urine sample was prepared and evaluated at 510 nm singly or in duplicates (five duplicates per set of 20 samples) according to the manufacturer's instructions. The inter-assay coefficient of variation (CV) ranged from 4.0 to 6.8% and the intra-assay CV ranged from 0.3 to 16.1%, with an average of 4.9%. The assay standard accuracy ranged from 0.2 to 8.4%. Urinary PFOA is reported as corrected for creatinine concentrations (ng PFOA/g creatinine).
2.6. Computations and statistics
Reported PFOA concentrations have been adjusted for dilution or concentration factors, as well as creatinine levels (ng/g; urine), or the weight of the tissue evaluated (ng/g; mammary tissue and whole pups). Serum, amniotic fluid, milk and urinary concentrations are reported as ng/ml. Averages, proportions, and statistical comparisons were calculated with SAS 9.1 (SAS Institute; Cary, NC). Statistical significance was determined using a Proc GLM ANOVA, with a Dunnett's post hoc comparison, and significance was set at p < 0.05.
3. Results
3.1. Biological outcomes
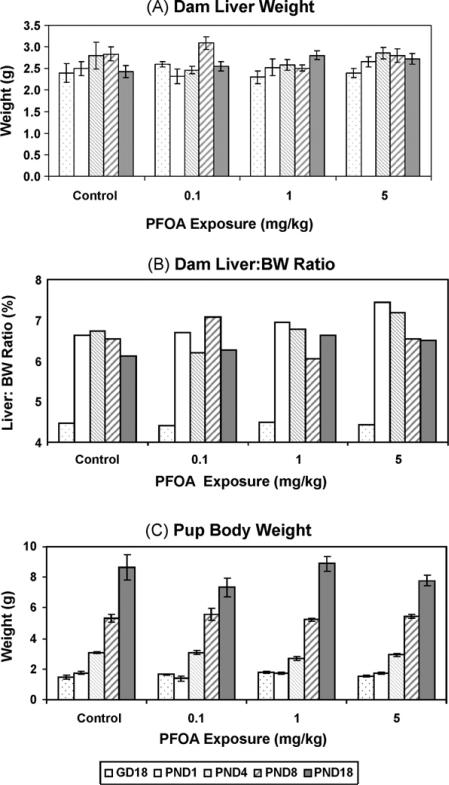
This is the first study to report single dose disposition of PFOA in pregnant and lactating mice and their offspring. The doses chosen were based on previous reports in CD-1 mice [3,14,16] demonstrating developmental health outcomes following multiple gestational PFOA exposures. A single PFOA exposure on GD17 did not affect the number of live fetuses (on GD18), implantation sites, or live-born pups (on PND1), or dam body weights (data not shown). Unlike studies using multiple gestational PFOA exposures [13,25], there was no change in pup body weight, dam liver weight, and dam liver:BW ratios, within the PFOA dose range administered in this study (Fig. 1). The rise in dam liver:BW ratio between GD18 and PND1, which persisted until weaning, was due to the dramatic decrease in body weight at parturition, as this single late gestation PFOA exposure failed to change mean liver weight in exposed dams, compared to control values, at any time evaluated.
Fig. 1.
Dam tissue weights and average pup weights following a single gavage PFOA exposure on GD17. PFOA was without effect on several biological end points (p > 0.05), such as dam body weight measured on several postnatal days (PND) and on gestation day (GD)18 (not shown). (A) Dam liver weight, (B) liver:body weight ratio, and (C) pup body weight over time or numbers of live pups or fetuses (not shown) were also unchanged by a single PFOA exposure. Data are shown as mean ± SEM or as a mean ratio.
3.2. PFOA concentrations prior to birth
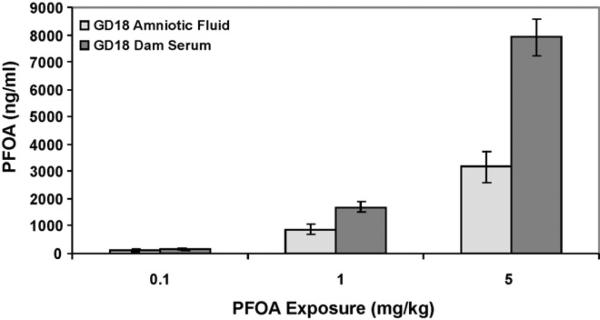
The mean concentration of PFOA in the amniotic fluid and serum of the exposed dams 24 h after exposure is shown (Fig. 2; amniotic fluid controls average 3.8 ng/ml). The average concentration of PFOA detected in dam serum was about twice the amniotic fluid concentration at each dose evaluated (amniotic fluid was 68.8, 51.8, and 40% of dam serum levels at 0.1, 1, and 5 mg PFOA/kg BW, respectively). A comparison of the amount of PFOA in an entire GD18 fetus (body burden/pup ± standard error of the mean [SEM]; Fig. 5) to the GD18 PFOA concentration in amniotic fluid (ng/ml; assuming 1 ml total volume) reveals 2.3-, 3.1-, and 2.7-fold increased PFOA in the pup vs. the fluid in which it was contained in utero for 0.1, 1, and 5 mg/kg dose groups, respectively.
Fig. 2.
Comparison of gestation day (GD)18 dam serum and amniotic fluid PFOA concentrations. PFOA concentrations were significantly higher in dam serum than amniotic fluid at all doses evaluated (p < 0.05). Data are shown as mean ± SEM.
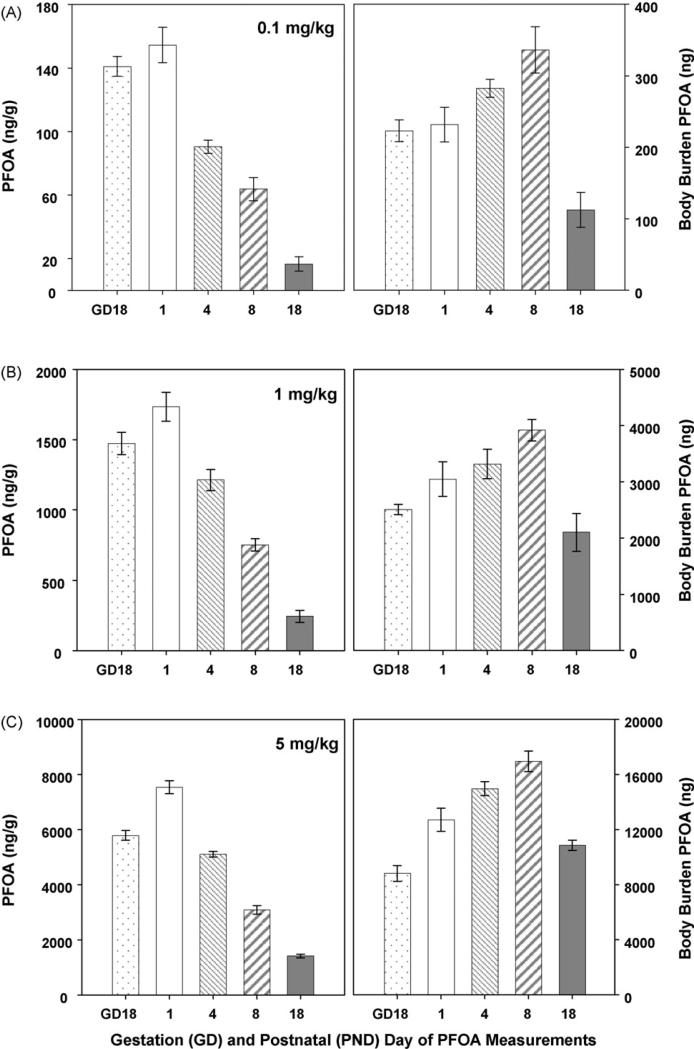
Fig. 5.
Whole pup PFOA concentrations. PFOA concentrations were measured in a representative whole pup (pup and blood; ng/g; left panels) from each litter. Although there is a consistent downward trend in PFOA concentration over time, the rapidly increasing blood volume and body weight changes must be taken into consideration when interpreting these data. Body weight-adjusted values (right panels; [ng/g PFOA measures × g body weight = body burden]) demonstrate an accumulation of exposure until late in the lactational period. Data are shown as mean ± SEM.
3.3. PFOA concentrations in the dams
The serum and urine PFOA concentrations were evaluated in dams that were nursing litters of approximately 10 pups (PND1 equalized; minimal pup loss over time). As expected, dam sera contained the highest PFOA concentrations of any matrix evaluated, regardless of dose (Fig. 3; all serum controls < LOQ). The rise in circulating serum PFOA with increasing dam exposures was proportional to the change in dose delivered, regardless of stage of lactation (i.e., mean 9.9-fold and 5.1-fold increases between 0.1 to 1.0 mg/kg and 1.0 to 5.0 mg/kg exposures, respectively).
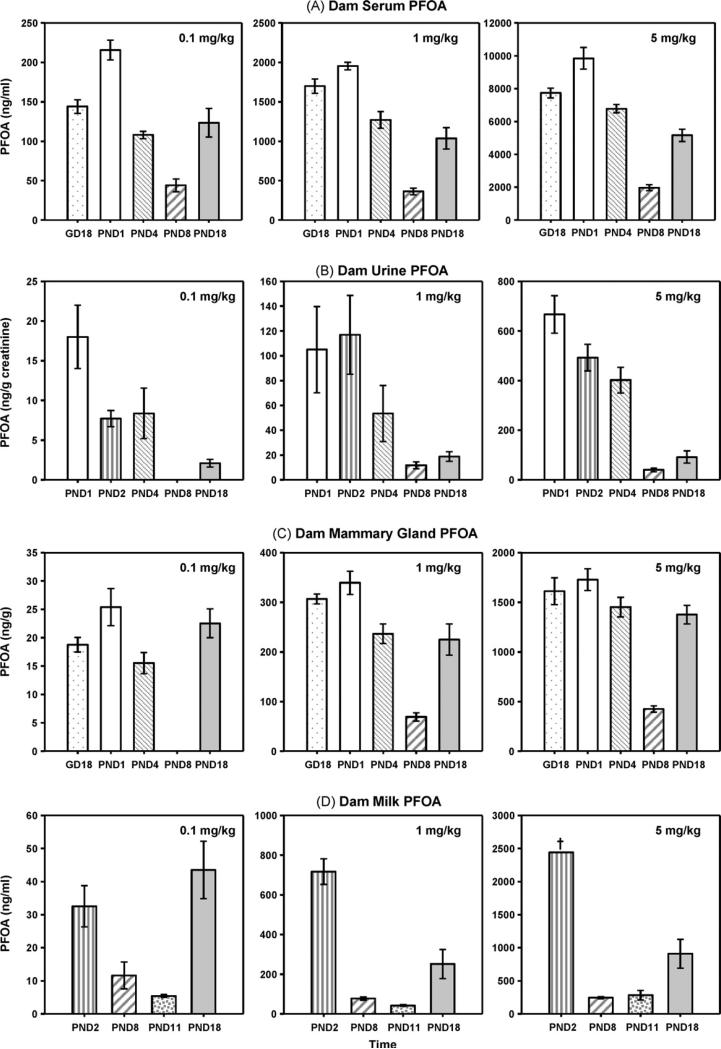
Fig. 3.
PFOA concentrations in exposed dams. PFOA concentrations were measured in dam serum (A; ng/ml), urine (B; ng/g creatinine), and mammary tissue (C; ng/g tissue weight) on postnatal days (PND) 1, 4, 8 and 18. PFOA concentrations were measured in aspirated milk samples collected on PNDs 2, 8, 11, and 18 (D; ng/ml). Although panels A and C and B and D cannot be directly compared (due to different units), the U-shaped concentration curve present in dam serum (regardless of dose) was also detected in mammary tissue and aspirated milk. Data are shown as mean ± SEM. †Denotes a single reliable measurement at this time due to insufficient volumes in other dams at this dose and time.
A one-time PFOA exposure of 0.1 mg/kg produced an average dam serum PFOA concentration (Fig. 3A) of 144–226 ng/ml at 24 and 48 h after exposure, respectively, which was reduced to an average of 44 ng/ml near the peak of lactation (PND8), and had risen to a mean of 123 ng/ml by PND18, a time when the pups’ primary caloric intake came from rodent chow and not milk. The U-shaped serum concentration curve observed in the 0.1 mg PFOA/kg dose group was also present in the 1 and 5 mg/kg exposure groups.
As shown in Fig. 3 (A–C; control urine and mammary gland PFOA < LOQ), although the concentrations of PFOA cannot be compared directly between serum, urine, and mammary tissue, due to the difference in units, it was evident that much less PFOA was being excreted in dam urine than was present in dam serum, and that mammary tissue contained a considerable amount of PFOA. While a U-shaped response in dam excretion of PFOA (urine) was not as pronounced as that of serum, mammary tissue demonstrated a strong U-shaped response, with the lowest concentrations measured near the peak of lactation, and a significant rise in concentration apparent again at PND18 (p < 0.05).
When aspirated milk PFOA values were evaluated (Fig. 3D; 1 control > LOQ), a U-shaped curve over time was again evident. As depicted in Table 1, the percentage of PFOA in milk (compared to serum) was substantial. Comparing the milk concentrations to the closest matched dam serum concentrations (by time), the amount of PFOA in the milk consistently ranged from 1/10 to 1/2 that of dam serum PFOA across dose and time. It appeared that the day of lactation on which milk PFOA was measured had an important influence on this relationship. The milk:serum PFOA ratio was greatest in early and late lactation (PND2 and PND18), ranging from 15 to 56% (means of 33% early and 26% late), while near the peak of lactation (PND8 and 11), the PFOA milk:serum ratio ranged from 11 to 27% (mean 17.7%). It was not possible to accurately measure the volume of milk obtained at aspiration, but precise weights were compared. On PNDs 2, 8, 11, and 18, the average weight of milk obtained via aspiration of control mice was 0.072, 0.1906, 0.2547, and 0.0457 g, respectively, demonstrating over a 3.5-fold increase in weight from PND2 to 11 and a 5.6-fold drop from PND11 to 18.
Table 1.
Milk-borne PFOAa as a percentage of dam serum concentrations over lactation.
| Single GD17 PFOA exposure | PND1 serum | PND4 serum | PND8 serum | PND8 serum | PND18 serum |
|---|---|---|---|---|---|
| PND2 milk | PND8 milk | PND11 milk | PND18 milk | ||
| 0.1 mg PFOA/kg | 15% | 31% | 27% | 11% | 36% |
| 1.0 mg PFOA/kg | 37% | 56% | 21% | 21% | 24% |
| 5.0 mg PFOA/kg | 25% | 36% | 13% | 13% | 18% |
PFOA = perfluorooctanoic acid, GD = gestational day, PND = postnatal day. The milk:serum PFOA ratio reported above was calculated as: [concentration of milk PFOA/concentration of serum PFOA] × 100 = % milk:serum for each dam within a dose group. These values were averaged and reported above.
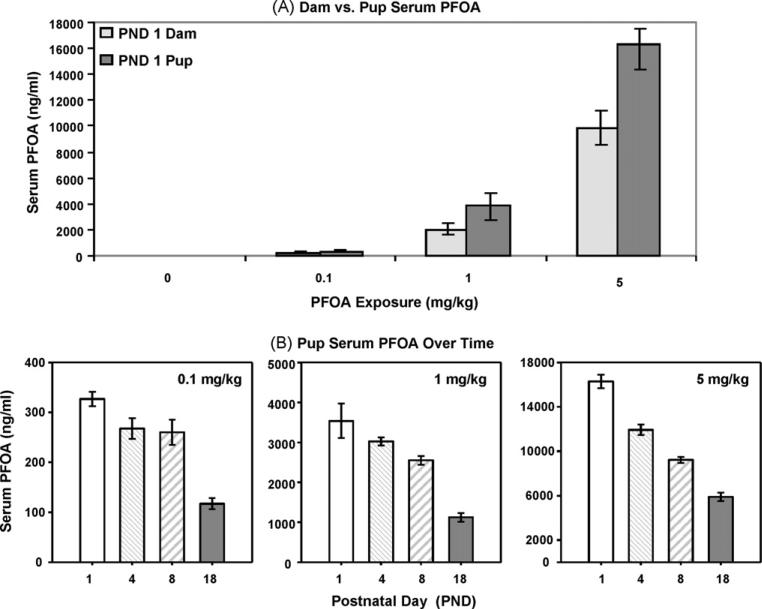
3.4. PFOA concentrations in the pups
Pup serum PFOA concentration was evaluated on PNDs 1, 4, 8, and 18. In comparing the average PFOA concentrations in PND1 pups vs. their respective dams (Fig. 4A; whole control pups and control serum < LOQ), it appeared that circulating pup serum PFOA concentrations were significantly higher than those measured in dams, regardless of dose (p < 0.05). Although pups possessed a substantially higher serum PFOA concentration than dams, the difference in pup and dam blood volumes at those stages of pup development are considerable. Regardless of those differences, heightened circulating PFOA in pup sera reflected increased exposures, proportional to dose throughout lactation (i.e., mean 10.4-fold and 4.3-fold increases between 0.1 to 1.0 mg/kg and 1.0 to 5.0 mg/kg exposures, respectively).
Fig. 4.
Neonatal transfer of PFOA to pups. (A) A significantly higher PFOA concentration in pup vs. dam serum on PND1 was noted (p < 0.05; v:v). (B) Pooled pup serum PFOA concentrations did not demonstrate a U-shaped curve, but gradually declined over time, presumably due to dilution of dose by increased growth-related blood volume. Data are shown as mean ± SEM.
Unlike their dams, pups did not demonstrate U-shaped serum PFOA concentration curves (Fig. 4B). Pup serum PFOA concentrations continued to exceed the average dam serum PFOA concentrations over time, until PND18 when the pup and dam concentrations approached 1:1. When the PFOA concentration (ng/g) was evaluated in whole pups (pup and blood; Fig. 5 left panels), a decline in PFOA concentration was detected over time, across all doses. However, when the rapidly increasing body weight of the pups was taken into consideration to calculate the total amount of PFOA in the neonate (as shown in Fig. 1), a completely different trend was noted (Fig. 5 right panels). Regardless of exposure dose, PFOA body burden (adjusted for weight) rose through the peak of lactation and had begun to decline by PND18, demonstrating an inverse U-shaped curve. When the administered PFOA dose and measured body burden in whole pups (body weight taken into effect) were compared, the administered PFOA:measured PFOA ratio was no longer proportional throughout lactation, and were unlike the ratios reported for dam and pup serum PFOA. Mean body burden ratios of 13.2-fold (range 11.1–17.8) and 4.3-fold (range 3.2–5.1) increases between 0.1 to 1.0 mg/kg and 1.0 to 5.0 mg/kg exposures, respectively, were determined.
4. Discussion
These data confirm that on a concentration-based comparison, gestationally PFOA-exposed pups exhibited a significantly larger serum PFOA load than their dam. That substantial serum PFOA load in pups was evident 24 h after a single exposure, and was apparently due to blood-borne (transplacental) transfer. Another important discovery is the U-shaped PFOA concentration over time, regardless of dose, in the dam mammary tissue, milk, and serum. This unique PFOA response was not detected in pups or pup serum, and was evident to a lesser extent in the dams’ urinary excretion curves. However, when PFOA body burden in whole pups was the unit of measure, an inverse U-shaped curve was apparent, and the PFOA burden of pups is proposed to increase due to milk-borne PFOA intake.
The decline in concentration seen in the milk, mammary and serum U-shaped curves is hypothesized to be due to hydro-dilution associated with increased blood and milk volumes. Several physiological conditions are changed during lactation that have been well documented in rats and directly relate to mice, as their lactation period is of the same length. A decrease in total plasma proteins due to increased blood volume, cardiac output, and blood flow to certain tissues, such as the mammary gland, has been reported in rats [26,27]. Elevated blood volume is due to increased plasma volume [27]. Milk yield (g/h) in rats was reported to reach its peak by PND10 [27] and the rat mammary gland reaches its maximum size (as % body weight) by PND15 [26], with a steep rise in size from PND5–15. Rat mammary gland blood flow and volume of milk produced are directly related, when measured on PND15 [26]. Total serum proteins are lower in lactating rats that those measured in non-lactating rats [27], and in humans, serum albumin concentration decreases during pregnancy and early lactation [28]. Further, at 14 days postpartum, the cardiac output of lactating rabbits was 30% higher than that in non-lactating animals, and the mammary gland was the only organ shown to increase in weight, relative to body weight [29].
Although a complete set of data that could address the exact reason for the U-shaped curves during lactation was not collected in this study, the aspirated milk weights did reveal a dramatic increase in milk volume (assumed due to weight change) from PND2 up to the peak of lactation (PND11). This dramatic change in volume (weight) may explain the decrease in milk PFOA concentration seen between PND2 and PND11. PFOA also appears to concentrate in serum and milk near the end of lactation (PND18, for example) when pups are eating more chow and suckle less often. Mammary gland blood flow has been reported to decrease by half in a 24 h period, after suckling rat offspring are removed from their dam [26], and this fall in mammary blood flow is directly associated with decreased cardiac output and % blood flow used by the mammary gland. In this study a precipitous drop in weight of milk collected between the peak of lactation and PND18 was noted, indicating a rapid decrease in milk volume. Therefore, the U-shape of the dam PFOA curves are proposed to be driven by physiological dilution and concentration of the PFOA load over the period of lactation, reaching the greatest dilution at or near the peak of lactation when the milk volume produced by the dam and consumed by the pups is the greatest. Increased consumption of milk up to PND11 likely directly contributed to the accumulation of body burden in the pup over this life stage.
A significant contribution of milk-borne PFOA transfer in CD-1 mice was detected in these studies. Previous reports in rats [22] and humans [18] have estimated that the dam PFOA milk:serum distribution ratio was 0.1 and 0.01, respectively. In the present study, the distribution ratio ranged from slightly more than 0.1 to over 0.5 in mice, depending on dose, with the lowest doses tested demonstrating the highest ratios over time. If the milk PFOA concentrations had been measured near the peak of lactation only (PND 8–11), the 0.1 milk:sera distribution estimate previously reported for rats in midlactation [22] may have also been presumed true in mice. However, at two periods during lactation (early and late) spikes of increased milk:serum ratios appeared, regardless of dose, with a substantial peak in milk PFOA concentrations on PND2. Although volumes of milk large enough to perform analytical measures prior to PND2 were not able to be obtained, we suspect, based on the significant PFOA concentrations in the PND1 mammary gland, that substantial milk PFOA concentrations would have been evident on PND1, as well, primarily due to being condensed in small milk volumes.
In previous reports by Lau et al. [13], Wolf et al. [16], and White et al. [14], decreased body weight gain and neonatal mortality were evident on several days just after birth in CD-1 mouse litters gestationally exposed to 3 mg/kg PFOA and higher. In fact, in a cross-foster study [16] demonstrating decreased body weight gain at 5 mg/kg from in utero exposure only, significant decreases in body weight gain were detected in the 3 mg/kg dose group only when in utero-exposed mice were also allowed to nurse from a PFOA exposed dam. Even at 5 mg/kg, there was no evidence of decreased pup body weight or neonatal mortality in the current study, following a single gestational PFOA exposure. Our PFOA measurements in whole pups indicate that the PFOA body burden accumulates in early life, and starts to decline as pups mature, open their eyes, and begin to eat chow and drink water. Our data and those demonstrating deleterious health outcomes suggest that the milk of gestationally PFOA-exposed mice was a major source of continued exposure to this compound for the developing pups.
As expected, large differences in dam and pup serum PFOA concentrations from those previously reported [14,16] were noted, and those differences bring to light the issue of single vs. multiple dose kinetics. As noted for PFOS, single dose kinetics may differ substantially from those involving repeated doses [30]. Concentration dependent changes in clearance can result in discrepancies between single and repeated dose kinetics.
A limited number of epidemiological studies have revealed associations between health outcomes (birth weight, head circumference) and cord blood or maternal serum PFOA concentrations in humans [20,21], while other studies failed to detect associations with later developmental milestones in infants [31]. Several studies have now measured PFAAs in human milk [17–19,32,33], however, only one study has been able to approximate the milk:serum relationship of PFOA transfer [18]. The reported 0.01 (1/100th) relationship was determined from a single voluntarily contributed sample at 3 weeks postpartum. According to the mouse milk:serum PFOA distribution over time that we report herein, the values reported in one human [18] and rats [22] may not be representative of the PFOA distribution to milk throughout lactation in those species.
In conclusion, these studies confirmed and further defined considerable PFOA exposures to mouse offspring following a single gestational exposure. They also demonstrated the accumulation of chemical over time in whole pups, which likely results from milk-borne PFOA, an exposure that had previously been incompletely assessed in other species. A single 0.1 mg/kg PFOA exposure to a pregnant mouse induced circulating serum PFOA concentrations of 44–216 ng/ml in dams and 117–326 ng/ml in pups; values comparable to serum PFOA concentrations of children that were accidentally exposed via DuPont production plant emission [34]. Because of evidence [15,35] demonstrating neonatal and latent health effects following developmental exposures to PFOA in mice, associated with higher circulating PFOA levels than those reported here, continued studies evaluating exposure–effect relationships are warranted in children.
Acknowledgements
The authors would like to thank Drs. Barbara Abbott (US EPA, Reproductive Toxicology Division) and Chester Rodriguez (National Center for Computational Toxicology, US EPA) for their constructive criticisms of this manuscript. We acknowledge the excellent care of these animals by New Year Tech., Inc. (Restin, VA). The research in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, US Environmental Protection Agency (EPA), and approved for publication. Findings in this report are those of the authors and approval does not signify this report reflects EPA policy. The use of trade names or commercial products does not constitute endorsement or recommendation for use.
Abbreviations
- ANOVA
analysis of variance
- BW
body weight
- GD
gestational day
- LOD
limit of detection
- LOQ
limit of quantitation
- MS
mass spectrometer
- PFAA
perfluoroalkyl acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctane sulfonate
- PND
postnatal day
- SEM
standard error of the mean
- UPLC
ultra-performance liquid chromatography
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
Conflict of interest statement
The author declares that there are no conflicts of interest.
References
- 1.U.S. Environmental Protection Agency [2/3/2009];Announcement of the 2010/15 PFOA Stewardship Program by Administrator Stephen L. Johnson (2006) Available at http://www.epa.gov/opptintr/pfoa/pubs/pfoastewardship.htm.
- 2.Andersen ME, Butenhoff JL, Chang SC, Farrar DG, Kennedy GL, Jr, Lau C, et al. Perfluoroalkyl acids and related chemistries—toxicokinetics and modes of action. Toxicol Sci. 2008;102:3–14. doi: 10.1093/toxsci/kfm270. [DOI] [PubMed] [Google Scholar]
- 3.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99:366–94. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 4.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuhler K. Estimating consumer exposure to PFOS and PFOA. Risk Anal. 2008;28:251–69. doi: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 6.Guruge KS, Manage PM, Yamanaka N, Miyazaki S, Taniyasu S, Yamashita N. Species-specific concentrations of perfluoroalkyl contaminants in farm and pet animals in Japan. Chemosphere. 2008;73:S210–215. doi: 10.1016/j.chemosphere.2006.12.105. [DOI] [PubMed] [Google Scholar]
- 7.Olsen GW, Huang HY, Helzlsouer KJ, Hansen KJ, Butenhoff JL, Mandel JH. Historical comparison of perfluorooctanesulfonate, perfluorooctanoate, and other fluorochemicals in human blood. Environ Health Perspect. 2005;113:539–45. doi: 10.1289/ehp.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao L, Kannan K, Kajiwara N, Costa MM, Fillmann G, Takahashi S, et al. Perfluorooctanesulfonate and related fluorochemicals in albatrosses, elephant seals, penguins, and polar skuas from the Southern Ocean. Environ Sci Technol. 2006;40:7642–8. doi: 10.1021/es061513u. [DOI] [PubMed] [Google Scholar]
- 9.Yeung LW, So MK, Jiang G, Taniyasu S, Yamashita N, Song M, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood samples from China. Environ Sci Technol. 2006;40:715–20. doi: 10.1021/es052067y. [DOI] [PubMed] [Google Scholar]
- 10.Olsen GW, Mair DC, Church TR, Ellefson ME, Reagen WK, Boyd TM, et al. Decline in perfluorooctanesulfonate and other polyfluoroalkyl chemicals in American Red Cross adult blood donors, 2000–2006. Environ Sci Technol. 2008;42:4989–95. doi: 10.1021/es800071x. [DOI] [PubMed] [Google Scholar]
- 11.Butenhoff JL, Gaylor DW, Moore JA, Olsen GW, Rodricks J, Mandel JH, et al. Characterization of risk for general population exposure to perfluorooctanoate. Regul Toxicol Pharmacol. 2004;39:363–80. doi: 10.1016/j.yrtph.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 2004;198:231–41. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, et al. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90:510–8. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 14.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96:133–44. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 15.White SS, Kato K, Jia LT, Basden BJ, Calafat AM, Hines EP, et al. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod Toxicol. 2009;27:289–98. doi: 10.1016/j.reprotox.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf CJ, Fenton SE, Schmid JE, Calafat AM, Kuklenyik Z, Bryant XA, et al. Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol Sci. 2007;95:462–73. doi: 10.1093/toxsci/kfl159. [DOI] [PubMed] [Google Scholar]
- 17.So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, et al. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ Sci Technol. 2006;40:2924–9. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- 18.Karrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, et al. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect. 2007;115:226–30. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao L, Kannan K, Wong CM, Arcaro KF, Butenhoff JL. Perfluorinated compounds in human milk from Massachusetts, U.S.A. Environ Sci Technol. 2008;42:3096–101. doi: 10.1021/es702789k. [DOI] [PubMed] [Google Scholar]
- 20.Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115:1670–6. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fei C, McLaughlin JK, Tarone RE, Olsen J. Fetal growth indicators and perfluorinated chemicals: a study in the Danish National Birth Cohort. Am J Epidemiol. 2008;168:66–72. doi: 10.1093/aje/kwn095. [DOI] [PubMed] [Google Scholar]
- 22.Hinderliter PM, Mylchreest E, Gannon SA, Butenhoff JL, Kennedy Jr GL. Perfluorooctanoate: placental and lactational transport pharmacokinetics in rats. Toxicology. 2005;211:139–48. doi: 10.1016/j.tox.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J Biochem Toxicol. 1991;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- 24.Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Fenton SE, Lindstrom AB, et al. Analysis of PFOA in dosed CD1 mice. Part 1. Methods development for the analysis of tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol. 2009;27:360–4. doi: 10.1016/j.reprotox.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott BD, Wolf CJ, Schmid JE, Das KP, Zehr RD, Helfant L, et al. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicol Sci. 2007;98:571–81. doi: 10.1093/toxsci/kfm110. [DOI] [PubMed] [Google Scholar]
- 26.Hanwell A, Linzell JL. The effects of engorgement with milk and of suckling on mammary blood flow in the rat. J Physiol. 1973;233:111–25. doi: 10.1113/jphysiol.1973.sp010300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki K, Hirose H, Hokao R, Takemura N, Motoyoshi S. Changes of plasma osmotic pressure during lactation in rats. J Vet Med Sci. 1993;55:561–4. doi: 10.1292/jvms.55.561. [DOI] [PubMed] [Google Scholar]
- 28.Dean M, Stock B, Patterson RJ, Levy G. Serum protein binding of drugs during and after pregnancy in humans. Clin Pharmacol Ther. 1980;28:253–61. doi: 10.1038/clpt.1980.158. [DOI] [PubMed] [Google Scholar]
- 29.Jones CS, Parker DS. Mammary blood flow and cardiac output during initiated involution of the mammary gland in the rabbit. Comp Biochem Physiol A: Comp Physiol. 1988;91:21–5. doi: 10.1016/0300-9629(88)91586-1. [DOI] [PubMed] [Google Scholar]
- 30.Harris LA, Barton HA. Comparing single and repeated dosimetry data for perfluorooctane sulfonate in rats. Toxicol Lett. 2008;181:148–56. doi: 10.1016/j.toxlet.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115:1677–82. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkel W, Genzel-Boroviczeny O, Demmelmair H, Gebauer C, Koletzko B, Twardella D, et al. Perfluorooctane sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human breast milk: results of a pilot study. Int J Hyg Environ Health. 2008;211:440–6. doi: 10.1016/j.ijheh.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 33.von Ehrenstein OS, Fenton SE, Kato K, Kuklenyik Z, Calafat AM, Hines EP. Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod Toxicol. 2009;27:239–45. doi: 10.1016/j.reprotox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J Occup Environ Med. 2006;48:759–70. doi: 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 2009;27:365–72. doi: 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]