Abstract
Problems in the perception of emotional material, in particular deficits in the recognition of negative stimuli, have been demonstrated in schizophrenia including in first-episode samples. However, it is largely unknown if emotion recognition impairment is present in people with subthreshold psychotic symptoms. Here, we examined the capacity to recognize facially expressed emotion and affective prosody in 79 individuals at ultra high-risk for psychosis, 30 clinically stable individuals with first-episode schizophrenia assessed as outpatients during the early recovery phase of illness, and 30 unaffected healthy control subjects. We compared (1) scores for a combined fear-sadness aggregate index across face and voice modalities, (2) summary scores of specific emotions across modalities, and (3) scores for specific emotions for each sensory modality. Findings supported deficits in recognition of fear and sadness across both modalities for the clinical groups (the ultra high-risk and first-episode group) as compared with the healthy controls. Furthermore, planned contrasts indicated that compared with the healthy control subjects, both clinical groups had a significant deficit for fear and sadness recognition in faces and for anger recognition in voices. Specific impairments in emotion recognition may be apparent in people at clinical high-risk for schizophrenia before the full expression of psychotic illness. The results suggest a trait deficit and an involvement of the amygdala in the pathology of ultra high-risk states.
Keywords: facial emotion labeling, affective prosody, ultra high-risk, schizophrenia, adolescents
Introduction
Individuals with schizophrenia experience problems in the perception of emotional material.1–3 The ability to recognize others’ emotional states is essential for social cognition to guide social functioning and behavior. Impairments in emotion recognition may therefore contribute to deficits in social functioning, which are present in people with schizophrenia throughout the course of the disorder,4,5 as well as in individuals ‘at-risk’ for psychosis.6–8
Rather than a general deficit that encompasses all emotions, schizophrenia may be associated with a more specific deficit in the processing of a subset of negative emotions including anger, disgust, sadness, and/or fear.1,9–12 For example, Edwards et al11 examined facial affect and affective prosody recognition in a representative sample of individuals with first-episode psychosis assessed as outpatients during the recovery phase of illness. Their findings supported small but consistent deficits in the recognition of fear and sadness in faces and across both sensory modalities for the combined schizophrenia and other psychotic disorders groups as compared with the affective psychoses group and nonpatients, independent of intelligence. The fact that the amygdala plays an important role in the recognition of fear13,14 and sadness14,15 and has also been suggested to be a relevant factor in the pathogenesis of schizophrenia on the basis of imaging studies16–18 provides a theoretical rationale for a fear/sadness recognition deficit in schizophrenia. However, there are also studies that have shown deficits for other emotions (eg, happiness, surprise, and neutral).1,19 One reason contributing to these inconsistencies in the literature is the substantial heterogeneity of applied tasks that vary in the number of emotions examined, response time, response format, and stimulus complexity with only few instances of identical procedures being adopted in more than 1 study.1 To avoid the influence of varying emotion recognition assessments, the present study used the same number of items and exposure times for the facial emotion–labeling task and the affective prosody task as proposed by Edwards and colleagues.11
The neurodevelopmental model of schizophrenia postulates that some deficits may occur before the onset of illness.20 If emotion recognition deficits represent a vulnerability-linked impairment, they may be apparent in people with subthreshold psychotic symptoms who are at elevated risk for schizophrenia.21 Two previous studies have investigated emotion perception in clinical high-risk populations. The first study, by Pinkham et al,8 did not detect differences between clinical high-risk individuals and control individuals for both emotion identification (ie, to identify which emotion a face expresses) and emotion discrimination (ie, to determine if 2 faces are displaying the same or different emotions). The second study, by Addington et al,22 reported that in high-risk individuals, the ability to identify emotions, but not the ability to discriminate between them, was significantly impaired compared with a nonpsychiatric control group and did not differ from patients with first-episode schizophrenia or chronic schizophrenia.
The present study extends the research in clinical at-risk groups by investigating specific emotions (fear, sadness, anger, disgust, surprise, happiness, and neutral) including stimuli in multiple modalities (eg, faces and voices). The aim of our study was to test the hypotheses that (1) ultra high-risk patients and (2) clinically stable patients with first-episode schizophrenia are characterized by impaired recognition of fear and sadness but not of other emotions using tasks previously used by Edwards et al.11 Confirmation of these hypotheses would be consistent with the view that a dysfunction of the amygdala underlies the emotion perception deficit in schizophrenia16 and provide new information on specific deficits in emotion perception in people with subthreshold manifestations of psychosis, as well as validate previous findings in first-episode schizophrenia.
Methods
Participants
The study was conducted in 79 individuals at ultra high-risk of psychosis, 30 individuals with first-episode schizophrenia, and 30 healthy controls. The ultra high-risk group comprised individuals who were aged 13–25 years and met the criteria for 1 or more of 3 operationally defined and well-validated23,24 groups of risk factors for psychosis: attenuated positive psychotic symptoms, transient psychosis, and genetic risk plus a decrease in functioning (table 1). The presence of attenuated psychotic symptoms (group 1) and transient psychosis (group 2) were determined in a semistructured interview by applying the Positive and Negative Syndrome Scale (PANSS)25 cut-off scores for symptom severity proposed by Morrison and colleagues26 and frequency and duration criteria of Yung et al.27 Group 3 comprised individuals with a family history of psychotic disorder in a first-degree relative (assessed with the Family History Research Diagnostic Criteria)28 or having a schizotypal personality disorder (as defined by Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM]-IV) and a decrease in functioning of 30% or more on the Global Assessment of Functioning Scale within the past year. The detailed ultra high-risk criteria are listed in table 1.
Table 1.
Ultra High-Risk Criteria
| Group 1: Attenuated psychotic symptoms |
| Presence of symptom scores of 3 on the Positive and Negative Syndrome Scale (PANSS) delusions scale, 2–3 on the PANSS hallucinations scale, 3–4 on PANSS suspiciousness or 3–4 on PANSS conceptual disorganization scale (frequency of symptoms ≥2 times per week for a period of at least a week and not longer than 5 years and to have occurred within the last year). |
| Group 2: Transient psychosis |
| Presence of symptoms scores of ≥4 on PANSS hallucinations scale, ≥4 on PANSS delusions scale, or ≥5 on PANSS conceptual disorganization scale (symptoms not sustained beyond a week and resolve without antipsychotic medication and have occurred within the last year). |
| Group 3: Trait plus state risk factors |
| Having a schizotypal personality disorder (as defined by DSM-IV) or a first-degree relative with a DSM-IV psychotic disorder and a significant decrease in functioning from premorbid level, resulting in a decrease of 30% on the Global Assessment of Functioning Scale, maintained for at least a month and not longer than 5 years. The decrease in functioning needed to have occurred within the past year. |
Note: PANSS, Positive and Negative Syndromes of Schizophrenia Scale; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.
The distribution of the criteria met by the subjects were as follows: attenuated psychotic symptoms (group 1), 50.6% (40/79); transient psychosis (group 2), 7.6% (6/79); trait plus state risk factors (group 3), 2.5% (2/71); attenuated psychotic symptoms plus transient psychosis (group 1 and group 2), 34.2% (27/79); attenuated psychotic symptoms plus trait plus state risk factors (group 1 and group 3), 5.1% (4/79). Diagnosis of first-episode schizophrenia was established on the basis of the Structured Clinical Interview for DSM-IV (SCID-P).29 Healthy controls were recruited from among the patient participants’ friends and patients’ schools; they underwent the same assessment procedure as the clinical groups. Study exclusion criteria were significant impaired vision (ie, blurred or less than 20/20 vision with correction), impaired auditory acuity, organic mental disorder, mental retardation, the presence of any documented neurological condition.
Procedure
The study was approved by the Ethics Committee of the Medical University of Vienna. All patients were consecutive admissions to a specialized psychosis detection and treatment unit at the Department of Child and Adolescent Psychiatry, Medical University of Vienna, Austria, between May 2003 and May 2006. All participants provided written informed consent, including parental consent for those less than 18 years of age. The ultra high-risk group was recruited for a randomized controlled trial of omega-3 fatty acids vs placebo which is described in detail elsewhere.30 The emotion recognition assessment in the ultra high-risk group was conducted as part of the trial baseline assessment, and all ultra high-risk individuals were free of antipsychotic medication. The first-episode schizophrenia group was assessed as outpatients during the early recovery phase of illness.
Measures
Psychopathology
The PANSS25 was used to assess psychiatric symptoms. Raters (M.R.S., K.P., M.S.) were experienced clinicians who were thoroughly trained in the administration of the PANSS before the beginning of the study. Interrater reliability estimates for PANSS subscales were excellent (all intraclass correlation coefficients >0·92). To maintain reliability between raters, videotaped interviews were used approximately every 3 months across the entire study period to avoid rater drift. The SCID-P for DSM-IV29 was used to ascertain psychiatric diagnoses.
Emotion Recognition
We applied emotion recognition assessments used by Edwards and colleagues.11 The facial emotion–labeling task was a computerized modification of the Feinberg et al31 procedure. Stimuli, comprising 21 slides from 110 slides of Ekman and Friesen,32 were photographs of faces representing standardized poses of fundamental emotions and included sadness, anger, happiness, disgust, surprise, fear, and neutral. Exposure time was 0.5 seconds. Three randomly selected practice items preceded the task and an attention-control task was administered which consisted of 4 single slides, showing numbers 1–10, interspersed throughout the labeling task. Near vision was tested using magazines. Distant vision was estimated by the ability to read the standard Snellen types at a distance of 20 feet. Prosody tasks were developed by Edwards and colleagues who hired four professional actors to speak 16 simple sentences—variations of the four sentences used by Roberts et al33: ‘they must stay here’; ‘he will come soon’; ‘she will drive fast’; and ‘we must go there’—each sentence being spoken by the actors in different moods. Item selections were based on responses of undergraduate students. The final task used 3 of the 4 actors and comprised 60 items across the 5 emotion categories of fear, sadness, anger, surprise, and neutral. Eight seconds of silence were inserted between items, and each speaker and task was preceded by 3 practice items, resulting in a recording of 23 minutes. Current IQ was measured with the Number-Combination Test.34
Statistical Analysis
Following research by Edwards et al,11 which showed that the greatest group differences between patients with schizophrenia and other psychotic disorders as compared with affective psychosis and healthy controls, were evident for fear and sadness recognition and that these response patterns were consistent across the facial affect and prosody tasks, an aggregate index was created by summing the respective facial and vocal subscale mean scores. More information on the rationale and construction of this index is provided by Edwards et al.11 (p247) In accordance with this index and with research showing a relationship between facial affect labeling and prosody recognition,35–38 we calculated summary scores across the emotion modalities, unweighted for facial affect and prosody. Facial affect and prosody recognition were also investigated independently. On the basis of findings from Edwards et al,11 we tested the hypotheses that in contrast to healthy controls, both patient groups (individuals at ultra high-risk for psychosis and individuals with first-episode schizophrenia) would be characterized by significant impairment in the fear/sadness (aggregate scale) recognition.
One-way ANOVA and subsequent ANCOVA with adjustments for age, current IQ, and psychopathology (PANSS total score) with sex as additional factor were applied to compare the percentages of correct emotion answers in ultra high-risk patients, first-episode schizophrenia patients, and healthy control subjects. Planned simple contrasts were undertaken comparing each of the ultra high-risk and first-episode schizophrenia groups to the control group. Effect sizes were calculated by dividing the adjusted mean difference for our particular contrasts of interest by the pooled standard deviation of all groups.39 This measure is similar to Cohen’s d where a value of 0.8 indicates a large effect size. Prior to this, the groups were compared on continuous demographic and symptom variables using ANOVA, with comparisons on categorical variables made using chi-square analysis. Pearson correlations were performed to determine associations between psychopathology and emotion tasks. A significance level of 0.05 was used for all statistical tests, and two-tailed tests were applied. Tests were carried out with the statistical package SPSS (version 17.0.2).
Results
Demographic and clinical characteristics are displayed in table 2. The groups differed significantly on sex (with a lower proportion of males in the ultra high-risk group), on current IQ (with lowest scores in the first-episode group), and on all PANSS subscales (with highest scores on the positive, global, and total symptom scale in the ultra high-risk group and highest scores on negative symptoms in the first-episode group).
Table 2.
Demographic Characteristics and Psychiatric Symptoms in Individuals at Ultra High-Risk for Psychosis, in Individuals With First-Episode Schizophrenia, and Healthy Controls
| Variable | Ultra High-Risk Subjectsa (N = 79) | First-Episode Schizophrenia Subjects (N = 30) | Healthy Control Subjects (N = 30) | Analysesb | ||||||||
| N | % | N | % | N | % | χ2 | df | P | ||||
| Demographic characteristics | ||||||||||||
| Male | 26 | 32.9 | 18 | 60.0 | 15 | 50.0 | 7.43 | 2 | 0.02 | |||
| Education | 0.40 | 2 | 0.82 | |||||||||
| Basic | 37 | 46.8 | 16 | 53.3 | 14 | 46.7 | ||||||
| Higher | 42 | 53.2 | 14 | 46.7 | 16 | 53.3 | ||||||
| Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI | F | df | P | |
| Age | 16.5 | 2.1 | 16–17 | 16.8 | 1.41 | 16–17 | 15.6 | 2.01 | 15–16 | 2.87 | 2, 136 | 0.06 |
| Current IQ | 99.3 | 15.6 | 96–103 | 90.8 | 10.0 | 87–95 | 104.4 | 11.1 | 100–109 | 7.64 | 2, 136 | 0.001 |
| Symptoms | ||||||||||||
| PANSS scores | ||||||||||||
| Positive symptom score | 14.5 | 3.1 | 12.4 | 4.1 | 7.2 | 0.9 | 60.3 | 2, 136 | <0.001 | |||
| Negative symptom score | 14.0 | 5.8 | 16.2 | 4.7 | 7.1 | 0.4 | 29.3 | 2, 136 | <0.001 | |||
| Global symptom score | 30.2 | 7.0 | 28.5 | 5.5 | 16.9 | 2.6 | 55.5 | 2, 136 | <0.001 | |||
| Total symptom score | 58.7 | 13.4 | 57.0 | 12.4 | 31.2 | 3.7 | 62.0 | 2, 136 | <0.001 | |||
Note:
Assessment of ultra high-risk subjects was based on state plus trait risk factors, attenuated symptoms, and/or brief limited intermittent psychotic symptoms.
One-way ANOVA or chi-square analysis for categorical data.
To test our hypotheses, each clinical group was compared with the group of healthy controls. Planned contrasts within the ANOVA framework indicated that as expected, both the ultra high-risk group (P = .005) and the first-episode schizophrenia group (P = .006) achieved a significantly lower percentage of correct fear/sadness emotion answers (as measured by the aggregate index across modalities) than the control group. Next, we determined if these group differences were influenced by demographic or illness-related factors. Planned contrasts from a subsequent ANCOVA, adjusting for the effects of age, current IQ, and psychiatric symptoms (PANSS total score) with diagnostic group and sex as factors confirmed significant group differences (ultra high-risk group vs controls, P = .002; first-episode group vs controls, P = .004), with no significant sex difference or sex-diagnostic group interaction being observed. Next, planned comparisons were undertaken investigating the summary scores across modalities (facial affect recognition and prosody tasks) for sadness, anger, surprise, fear, and neutrality. Both ANOVA and ANCOVA-derived planned contrasts were consistent in their results for each emotion. The adjusted mean percentages of correct emotion answers by group, and the P values of the planned contrasts, are displayed in table 3.
Table 3.
Adjusted Mean Percentages of Correct Emotion Answers in Individuals at Ultra High-Risk for Psychosis, in Individuals With First-Episode Schizophrenia, and Healthy Controls
| Planned Contrasts with Healthy Control Groupb | |||||||||||
| Variable | Ultra High-Risk Subjectsa (N = 79) | First-EpisodeSchizophreniaSubjects (N = 30) | Healthy Control Subjects (N = 30) | Ultra High-Risk Group | First-Episode Schizophrenia Group | ||||||
| Mean | SE | 95% CI | Mean | SE | 95% CI | Mean | SE | 95% CI | P | P | |
| Emotion recognitionc | |||||||||||
| Fear/Sadness score | 58.2 | 1.9 | 55–62 | 58.6 | 2.7 | 53–64 | 72.3 | 3.5 | 65–79 | 0.002 | 0.004 |
| Sadness score | 57.3 | 2.3 | 53–62 | 57.1 | 3.4 | 50–64 | 71.5 | 4.3 | 63–80 | 0.009 | 0.014 |
| Anger score | 79.4 | 1.8 | 76–83 | 71.0 | 2.7 | 66–76 | 79.7 | 3.3 | 73–86 | 0.954 | 0.059d |
| Surprise score | 81.0 | 2.1 | 77–85 | 87.2 | 3.2 | 81–93 | 84.8 | 4.0 | 77–93 | 0.452 | 0.650 |
| Fear score | 59.1 | 2.4 | 54–64 | 60.1 | 3.6 | 53–67 | 73.1 | 4.5 | 64–82 | 0.014 | 0.033 |
| Neutral score | 79.1 | 1.9 | 75–83 | 82.7 | 2.7 | 77–88 | 84.7 | 3.5 | 78–92 | 0.199 | 0.662 |
Note:
Assessment of ultra high-risk subjects was based on state plus trait risk factors, attenuated symptoms, and/or brief limited intermittent psychotic symptoms.
P values of ANCOVA-derived planned contrasts adjusted for age, current IQ, and PANSS total symptom score with sex as additional factor.
Summary scores across faces and voices.
The ANOVA-derived unadjusted P value was statistically significant.
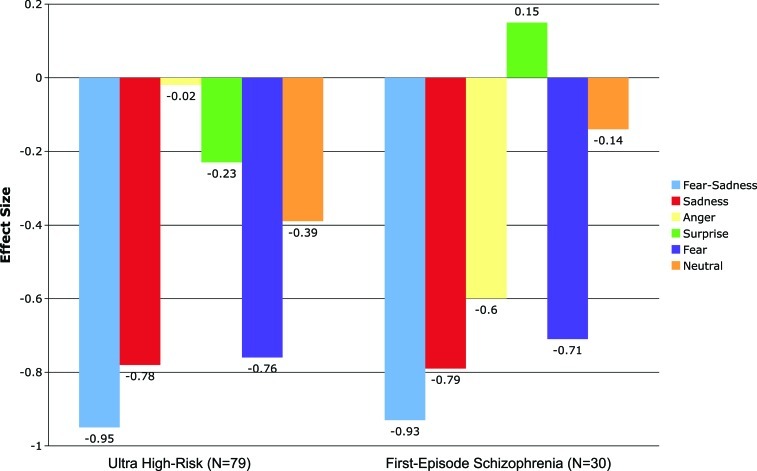
Consistent with our hypotheses, the planned contrasts indicated statistically significant differences between both clinical groups (the ultra high-risk and first-episode group) and the healthy control group in the recognition of fear and sadness. For all other emotions, the planned contrasts failed to attain statistical significance. The effect sizes for the differences of correct emotion answers across modalities between the clinical groups and the healthy control group are presented in figure 1. For the fear/sadness aggregate index effect sizes were large which is similar to the effect sizes reported in two recent meta-analyses.2,3
Fig. 1.
Effect sizes for emotion perception deficits across facial affect recognition and prosody tasks in ultra high-risk and first-episode schizophrenia patients.
Furthermore, we investigated facial emotion labeling and affective prosody recognition independently for each emotion. The adjusted mean percentages of correct emotion answers by group, and the P values of the planned contrasts for facial affect recognition and prosody tasks, are displayed in table 4.
Table 4.
Adjusted Mean Percentages of Correct Emotion Answers for Facial Affect and Affective Prosody Recognition in Individuals at Ultra High-Risk for Psychosis, in Individuals With First-Episode Schizophrenia, and Healthy Controls
| Planned Contrasts with Healthy Control Groupb | |||||||||||
| Variable | Ultra High-Risk Subjectsa (N = 79) | First-Episode SchizophreniaSubjects (N = 30) | Healthy Control Subjects (N = 30) | Ultra High-Risk Group | First-Episode Schizophrenia Group | ||||||
| Mean | SE | 95% CI | Mean | SE | 95% CI | Mean | SE | 95% CI | P | P | |
| Facial affect recognition | |||||||||||
| Sadness score | 63.7 | 0.03 | 57–70 | 63.8 | 0.05 | 54–73 | 82.2 | 0.06 | 70–94 | 0.017 | 0.028 |
| Anger score | 68.1 | 0.03 | 62–74 | 52.4 | 0.05 | 43–62 | 61.7 | 0.06 | 50–73 | 0.386 | 0.248c |
| Happiness score | 95.1 | 0.01 | 92–98 | 98.6 | 0.02 | 95–100 | 100.0 | 0.03 | 96–100 | 0.074 | 0.489 |
| Disgust score | 63.5 | 0.04 | 56–71 | 62.5 | 0.06 | 51–74 | 69.2 | 0.07 | 55–83 | 0.523 | 0.488 |
| Surprise score | 83.4 | 0.03 | 77–90 | 91.3 | 0.05 | 82–100 | 78.7 | 0.06 | 67–90 | 0.522 | 0.109 |
| Fear score | 56.6 | 0.05 | 48–65 | 55.8 | 0.07 | 43–69 | 81.9 | 0.08 | 66–98 | 0.017 | 0.022 |
| Neutral score | 86.2 | 0.02 | 81–91 | 91.5 | 0.04 | 85–99 | 91.7 | 0.05 | 83–100 | 0.328 | 0.976 |
| Affective prosody recognition | |||||||||||
| Sadness score | 51.0 | 0.03 | 45–57 | 50.4 | 0.04 | 42–59 | 60.8 | 0.06 | 50–72 | 0.158 | 0.163 |
| Anger score | 90.8 | 0.01 | 88–94 | 89.6 | 0.02 | 86–94 | 97.7 | 0.03 | 93–100 | 0.037 | 0.024 |
| Surprise score | 78.6 | 0.02 | 74–83 | 83.1 | 0.03 | 76–90 | 90.9 | 0.04 | 82–99 | 0.024 | 0.182c |
| Fear score | 61.6 | 0.02 | 58–66 | 64.3 | 0.03 | 58–70 | 64.4 | 0.04 | 57–72 | 0.562 | 0.984 |
| Neutral score | 72.1 | 0.02 | 68–77 | 73.8 | 0.03 | 67–81 | 77.7 | 0.04 | 69–86 | 0.298 | 0.504 |
Note:
Assessment of ultra high-risk subjects was based on state plus trait risk factors, attenuated symptoms, and/or brief limited intermittent psychotic symptoms.
P values of ANCOVA-derived planned contrasts adjusted for age, current IQ, and PANSS total symptom score with sex as additional factor.
The ANOVA-derived unadjusted P value was statistically significant.
For facial affect recognition, the planned contrasts indicated statistically significant differences between both clinical groups and the healthy control group for fear and sadness. Consistent with the findings combining both modalities, the planned contrasts failed to attain statistical significance for the other emotions. For affective prosody recognition, however, the planned contrasts revealed statistically significant differences between both clinical groups compared with the healthy control group for anger and between the ultra high-risk group and the control group for surprise. No significant differences were observed for affective prosody recognition of fear, sadness, and neutral. Finally, Pearson correlations between psychopathological measures (ie, PANSS scores) and emotion recognition tasks that significantly differed from the healthy comparisons were examined in the clinical groups. No significant correlations were observed in both groups.
Discussion
To our knowledge, this is the first study that has investigated the perception of specific emotions in 2 modalities in young people at ultra high-risk of psychosis. In line with the neurodevelopmental model of schizophrenia, our findings suggest that impairments in emotion recognition may be apparent before the full expression of psychotic illness. The observation that such deficits may be present in individuals with subthreshold psychotic symptoms, of whom only some will progress to schizophrenia or other psychoses, is consistent with the study by Addington and colleagues.22 However, their study did not determine if the deficit was specific to certain emotions. Deficits in emotion recognition have been reported in people with schizophrenia throughout the course of their illness, including in first-episode patients.1–3 However, because the adoption of identical emotion recognition assessment procedures across sites is rare in this field of research,1 our replication of previous findings (ie, for facial emotion recognition and across sensory modalities) in patients with first-episode schizophrenia11 is another important result of the present study.
Emotions are perceived bimodal by the ear and the eye. Most of our social interactions involve combining information from the face and the voice of other people (with the notable exception of telephone conversations).38 Research indicates that there are differences in the effectiveness with which the face and the voice convey different emotions.1,40 For example, happiness is the easiest facial expression to recognize, but when it comes to expression in the voice, happiness is harder to distinguish from other emotions. However, an intuitive rationale for creating summary scores across communication channels is provided by the common quality of an emotion: eg, both an angry face as well as an angry voice convey the information that the person is angry.40 Additional empirical support for the evaluation across sensory modalities is provided by research showing a relationship between face and voice expression recognition.11,35–38
Emotion recognition is fundamental to social cognition and social functioning. It is well established that deficits in social functioning often predate the onset of illness in schizophrenia.6,8 The nature of the underlying dysfunction is still only partially understood. Impaired emotion recognition has been related to social dysfunction in people with schizophrenia.41 Impaired emotion recognition may therefore be one mechanism among the factors contributing to social impairment in people with schizophrenia. In social situations, inaccurate decoding of emotional expression is a source of stress and a barrier to social interactions and communication.42 Stress may exacerbate symptoms in people with schizophrenia,43 and possibly also play a role in the onset of frank psychosis in high-risk individuals.44 Although we did not observe significant associations between emotion recognition deficits and PANSS summary scores the inaccurate decoding of emotions may also serve as a building block in delusion formation.26,45 We will address this hypothesis in detail in a future analysis investigating individual symptoms selected from the PANSS.
Three anatomical regions of the brain—the lateral fusiform gyrus, the superior temporal sulcus, and the amygdala—have been associated with facial and emotional perception.46,47 The lateral fusiform gyrus subserves selective activation to faces and predominately processes static aspects of the face. The superior temporal sulcus is more involved in the dynamic aspects of the face, such as changes in the shape of the eyes and mouth which indicate different emotions. The amygdala is a multimodal structure, receiving information from several sensory sources including the visual, auditory, gustatory, and olfactory systems.48 Although one recent functional imaging study has demonstrated amygdala activation to all emotions presented on faces,49 the amygdala may have a particular importance in the recognition of negative and threatening stimuli.50 More specifically, lesion and imaging studies suggest that the amygdala may be closely linked to the recognition of fearful13,51 and sad facial expressions.15,52 However, a recent meta-analysis reported that happy faces, as well as fearful and sad faces, also activated the amygdala, whereas angry or disgusted faces had no effect on this brain region.14 Structural and functional abnormalities of the amygdala and the fusiform gyrus, and impaired facial emotion recognition, have been both reported in schizophrenia and ultra high-risk states.16,24,53 Hence, these abnormalities may underlie the dysfunction in facial emotion recognition observed in the present study. Support for this view is provided by a high-resolution magnetic resonance imaging (MRI) study that found a correlation between left amygdala volume and the recognition of sadness in facial expressions.54 Our results for facial emotion labeling are also consistent with imaging findings reporting an involvement of the amygdala and the fusiform gyrus in the pathology of ultra high-risk states.24
In the study by Edwards et al,11 the group with schizophrenia, like both clinical groups in our study, did not perform significantly worse than nonpatients on identifying fear and sadness in the auditory tasks. Positron emissions tomography and functional MRI studies have demonstrated the importance of the amygdala and anterior insula in processing vocal emotion.55 The amygdala has been shown to respond to emotional vocalizations of fear37,48,56 and sadness,48,57 although it may also be involved in the auditory recognition of other emotions (ie, anger).37,58 An unexpected finding of our study was that both clinical groups scored significantly lower than the healthy control group for prosodic recognition of anger. Similarly to the deficit in the recognition of facial expressions of fear and sadness, the deficit in the recognition of anger in voices is consistent with our hypothesis that the emotion recognition deficit in ultra high-risk individuals and in people with first-episode schizophrenia is associated with amygdala dysfunction. The deficit in anger recognition is even more interesting because anger, in contrast to fear or sadness in facial expressions, is the easiest emotion to recognize in a voice.1 It is therefore unlikely that the anger recognition deficit was due to a general cognitive impairment or task difficulty. Another unexpected finding of the study was that the ultra high-risk group had a significantly lower percentage of correct answers for surprise on the prosody task. To our knowledge, the functional neuroanatomy of perceiving surprise in voices has not yet been investigated.
Impairments in emotional processes are also evident in other psychiatric conditions59–61 as well as in neurological disorders which involve the dopaminergic system.62 For example, in patients with Parkinson’s disease emotion recognition deficits were found to be associated with decreased amygdala activation.63 In addition, the amygdala’s response to emotional tasks has been shown to be altered by the administration of dopaminergic drugs.64 These observations reinforce the relevance of the dopaminergic system to emotion recognition. The involvement of dopamine in emotional processes provides an important link between emotion recognition deficits and the pathophysiology of schizophrenia and subthreshold psychotic states.65 Anomalous dopaminergic states could contribute to both deficits in the recognition of emotional stimuli and to psychotic symptoms.66
Our study has several strengths. First, we investigated representative samples of consecutive ultra high-risk and first-episode patients recruited from a frontline public service, which supports the generalizability of the results. Second, all ultra high-risk patients were free of antipsychotic medication. Third, the emotion recognition tasks permitted examination across both the facial and vocal modalities. Fourth, the emotion recognition stimuli used have adequate well-documented reliability and validity.11 Fifth, the data analysis ruled out potential biases due to age, sex, current IQ, and psychiatric symptoms. Sixth, the design of the study was hypothesis driven. An important limitation of the study is its cross-sectional design that does not allow conclusions to be drawn if the emotion recognition deficit observed in individuals at ultra high-risk of psychosis is predictive of conversion to schizophrenia or other psychoses. Furthermore, we cannot rule out that the fear and sadness effects for the facial tasks may be specific to the shorter exposure times. However, Edwards et al11 reported the fear pattern for 500 ms was similar to the results for the 5 seconds exposure time, suggesting that the fear results, at least, were not simply a consequence of brief exposure times. There is also evidence suggesting that facial expressions can be perceived at much shorter stimulus exposure times67 (ie, 20 ms) and without conscious awareness.68 Another limitation of the study is that we cannot completely rule out that the results could reflect a difficulty effect, with fear and sadness being more difficult to recognize in faces than other emotions (eg, happiness). However, for anger and disgust, which are both equally difficult to recognize in faces, no differences between the clinical groups and the healthy controls were evident. The stage of illness of the sample (ie, ultra high-risk and first-episode patients) is a final limitation to the study findings. This seems relevant considering two recent studies providing support for a general perceptual deficit in samples of chronically ill patients with schizophrenia.69,70
This study is a first step toward elucidating the specific aspects of emotion perception impairment in young help-seeking people with subthreshold psychotic manifestations. To date, the knowledge of the interactions between emotion recognition, cognition, social functioning, and symptom formation in schizophrenia and other psychotic disorders is still very limited.4,45,71 These processes warrant further examination as they will enhance our understanding of illness development and provide new targets for the prevention of psychotic disorders.
Funding
Stanley Medical Research Institute (03T-315); Österreichische National Bank (National Bank of Austria) (9848). B.N. is supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award and a Ronald Phillip Griffith Fellowship.
Acknowledgments
We thank Dr Jane Edwards for providing us with the emotion recognition assessment tools used in this study and Dr Sherilyn Goldstone for careful editing of the final manuscript. The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 2.Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res. 2007;96:135–145. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 2003;160:815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- 6.Addington J, Penn D, Woods SW, Addington D, Perkins DO. Social functioning in individuals at clinical high risk for psychosis. Schizophr Res. 2008;99:119–124. doi: 10.1016/j.schres.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips LK, Seidman LJ. Emotion processing in persons at risk for schizophrenia. Schizophr Bull. 2008;34:888–903. doi: 10.1093/schbul/sbn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinkham AE, Penn DL, Perkins DO, Graham KA, Siegel M. Emotion perception and social skill over the course of psychosis: a comparison of individuals ‘at-risk’ for psychosis and individuals with early and chronic schizophrenia spectrum illness. Cogn Neuropsychiatry. 2007;12:198–212. doi: 10.1080/13546800600985557. [DOI] [PubMed] [Google Scholar]
- 9.Bediou B, Franck N, Saoud M, Baudouin JY, Tiberghien G, Dalery J, d'Amato T. Effects of emotion and identity on facial affect processing in schizophrenia. Psychiatry Res. 2005;133:149–157. doi: 10.1016/j.psychres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Bell M, Bryson G, Lysaker P. Positive and negative affect recognition in schizophrenia: a comparison with substance abuse and normal control subjects. Psychiatry Res. 1997;73:73–82. doi: 10.1016/s0165-1781(97)00111-x. [DOI] [PubMed] [Google Scholar]
- 11.Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res. 2001;48:235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- 12.Leppanen JM, Niehaus DJ, Koen L, Du Toit E, Schoeman R, Emsley R. Emotional face processing deficit in schizophrenia: a replication study in a South African Xhosa population. Schizophr Res. 2006;84:323–330. doi: 10.1016/j.schres.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 15.Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. J Cogn Neurosci. 2004;16:453–462. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- 16.Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Fudge JL, Powers JM, Haber SN, Caine ED. Considering the role of the amygdala in psychotic illness: a clinicopathological correlation. J Neuropsychiatry Clin Neurosci. 1998;10:383–394. doi: 10.1176/jnp.10.4.383. [DOI] [PubMed] [Google Scholar]
- 18.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 19.Pomarol-Clotet E, Hynes F, Ashwin C, Bullmore ET, McKenna PJ, Laws KR. Facial emotion processing in schizophrenia: a non-specific neuropsychological deficit? Psychol Med. 2010;40:911–919. doi: 10.1017/S0033291709991309. [DOI] [PubMed] [Google Scholar]
- 20.Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol. 2000;12:501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- 21.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 22.Addington J, Penn D, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for psychosis. Br J Psychiatry. 2008;192:67–68. doi: 10.1192/bjp.bp.107.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 24.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 25.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 26.Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185:291–297. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- 27.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 28.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JBW. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID I/P) [Google Scholar]
- 30.Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 31.Feinberg TE, Rifkin A, Schaffer C, Walker E. Facial discrimination and emotional recognition in schizophrenia and affective disorders. Arch Gen Psychiatry. 1986;43:276–279. doi: 10.1001/archpsyc.1986.01800030094010. [DOI] [PubMed] [Google Scholar]
- 32.Ekman P, Friesen WV. Palo Alto, CA: Consulting Psychologists Press; 1976. Pictures of Facial Affect. [Google Scholar]
- 33.Roberts C, Kinsella G, Wales R. Disturbances in processing prosodic features of language following right hemisphere lesions. Bull Post-graduate Com Med Univ Sydney. 1981;(suppl):172–183. [Google Scholar]
- 34.Oswald WD, Roth E. Number Combination Test (Zahlen-Verbindungs-Test; ZVT) Göttingen, Germany: Testzentrale; 1987. [Google Scholar]
- 35.Haskins B, Shutty MS, Kellogg E. Affect processing in chronically psychotic patients: development of a reliable assessment tool. Schizophr Res. 1995;15:291–297. doi: 10.1016/0920-9964(94)00081-i. [DOI] [PubMed] [Google Scholar]
- 36.Kucharska-Pietura K, David AS, Masiak M, Phillips ML. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br J Psychiatry. 2005;187:523–528. doi: 10.1192/bjp.187.6.523. [DOI] [PubMed] [Google Scholar]
- 37.Scott SK, Young AW, Calder AJ, Hellawell DJ, Aggleton JP, Johnson M. Impaired auditory recognition of fear and anger following bilateral amygdala lesions. Nature. 1997;385:254–257. doi: 10.1038/385254a0. [DOI] [PubMed] [Google Scholar]
- 38.Campanella S, Belin P. Integrating face and voice in person perception. Trends Cogn Sci. 2007;11:535–543. doi: 10.1016/j.tics.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Cortina JM, Nouri H. Effect Sizes for ANOVA Designs. Thousand Oaks, CA: Sage; 2000. [Google Scholar]
- 40.de Gelder B, Vroomen J. The perception of emotions by ear and by eye. Cognition & Emotion. 2000;14:289–311. [Google Scholar]
- 41.Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112:41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 42.Bediou B, Asri F, Brunelin J, et al. Emotion recognition and genetic vulnerability to schizophrenia. Br J Psychiatry. 2007;191:126–130. doi: 10.1192/bjp.bp.106.028829. [DOI] [PubMed] [Google Scholar]
- 43.van Os J, McGuffin P. Can the social environment cause schizophrenia? Br J Psychiatry. 2003;182:291–292. doi: 10.1192/bjp.182.4.291. [DOI] [PubMed] [Google Scholar]
- 44.Phillips LJ, McGorry PD, Garner B, et al. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. 2006;40:725–741. doi: 10.1080/j.1440-1614.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- 45.Blackwood NJ, Howard RJ, Bentall RP, Murray RM. Cognitive neuropsychiatric models of persecutory delusions. Am J Psychiatry. 2001;158:527–539. doi: 10.1176/appi.ajp.158.4.527. [DOI] [PubMed] [Google Scholar]
- 46.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 47.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 48.Fecteau S, Belin P, Joanette Y, Armony JL. Amygdala responses to nonlinguistic emotional vocalizations. Neuroimage. 2007;36:480–487. doi: 10.1016/j.neuroimage.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 49.Derntl B, Habel U, Windischberger C, et al. General and specific responsiveness of the amygdala during explicit emotion recognition in females and males. BMC Neurosci. 2009;10:91. doi: 10.1186/1471-2202-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- 51.Morris JS, Friston KJ, Buchel C, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, McCarthy G, Song AW, Labar KS. Amygdala activation to sad pictures during high-field (4 tesla) functional magnetic resonance imaging. Emotion. 2005;5:12–22. doi: 10.1037/1528-3542.5.1.12. [DOI] [PubMed] [Google Scholar]
- 53.McDonald B, Highley JR, Walker MA, et al. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: a postmortem study. Am J Psychiatry. 2000;157:40–47. doi: 10.1176/ajp.157.1.40. [DOI] [PubMed] [Google Scholar]
- 54.Namiki C, Hirao K, Yamada M, et al. Impaired facial emotion recognition and reduced amygdalar volume in schizophrenia. Psychiatry Res. 2007;156:23–32. doi: 10.1016/j.pscychresns.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Belin P, Fecteau S, Bedard C. Thinking the voice: neural correlates of voice perception. Trends Cogn Sci. 2004;8:129–135. doi: 10.1016/j.tics.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Phillips ML, Young AW, Scott SK, et al. Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sander K, Scheich H. Auditory perception of laughing and crying activates human amygdala regardless of attentional state. Brain Res Cogn Brain Res. 2001;12:181–198. doi: 10.1016/s0926-6410(01)00045-3. [DOI] [PubMed] [Google Scholar]
- 58.Sander D, Grandjean D, Pourtois G, et al. Emotion and attention interactions in social cognition: brain regions involved in processing anger prosody. Neuroimage. 2005;28:848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 59.Aigner M, Sachs G, Bruckmuller E, et al. Cognitive and emotion recognition deficits in obsessive-compulsive disorder. Psychiatry Res. 2007;149:121–128. doi: 10.1016/j.psychres.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 60.McClure EB, Pope K, Hoberman AJ, Pine DS, Leibenluft E. Facial expression recognition in adolescents with mood and anxiety disorders. Am J Psychiatry. 2003;160:1172–1174. doi: 10.1176/appi.ajp.160.6.1172. [DOI] [PubMed] [Google Scholar]
- 61.Rocca CC, Heuvel E, Caetano SC, Lafer B. Facial emotion recognition in bipolar disorder: a critical review. Rev Bras Psiquiatr. 2009;31:171–180. doi: 10.1590/s1516-44462009000200015. [DOI] [PubMed] [Google Scholar]
- 62.Salgado-Pineda P, Delaveau P, Blin O, Nieoullon A. Dopaminergic contribution to the regulation of emotional perception. Clin Neuropharmacol. 2005;28:228–237. doi: 10.1097/01.wnf.0000185824.57690.f0. [DOI] [PubMed] [Google Scholar]
- 63.Jacobs DH, Shuren J, Bowers D, Heilman KM. Emotional facial imagery, perception, and expression in Parkinson's disease. Neurology. 1995;45:1696–1702. doi: 10.1212/wnl.45.9.1696. [DOI] [PubMed] [Google Scholar]
- 64.Sprengelmeyer R, Young AW, Mahn K, et al. Facial expression recognition in people with medicated and unmedicated Parkinson's disease. Neuropsychologia. 2003;41:1047–1057. doi: 10.1016/s0028-3932(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 65.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 66.Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophr Res. 2002;58:159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 67.Hoschel K, Irle E. Emotional priming of facial affect identification in schizophrenia. Schizophr Bull. 2001;27:317–327. doi: 10.1093/oxfordjournals.schbul.a006877. [DOI] [PubMed] [Google Scholar]
- 68.Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 69.Butler PD, Abeles IY, Weiskopf NG, et al. Sensory contributions to impaired emotion processing in schizophrenia. Schizophr Bull. 2009;35:1095–1107. doi: 10.1093/schbul/sbp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leitman DI, Laukka P, Juslin PN, Saccente E, Butler P, Javitt DC. Getting the cue: sensory contributions to auditory emotion recognition impairments in schizophrenia. Schizophr Bull. 2010;36:545–556. doi: 10.1093/schbul/sbn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Addington J, Saeedi H, Addington D. Influence of social perception and social knowledge on cognitive and social functioning in early psychosis. Br J Psychiatry. 2006;189:373–378. doi: 10.1192/bjp.bp.105.021022. [DOI] [PubMed] [Google Scholar]