Abstract
Over the last 20 years, glutamatergic models of schizophrenia have become increasingly accepted as etiopathological models of schizophrenia, based on the observation that phencyclidine (PCP) induces a schizophrenia-like psychosis by blocking neurotransmission at N-methyl-D-aspartate (NMDA)-type glutamate receptors. This article reviews developments in two key predictions of the model: first, that neurocognitive deficits in schizophrenia should follow the pattern of deficit predicted based on underlying NMDAR dysfunction and, second, that agents that stimulate NMDAR function should be therapeutically beneficial. As opposed to dopamine receptors, NMDAR are widely distributed throughout the brain, including subcortical as well as cortical brain regions, and sensory as well as association cortex. Studies over the past 20 years have documented severe sensory dysfunction in schizophrenia using behavioral, neurophysiological, and functional brain imaging approaches, including impaired generation of key sensory-related potentials such as mismatch negativity and visual P1 potentials. Similar deficits are observed in humans following administration of NMDAR antagonists such as ketamine in either humans or animal models. Sensory dysfunction, in turn, predicts impairments in higher order cognitive functions such as auditory or visual emotion recognition. Treatment studies have been performed with compounds acting directly at the NMDAR glycine site, such as glycine, D-serine, or D-cycloserine, and, more recently, with high-affinity glycine transport inhibitors such as RG1678 (Roche). More limited studies have been performed with compounds targeting the redox site. Overall, these compounds have been found to induce significant beneficial effects on persistent symptoms, suggesting novel approaches for treatment and prevention of schizophrenia.
Key words: schizophrenia, NMDA receptors, neurophysiology, cognition, negative symptoms, glycine, glycine transport inhibitors
Introduction
Glutamatergic models of schizophrenia were first proposed approximately 20 years ago1–4 based on the early observation that phencyclidine (PCP), ketamine, and related drugs induced schizophrenia-like psychotic effects (Domino, this volume), followed later by the observation that these compounds induce their unique behavioral effects by blocking neurotransmission at N-methyl-D-aspartate-type glutamate receptors (NMDAR) 2 (Coyle, this issue) and that NMDAR antagonists such as ketamine could reproduce specific aspects of the disorder (Moghaddam, this issue). Since that time, neurochemical models based on actions of PCP and ketamine have become increasingly well established, with increased focus on glutamatergic dysfunction as a basis for both symptoms and cognitive dysfunction in schizophrenia.
At the time when the PCP/NMDA model was first proposed, it led to two strong forward predictions. First, it predicted that patterns of brain dysfunction in schizophrenia should follow the distribution and function of NMDAR within brain. Second, it predicted that treatments aimed at potentiating brain NMDAR function should be therapeutically beneficial. This article reviews studies performed over the past 20 years in support of these early etiological and treatment predictions. Treatment approaches predicted based on PCP/NMDA models are only now reaching pivotal clinical trials, with hope for developing treatments that may modify course of illness and ongoing symptoms.
Pathophysiological and Conceptual Implications of the PCP/NMDA Model of Schizophrenia
Dopamine theories of schizophrenia were first proposed in the early 1960s based on the fortuitous discovery of the antipsychotic effects of chlorpromazine and other D2 antagonists. More recently, the dopamine theory has been reconceptualized to view dopaminergic dysfunction simply as a final common pathway to psychosis, without necessarily implying intrinsic dysfunction of dopaminergic circuits themselves. 5 In this model, both extrinsic factors,such as NMDAR dysfunction, and intrinsic dopaminergic factors, such as genetic polymorphisms, contribute to the presumed dopaminergic hyperactivity of schizophrenia. An ongoing acknowledged limitation of dopaminergic models is that negative symptoms and patterns of cognitive deficit associated with schizophrenia are poorly modeled by either dopamine agonists or antagonists in humans. D1 receptors, which interact functionally with NMDAR, may represent an effective therapeutic target in schizophrenia. 6 7 Nevertheless, cortical dysfunction is not limited to brain regions with predominant dopaminergic function, but are instead generalized throughout brain, suggesting need for alternative conceptualizations.
As opposed to dopamine receptors, which are highly localized to frontostriatal brain systems, NMDAR show a widespread distribution in brain with high density in subcortical and cortical brain regions and, in cortex, in sensory and associative brain regions (figure 1). Thus, NMDAR models of schizophrenia predict widespread cortical dysfunction, with deficits that are regionally diffuse, but restricted to NMDAR-mediated processes. In particular, NMDA receptors are known to play a critical role in dopaminergic regulation, coincidence detection, cortical plasticity, “context dependent” processing, and nonlinear gain mechanisms (reviewed by Kantrowitz and Javitt) 8 . Over the past 20 years, significant advancements have been made in determining the basis for NMDAR dysfunction in schizophrenia, along with understanding the role played by NMDAR within sensory and associative brain regions, leading to convergent support for NMDAR-based models.
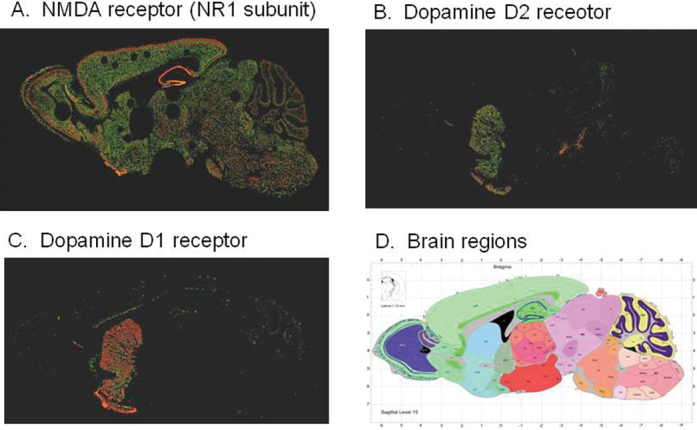
Fig. 1.
Relative distribution of N-methyl-D-aspartate (NMDAR) (A) and dopamine D2 (B) and D1 (C) receptors in brain. As opposed to dopamine receptors, NMDAR receptors are diffusely distributed throughout brain in both cortical and subcortical regions (D), consistent with recent findings of generalized brain dysfunction in schizophrenia. From Allen Brain Atlas (www.brain-map.org).
Evidence for NMDAR Dysfunction in Schizophrenia. Although mechanisms underlying NMDAR dysfunction in schizophrenia remain to be determined, both presynaptic, postsynaptic, and more general metabolic factors can be considered. Presynaptically, disturbances of glutamatergic release are reported (see Coyle, this volume), as well as associations with presynaptic genes such as dysbindin 9 10 may lead to impaired presynaptic function. Polymorphisms of the GRIN2B subunit have also been associated with schizophrenia, 11 12 as have abnormalities in genes regulating D-serine synthesis including serine racemase, D-amino acid oxidase (DAAO), 13 and DAAO activator/G72 14 and those regulating glutathione synthesis/oxidative stress. 15 NMDAR function also depend crucially on integrity of synaptic/dendritic function, and so can be influenced by growth factors such as neuregulin, actining through ErbB4 receptors. 16 Within each individual subject with schizophrenia, multiple factors may contribute, with NMDAR dysfunction representing a final common pathway to schizophrenia. 8
NMDAR Contributions to Dopaminergic Dysfunction in Schizophrenia. D2-blockers remain the primary treatments for schizophrenia. Nevertheless, mechanisms underlying dopaminergic dysfunction remain poorly understood. In vivo dopaminergic dysfunction has been demonstrated in schizophrenia by measurement amphetamine-induced dopamine release using D2 SPECT and/or PET radioreceptor imaging, 17 and, more recently using measures of presynaptic striatal dopaminergic metabolism. 18 Both effects are concentrated in associative, rather than limbic or sensorimotor, striatum. Deficits are presently conceptualized as being most related to expression of psychotic symptoms across disorders, rather than to the schizophrenia disease process. Thus, dopaminergic dysregulation is best viewed as final common pathway leading to psychosis in general, rather than specifically schizophrenia.
Despite the well-established nature of dopaminergic dysfunction in schizophrenia, underlying mechanisms remain unknown. Alterations in dopamine similar to those of schizophrenia are induced by ketamine administration in normal volunteers. 17 In addition, severity of psychotic symptoms observed after ketamine challenge correlates to levels of extracellular prefrontal dopamine release, 19 suggesting that NMDAR dysfunction on its own may be sufficient to account for dopaminergic dysfunction. In rodents, as in humans, PCP treatment leads to enhanced amphetamine-induced dopamine release in frontal cortex and dorsal—but not ventral—striatum, consistent with findings in schizophrenia. 20–22 Effects in rodents, moreover, are reversed by simultaneous treatment with NMDAR/glycine-site agonists, 21 23 supporting the role of NMDAR in dopaminergic regulation.
Hippocampal Dysfunction. At the time when the PCP/NMDA model was first proposed, the role of NMDAR in hippocampal long-term potentiation was already well established, as were analogous deficits in learning and long-term memory formation in schizophrenia. 2 These deficits continue to represent an area of pathology in schizophrenia that conforms poorly to dopaminergic models, but is accounted for well by underlying NMDAR dysfunction (see Tamminga, this volume). Regional changes in cerebral blood flow in the CA1 hippocampal subfield may also be among the earliest reflections of progression to psychosis. 24
Sensory Cortical Dysfunction in Schizophrenia. In addition to localization to striatum and hippocampus, NMDAR are diffusely distributed throughout cortex, with strong expression in sensory and higher cortical brain regions. Thus, whereas sensory function has classically been considered to be an “intact simple function” based on classical models in schizophrenia, 25 the significant expression of NMDAR within sensory regions predicts that significant deficits should be present even in early sensory processes and that sensory processes requiring NMDAR involvement should be particularly affected.
One of the first sensory processes shown to depend on intact NMDAR function is generation of the mismatch negativity (MMN) auditory event-related potential (ERP). MMN is generated in an auditory oddball paradigm, in which a sequence of repetitive tones is interrupted by physically or contextually deviant oddball stimuli. In this paradigm, therefore, the same physical tone elicits differential cortical response dependent on underlying context. MMN generation was first shown to depend specifically on NMDAR function based on studies in monkey auditory cortex. 26 This finding was subsequently confirmed in both humans and rodents (reviewed by Javitt et al.). 27 In contrast to ketamine, other psychotomimetic agents such as psilocybin 28 or LSD 29 do not disrupt MMN, although they do impair generation of other, frontoparietally generated potentials (eg, P3). Conversely, memantine, a compound that is often classified as an NMDAR antagonist but paradoxically does not produce psychosis in humans, 30 also paradoxically enhances MMN in humans 31 despite blocking MMN generation in rodents, 32 suggesting that MMN is sensitive to the net neurophysiological events associated with psychosis and cognitive impairments in humans, rather than to the a priori classification of a drug based on animal models.
Finally, in normal volunteers reduced MMN amplitude predicts susceptibility to ketamine-induced psychosis 33 while in patients with prodromal symptoms, reduced MMN amplitude predicts risk for psychosis, 34 suggesting that MMN indexes a subpopulation predisposed to development of schizophrenia. Other auditory ERP, including auditory N1 and the auditory steady-state response may also reflect underlying NMDAR dysfunction in both humans 35 and in animal models. 36
Similar deficits have also been observed within the early visual system. In particular, the magnocellular visual system functions primarily in a nonlinear gain mode, with large responses to low contrast (<12%) stimuli but saturating responses at high contrasts (>32%). In schizophrenia, gain of the magnocellular response function in blunted similar to observed in animal NMDAR models. 37 Over recent years, deficits in magnocellular function have been confirmed in schizophrenia using psychophysical-, neurophysiological-, and functional imaging-based approaches. 38–41
Finally, deficits in both auditory and visual processing in schizophrenia have been linked to impairments in higher order cognitive. Thus, deficits in auditory tone matching ability in schizophrenia lead to impairments in detection of tonal modulations in voice (“prosody”) which, in turn, lead to impairments in auditory emotion recognition and social cognition (figure 2A) 42 (Disturbances in visual processing lead to impairments in coding of visual information, which in turn affect such processes as face emotion recognition, 43 44 perceptual closure 40 (figure 2B) or continuous performance task performance 41 (figure 2C). Most recently, ketamine-induced reductions in a visual oddball task was associated primarily with activation reductions in sensory, as opposed to frontal, brain regions, 45 suggesting that many deficits that have been attributed to impairments of attention in schizophrenia may instead reflect impaired processing of physical stimulus features at the level of primary sensory cortex.
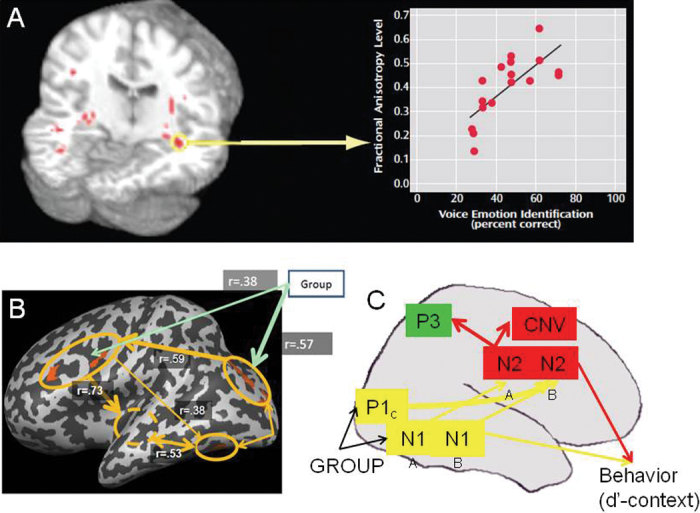
Fig. 2.
Sensory contributions to cognitive dysfunction in schizophrenia. In the auditory system, structural and functional disturbances at the level of primary auditory cortex correlate with impairments in higher auditory auditory function such as auditory emotion recognition 73 (A). In the visual system, deficits in early visual activation lead to impairments in frontal activation during tasks such as perceptual closure 40 (B) or AX-type continuous performance task (C) 41 performance.
Higher Cognitive Deficits in Schizophrenia. In addition to predicting that some, but not all, sensory level impairments will be impaired in schizophrenia, PCP/NMDA models predict that some, but not all, higher level cognitive functions will be impaired as well, dependent on underlying NMDAR involvement. NMDAR are present at high density within frontoparietal and temporal brain regions, suggesting that dysfunction of these receptors may underlie higher cognitive deficits associated in schizophrenia. For example, ketamine administration reproduces deficits in semantic priming, verbal fluency, auditory self-monitoring, and executive processing (reviewed by Kantrowitz et al.), 8 consistent with hypothesis that many of the higher cortical deficits in schizophrenia in fact reflect specifically impairments of NMDAR within these brain regions.
Notably, however, multiple paradigms have been identified that depend heavily on prefrontal function, which do not show impairments in schizophrenia. In particular, in task switching paradigms, subjects typically show a “switch cost” in which they are slower and less accurate responding immediately after a switch than they are immediately prior, related to functioning of underlying frontoparietal brain circuits. Despite the involvement of association brain regions in this task, patients do not show increased switch costs during task switching than controls, but do show increased slowing when the competing tasks would lead to conflicting vs identical responses. This pattern is similar to the pattern observed following ketamine administration in monkeys, 46 and suggests that even within higher brain regions only processes dependent on underlying NMDAR function are impaired, whereas NMDAR-independent functions are intact.
Brain Adaptations to Chronic NMDAR Function. Although many features of schizophrenia are reproduced by acute NMDAR antagonism, other features appear only following chronic administration. For example, schizophrenia-like auditory hallucinations are not observed during acute ketamine administration. In monkeys, such phenomena are observed during subchronic, but not acute ketamine administration, 47 suggesting that they may reflect secondary consequences of persistent NMDAR dysfunction. Psychotic symptoms, including hallucinations, are also observed in autoimmune disorders associated with anti-NMDA antibodies. 48 Although downstream consequences of NMDAR dysfunction have yet to be fully investigated, one critical mechanism appears to be oxidative stress, leading to downregulation of cortical parvalbumin (PV) neurotransmission. 49 This may lead particularly to impairments in generation of stimulus- and task-driven gamma in regions such as auditory and prefrontal cortex (see Lewis, this volume), reflecting local dysfunction within distributed brain regions.
Treatment Implications
A second major prediction of the PCP/NMDA model was that treatments that stimulate NMDAR receptor function should be therapeutically beneficial. The most direct test of this hypothesis comes from compounds that target specific binding sites on the NMDAR complex either directly or indirectly. Most studies have focused on the glycine/D-serine modulatory site, which was first characterized in 1987, 50 51 although a more recent study has targeted the redox/GSH site. 52 Most compounds studied to date have been compounds-of-convenience, which were able to be studied either because they are natural compounds or fortuitously cross-react with NMDAR as a secondary effect. These compounds have been used almost exclusively as add-on treatments, although one monotherapy study in acute patients has been reported. 53 Recently, however, high-affinity compounds have been developed for several proposed mechanisms, and entered into definitive clinical trials.
NMDAR Glycine Site Agonists. Initial controlled clinical studies with glycine were performed in the early 1990s. These studies showed significant proof of concept results, although doses required for treatment (approximately 60g/day) proved impractical for long-term use. 54 Subsequent studies were done with D-serine, which showed similar levels of benefit but at significantly lower doses (2–8g/day). A concern at higher doses is a potential for nephrotoxicity, although no significant adverse events have yet been observed at doses of ≤4g/day. Recent meta-analyses support use of full NMDAR agonists in combination with non-clozapine antipsychotics with moderate effect size across studies, not all of which were individually significant. 55–57
D-cycloserine, a partial NMDA/glycine-site agonist, has also been used for treatment of persistent symptoms (see Goff, this volume). Although less effective for symptomatic relief than full agonists during daily dosing, 55–57 they may be useful for cognitive remediation during persistent treatment. Drug companies have attempted repeatedly to develop novel, high potency direct agonists for the glycine binding site, but the small molecular size of this target has prevented further drug optimization.
Interestingly, potential beneficial effects of NMDAR agonists are not confined to behavioral symptoms of schizophrenia, but may extend to motor symptoms also. In most trials of NMDAR agonists, patients have had relatively low levels of motor symptoms because of inclusion/exclusion criteria and use of anticholinergics. However, in some trials, significant baseline Parkinsonian symptoms and tardive dyskinesia was observed. In such studies, highly significant, large effect size improvement in antipsychotic-induced motor symptoms was observed (figure 3). Although glycine-site agonists have been tested most for schizophrenia, the ability to manipulate NMDA receptors may be relevant to conditions others than schizophrenia, such as Parkinson’s disease, 58 where motor symptoms are primary.
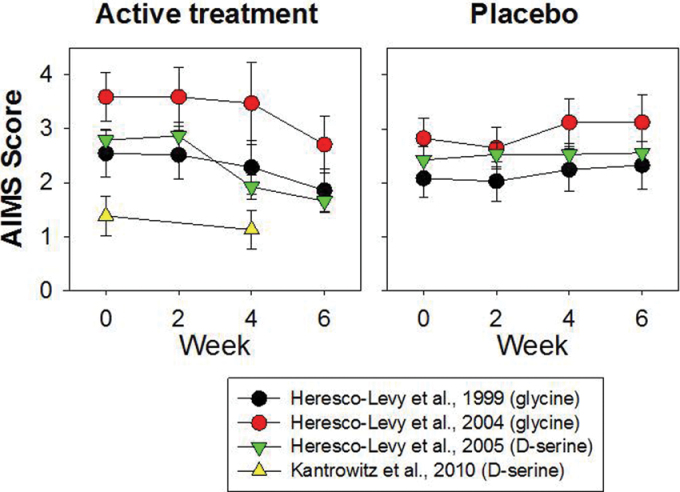
Fig. 3.
Effect of NMDAR glycine-site agonists on motor symptoms (tardive dyskinesia) as reflected in Abnormal Involuntary Movement Scale (AIMS) score. Data are from studies of glycine 74 75 or D-serine. 76 77 Across all studies, D-serine treatment led to a highly significant (t = 4.86, df = 192, P < .00001, d = .83) improvement in AIMS symptom, suggesting benefit of NMDAR agonist on motor and neuropsychological symptoms in schizophrenia.
Glycine Transport Inhibitors. Extracellular glycine levels in brain are typically above the level needed to fully saturate the glycine binding site of the NMDA receptor. NMDAR are protected from these circulating levels by glycine (GlyT1) transporters that are co-localized with NMDAR and serve to maintain low-subsaturating glycine levels within the local region of the NMDAR synapse (figure 4). Initial studies were performed with the low-affinity glycine derivative glycyldodecylamide (GDA). This compound was known to reverse PCP-induced hyperactivity at doses far lower than glycine itself, although mechanisms at the time were unknown. Studies initiated in the mid-1990s demonstrated that GDA induced its effects by blocking glycine transport, 59 thereby leading to increased glycine levels in the immediate vicinity of the NMDAR. A subsequent study demonstrated parallel structure activity relationships across a range of glycineamides, 60 as well as the ability of these compounds to modulate striatal dopamine release in vitro, 61 confirming the relevance of these compounds for schizophrenia.
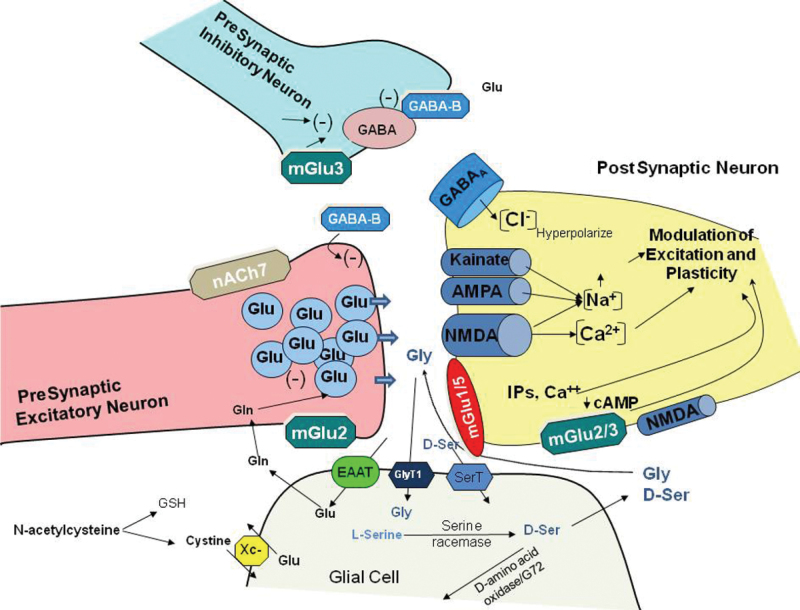
Fig. 4.
Schematic diagram of glutamate synapses showing potential sites of interaction for modulation of NMDAR function. mGlu, metabotropic glutamate receptor; nACh7, alpha7 nicotinic receptor; EAAT, excitatory amino acid transporter; GlyT1, glycine type 1 transporter; SerT, high-affinity D-serine transporter; Xc–, cystine/glutamate antiporter; Glu, glutamate; Gly, glycine; D-ser, D-serine; GSH, glutathione. Adapted from Javitt et al. 27
Initial follow-up studies were performed with the high-affinity compound NFPS, which, like GDA, was shown to enhance NMDA receptor-mediated neurotransmission and modulate dopamine in vitro and reverse PCP-induced hyperactivity and striatal dopamine release in vivo (reviewed by Javitt) 54 . Studies conducted throughout the past decade subsequently confirmed the effectiveness of high-affinity glycine transport inhibitors in a large variety of rodent 54 and primate 62 models relevant to schizophrenia. Most recently, the first of these compounds, bitopertin (RG1678, Roche) 63 has been entered into preclinical and clinical testing. As predicted, it was shown to induce significant beneficial effects on treatment refractory negative symptoms in individuals treated in accordance with protocol. Secondary outcome measures, including percentage responders, also favored bitopertin. GlyT1 occupancy associated with beneficial treatment response was in the range of 30%–50%, with loss of efficacy at higher occupancies suggesting a critical therapeutic window and a need for careful dose selection in phase 2 studies (Umbricht et al., unpublished data). 64 If confirmed in ongoing phase 3 studies, this will be the first compound developed specifically for treatment of schizophrenia based on PCP/NMDA models.
D-serine Modulators. The ability of glycine transport inhibitors to induce beneficial effects in animal models of schizophrenia as well as schizophrenia itself suggests that similar approaches to enhancement of NMDAR-mediated neurotransmission should also be possible through manipulation of brain D-serine levels. D-serine (SR) is synthesized in brain via serine racemase and degraded by D-amino acid oxidase (DAAO). Genes for both SR and DAAO have been linked to schizophrenia. In addition, reduced D-serine levels have been demonstrated in both serum and cerebrospinal fluid, 27 suggesting that D-serine disturbances may be closer to the pathophysiology of schizophrenia. Inhibition of DAAO is also reported to augment D-serine effectiveness in vitro, 65 although this approach may be limited by the low levels of DAAO expressed in cortex as opposed to cerebellum. Several D-serine transporters, including a novel alanine-sensitive transporters, 66 have also been identified, and thus may be appropriate targets for further treatment development.
Other Treatment Approaches. To the extent that schizophrenia results from underlying NMDAR dysfunction, manipulations that affect NMDAR function, presynaptic glutamate release or postsynaptic signal transduction may be therapeutically beneficial. In addition to the glycine/D-serine modulatory site, NMDAR contain a redox sensitive site that can be modulated by polyamines or glutathione (GSH). N-acetylcysteine (NAC), a precursor to GSH, has been studied in schizophrenia and observed to induce significant pro-therapeutic effects 52 and well as amelioration of neurophysiological deficits. 67 Elevations have been reported in levels of endogenous NMDAR antagonists such as homocysteine 68 or kynurenic acid, 69 suggesting that treatments designed to normalize such deficits may also be clinically beneficial.
Alpha7 nicotinic receptors are highly localized to presynaptic glutamate terminals and serve to enhance presynaptic glutamate release. Nicotinic activation potentiates NMDA receptor-mediated responses not only in prefrontal brain regions but also in visual and auditory regions, and so may affect sensory, as well as higher cognitive deficits associated with schizophrenia. Conversely, chronic PCP treatment downregulates frontal nicotinic receptor density in monkey cortex, 70 suggesting bidirectional interactions between nicotinic and NMDAR systems. Similarly, GABAB receptors may be located both presynaptically and postsynaptically, and may normalize presynaptic dopamine release, 71 leading to beneficial clinical response. 72
mGluR5 receptors are localized on postsynaptic terminals and serve to potentiate NMDAR response, suggesting potential efficacy for mGluR5 agonists or positive allosteric modulators, 27 while mGluR2/3 are located presynaptically and may serve to normalize presynaptic glutamate release (Moghaddam et al., this volume). Although many of these approaches are being tested in isolation, a critical issue is how these processes may interact, and which mechanisms produce synergistic vs competitive effects on underlying NMDAR function. At present, animal models are not required for further validation of these approaches because most are presently in early stage clinical development. In contrast, animal models may be critical to evaluate interaction between mechanisms and to enable next stages of clinical intervention.
Synthesis: Schizophrenia as the Clinical Condition Resulting from NMDAR Dysfunction
It has now been over 100 years since schizophrenia was first described, 50 years since the first description of PCP-induced psychotic symptoms, and 20 years since the realization of the potential role played by NMDAR dysfunction in the pathophysiology of schizophrenia. Despite this, the conceptualization of schizophrenia as a discrete illness continues to be elusive and available treatments for schizophrenia control symptoms, but do not appear to substantially modify the course of the underlying illness. Both genes and environmental influences have been identified that increase risk for schizophrenia, but typically with only small effect and large overlap between affected individuals and the general population. Fifty years after the PCP psychosis was first described, it remains the best acute intervention model for schizophrenia.
Rather than viewing schizophrenia as a disease with specific underlying pathophysiological process, findings to date suggest that the clinical syndrome of schizophrenia may reflect simply the behavioral pattern resulting from generalized disruption of NMDAR-mediated neurotransmission within brain. Similarly, ultimate treatment for schizophrenia may require correction of a range of underlying neurochemical disturbances that converge at the NMDAR, with direct stimulation of NMDAR representing only one component of a multipronged nutritional, psychopharmacological, and brain stimulation approach.
Funding
National Institute of Mental Health (R37 MH49334, P50 MH086385); National Institute on Drug Abuse (R01 DA03383 to DCJ).
Acknowledgments
Dr Javitt holds intellectual property rights for use of NMDA agonists, including glycine, D-serine, and glycine transport inhibitors in treatment of schizophrenia and related psychotic and movement disorders. Dr Javitt is a major shareholder in Glytech, Inc., and Amino Acids Solutions, Inc. Within the past year, Dr Javitt has served as a paid consultant to Sepracor, AstraZeneca, Cypress, Merck, Sunovion, Eli Lilly, Takeda, Guidepoint Global, and BMS. Dr Heresco holds intellectual property rights for use of D-serine in treatment of Parkinson’s disease and other movement disorders. Dr Zukin is an employee of Forest Research Institute. Dr Umbricht is an employee of Hoffman-La Roche.
References
- 1. Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia.Hillside J Clin Psychiatry 1987. 9 12–35 [PubMed] [Google Scholar]
- 2. Javitt DC,, Zukin SR. Recent advances in the phencyclidine model of schizophrenia.Am J Psychiatry 1991. 148 1301–1308 [DOI] [PubMed] [Google Scholar]
- 3. Olney JW,, Farber NB. Glutamate receptor dysfunction and schizophrenia.Arch Gen Psychiatry 1995. 52 998–1007 [DOI] [PubMed] [Google Scholar]
- 4. Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia.Harv Rev Psychiatry 1996. 3 241–253 [DOI] [PubMed] [Google Scholar]
- 5. Howes OD,, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway.Schizophr Bull 2009. 35 549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roberts BM, Seymour PA, Schmidt CJ, Williams GV, Castner SA. Amelioration of ketamine-induced working memory deficits by dopamine D1 receptor agonists.Psychopharmacology (Berl) 2010. 210 407–418 [DOI] [PubMed] [Google Scholar]
- 7. Slifstein M, Suckow RF, Javitch JA, Cooper T, Lieberman J, Abi-Dargham A. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride.J Cereb Blood Flow Metab 2011. 31 293–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kantrowitz JT,, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull 2010. 83 108–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnold SE, Talbot K, Hahn CG. Neurodevelopment, neuroplasticity, and new genes for schizophrenia.Prog Brain Res 2005. 147 319–345 [DOI] [PubMed] [Google Scholar]
- 10. Karlsgodt KH, Robleto K, Trantham-Davidson H, et al. Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance.Biol Psychiatry 2011. 69 28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database.Nat Genet 2008. 40 827–834 [DOI] [PubMed] [Google Scholar]
- 12. Li D,, He L. Association study between the NMDA receptor 2B subunit gene (GRIN2B) and schizophrenia: a HuGE review and meta-analysis.Genet Med 2007. 9 4–8 [DOI] [PubMed] [Google Scholar]
- 13. Shinkai T, De Luca V, Hwang R, et al. Association analyses of the DAOA/G30 and D-amino-acid oxidase genes in schizophrenia: further evidence for a role in schizophrenia.Neuromolecular Med 2007. 9 169–177 [DOI] [PubMed] [Google Scholar]
- 14. Li D,, He L. G72/G30 genes and schizophrenia: a systematic meta-analysis of association studies.Genetics 2007. 175 917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulak A, Cuenod M, Do KQ. Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder.Behav Brain Res 2012. 226 563–570 [DOI] [PubMed] [Google Scholar]
- 16. Hahn CG, Wang HY, Cho DS, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia.Nat Med 2006. 12 824–828 [DOI] [PubMed] [Google Scholar]
- 17. Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia.Arch Gen Psychiatry 2010. 67 231–239 [DOI] [PubMed] [Google Scholar]
- 18. Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia.Arch Gen Psychiatry 2009. 66 13–20 [DOI] [PubMed] [Google Scholar]
- 19. Aalto S, Ihalainen J, Hirvonen J, et al. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man.Psychopharmacology (Berl) 2005. 182 375–383 [DOI] [PubMed] [Google Scholar]
- 20. Balla A, Sershen H, Serra M, Koneru R, Javitt DC. Subchronic continuous phencyclidine administration potentiates amphetamine-induced frontal cortex dopamine release.Neuropsychopharmacology 2003. 28 34–44 [DOI] [PubMed] [Google Scholar]
- 21. Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycine-site agonists.Neuropsychopharmacology 2004. 29 300–307 [DOI] [PubMed] [Google Scholar]
- 22. Sershen H, Balla A, Aspromonte JM, Xie S, Cooper TB, Javitt DC. Characterization of interactions between phencyclidine and amphetamine in rodent prefrontal cortex and striatum: implications in NMDA/glycine-site-mediated dopaminergic dysregulation and dopamine transporter function.Neurochem Int 2008. 52 119–129 [DOI] [PubMed] [Google Scholar]
- 23. Balla A, Schneider S, Sershen H, Javitt DC. Effects of novel, high affinity glycine transport inhibitors on frontostriatal dopamine release in a rodent model of schizophrenia [published online ahead of print May 09, 2012].Eur Neuropsychopharmacol. doi:10.1016/j.euroneuro.2012.03.006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schobel SA, Kelly MA, Corcoran CM, et al. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia.Schizophr Res 2009. 114 110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Javitt DC. Sensory processing in schizophrenia: neither simple nor intact.Schizophr Bull 2009. 35 1059–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia.Proc Natl Acad Sci USA 1996. 93 11962–11967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment.Sci Transl Med 2011. 3 102mr102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Umbricht D, Vollenweider FX, Schmid L, et al. Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: implications for the neuropharmacology of cognitive deficits in schizophrenia.Neuropsychopharmacology 2003. 28 170–181 [DOI] [PubMed] [Google Scholar]
- 29. Heekeren K, Daumann J, Neukirch A, et al. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis.Psychopharmacology (Berl) 2008. 199 77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rammes G, Danysz W, Parsons CG. Pharmacodynamics of memantine: an update.Curr Neuropharmacol 2008. 6 55–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Korostenskaja M, Nikulin VV, Kicic D, Nikulina AV, Kähkönen S. Effects of NMDA receptor antagonist memantine on mismatch negativity.Brain Res Bull 2007. 72 275–283 [DOI] [PubMed] [Google Scholar]
- 32. Tikhonravov D, Neuvonen T, Pertovaara A, et al. Dose-related effects of memantine on a mismatch negativity-like response in anesthetized rats.Neuroscience 2010. 167 1175–1182 [DOI] [PubMed] [Google Scholar]
- 33. Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers.Biol Psychiatry 2002. 51 400–406 [DOI] [PubMed] [Google Scholar]
- 34. Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity.Biol Psychiatry 2011. 69 959–966 [DOI] [PubMed] [Google Scholar]
- 35. Hong LE, Summerfelt A, Buchanan RW, et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine.Neuropsychopharmacology.2010;35:632–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bickel S,, Javitt DC. Neurophysiological and neurochemical animal models of schizophrenia: focus on glutamate.Behav Brain Res 2009. 204 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia.Arch Gen Psychiatry 2005. 62 495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia.Annu Rev Clin Psychol 2009. 5 249–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martínez A, Hillyard SA, Bickel S, Dias EC, Butler PD, Javitt DC. Consequences of magnocellular dysfunction on processing attended information in schizophrenia.Cereb Cortex 2012;22:1282–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sehatpour P, Dias EC, Butler PD, et al. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study.Arch Gen Psychiatry 2010. 67 772–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia.Arch Gen Psychiatry 2011. 68 654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kantrowitz JT, Leitman DI, Lehrfeld JM, et al. Reduction in tonal discriminations predicts receptive emotion processing deficits in schizophrenia and schizoaffective disorder [published online ahead of print July 05, 2011].Schizophr Bull doi:10.1093/schbul/sbr060.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Butler PD, Abeles IY, Weiskopf NG, et al. Sensory contributions to impaired emotion processing in schizophrenia.Schizophr Bull 2009. 35 1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Norton D, McBain R, Holt DJ, Ongur D, Chen Y. Association of impaired facial affect recognition with basic facial and visual processing deficits in schizophrenia.Biol Psychiatry 2009. 65 1094–1098 [DOI] [PubMed] [Google Scholar]
- 45. Musso F, Brinkmeyer J, Ecker D, et al. Ketamine effects on brain function—simultaneous fMRI/EEG during a visual oddball task.Neuroimage 2011. 58 508–525 [DOI] [PubMed] [Google Scholar]
- 46. Wylie GR, Clark EA, Butler PD, Javitt DC. Schizophrenia patients show task switching deficits consistent with N-methyl-d-aspartate system dysfunction but not global executive deficits: implications for pathophysiology of executive dysfunction in schizophrenia.Schizophr Bull 2010. 36 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Linn GS, O’Keeffe RT, Lifshitz K, Schroeder C, Javitt DC. Behavioral effects of orally administered glycine in socially housed monkeys chronically treated with phencyclidine.Psychopharmacology (Berl) 2007. 192 27–38 [DOI] [PubMed] [Google Scholar]
- 48. Wandinger KP, Saschenbrecker S, Stoecker W, Dalmau J. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis.J Neuroimmunol 2011. 231 86–91 [DOI] [PubMed] [Google Scholar]
- 49. Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia.Neuropharmacology.2012;62:1322–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Johnson JW,, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons.Nature 1987. 325 529–531 [DOI] [PubMed] [Google Scholar]
- 51. Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release.Proc Natl Acad Sci USA 1995. 92 3948–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial.Biol Psychiatry 2008. 64 361–368 [DOI] [PubMed] [Google Scholar]
- 53. Lane HY, Liu YC, Huang CL, et al. Sarcosine (N-methylglycine) treatment for acute schizophrenia: a randomized, double-blind study.Biol Psychiatry 2008. 63 9–12 [DOI] [PubMed] [Google Scholar]
- 54. Javitt DC. Glycine transport inhibitors for the treatment of schizophrenia: symptom and disease modification.Curr Opin Drug Discov Devel 2009. 12 468–478 [PubMed] [Google Scholar]
- 55. Tuominen HJ, Tiihonen J, Wahlbeck K. Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis.Schizophr Res 2005. 72 225–234 [DOI] [PubMed] [Google Scholar]
- 56. Tsai GE,, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis.Curr Pharm Des 2010. 16 522–537 [DOI] [PubMed] [Google Scholar]
- 57. Singh SP,, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia.CNS Drugs 2011. 25 859–885 [DOI] [PubMed] [Google Scholar]
- 58. Gelfin E, Kaufman Y, Korn-Lubetzki I, et al. D-serine adjuvant treatment alleviates behavioural and motor symptoms in Parkinson’s disease.Int J Neuropsychopharmacol.2011. 1–7 [DOI] [PubMed] [Google Scholar]
- 59. Javitt DC, Sershen H, Hashim A, Lajtha A. Reversal of phencyclidine-induced hyperactivity by glycine and the glycine uptake inhibitor glycyldodecylamide.Neuropsychopharmacology 1997. 17 202–204 [DOI] [PubMed] [Google Scholar]
- 60. Javitt DC, Balla A, Sershen H, Lajtha A. A.E. Bennett Research Award. Reversal of phencyclidine-induced effects by glycine and glycine transport inhibitors.Biol Psychiatry 1999. 45 668–679 [DOI] [PubMed] [Google Scholar]
- 61. Javitt DC, Sershen H, Hashim A, Lajtha A. Inhibition of striatal dopamine release by glycine and glycyldodecylamide.Brain Res Bull 2000. 52 213–216 [DOI] [PubMed] [Google Scholar]
- 62. Roberts BM, Shaffer CL, Seymour PA, Schmidt CJ, Williams GV, Castner SA. Glycine transporter inhibition reverses ketamine-induced working memory deficits.Neuroreport 2010. 21 390–394 [DOI] [PubMed] [Google Scholar]
- 63. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfon yl-2-((S)-2,2,2-trifluoro-1- methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia.J Med Chem. 2010. 53 4603–4614 [DOI] [PubMed] [Google Scholar]
- 64. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia.Neuropsychopharmacol.2010. 35 s320–321 [Google Scholar]
- 65. Hashimoto K, Fujita Y, Horio M, et al. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine.Biol Psychiatry 2009. 65 1103–1106 [DOI] [PubMed] [Google Scholar]
- 66. Javitt DC, Balla A, Sershen H. A novel alanine-insensitive D-serine transporter in rat brain synaptosomal membranes.Brain Res 2002. 941 146–149 [DOI] [PubMed] [Google Scholar]
- 67. Lavoie S, Murray MM, Deppen P, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients.Neuropsychopharmacology 2008. 33 2187–2199 [DOI] [PubMed] [Google Scholar]
- 68. Roffman JL, Weiss AP, Purcell S, et al. Contribution of methylenetetrahydrofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia.Biol Psychiatry 2008. 63 42–48 [DOI] [PubMed] [Google Scholar]
- 69. Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia.Schizophr Bull 2010. 36 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hashimoto K, Nishiyama S, Ohba H, et al. [11C]CHIBA-1001 as a novel PET ligand for alpha7 nicotinic receptors in the brain: a PET study in conscious monkeys.PLoS ONE 2008. 3 e3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Balla A, Nattini ME, Sershen H, Lajtha A, Dunlop DS, Javitt DC. GABAB/NMDA receptor interaction in the regulation of extracellular dopamine levels in rodent prefrontal cortex and striatum.Neuropharmacology 2009. 56 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kantrowitz JT, Oakman E, Bickel S, et al. The importance of a good night’s sleep: an open-label trial of the sodium salt of gamma-hydroxybutyric acid in insomnia associated with schizophrenia.Schizophr Res 2010. 120 225–226 [DOI] [PubMed] [Google Scholar]
- 73. Leitman DI, Hoptman MJ, Foxe JJ, et al. The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents.Am J Psychiatry 2007. 164 474–482 [DOI] [PubMed] [Google Scholar]
- 74. Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia.Arch Gen Psychiatry 1999. 56 29–36 [DOI] [PubMed] [Google Scholar]
- 75. Heresco-Levy U. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia.Biol Psychiatr. 2004. 55 165–171 [DOI] [PubMed] [Google Scholar]
- 76. Heresco-Levy U, Javitt DC, Ebstein R, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia.Biol Psychiatry 2005. 57 577–585 [DOI] [PubMed] [Google Scholar]
- 77. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia.Schizophr Res 2010. 121 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]