Abstract
At present, all medications for schizophrenia function primarily by blocking dopamine D2 receptors. Over 50 years ago, the first observations were made that subsequently led to development of alternative, glutamatergic conceptualizations. This special issue traces the historic development of the phencyclidine (PCP) model of schizophrenia from the initial description of the psychotomimetic effects of PCP in the early 1960s, through discovery of the link to N-methyl-D-aspartate-type glutamate receptors (NMDAR) in the 1980s, and finally to the development of NMDA-based treatment strategies starting in the 1990s. NMDAR antagonists uniquely reproduce both positive and negative symptoms of schizophrenia, and induce schizophrenia-like cognitive deficits and neurophysiological dysfunction. At present, there remain several hypotheses concerning mechanisms by which NMDAR dysfunction leads to symptoms/deficits, and several theories regarding ideal NMDAR-based treatment approaches as outlined in the issue. Several classes of agent, including metabotropic glutamate agonists, glycine transport inhibitors, and D-serine-based compounds are currently in late-stage clinical development and may provide long-sought treatments for persistent positive and negative symptoms and cognitive dysfunction in schizophrenia.
Key words: schizophrenia, glutamate, NMDA receptor, glycine, D-serine, glycine transport inhibitor, metabotropic
The mid-20th century was an exciting period for drug development in psychiatry. Antipsychotics were developed based on the seminal observations of Delay and Deniker and linked to D2 blockade shortly thereafter. By 1971, clozapine, the current “gold standard” treatment for schizophrenia, had already been marketed. Antidepressants were developed based on clinical observations with isoniazid (INH) in the 1950s; benzodiazepines were developed based upon GABA receptor-binding assays in the 1960s; and definitive studies demonstrating efficacy of lithium were performed by the early 1970s. Decades later, these classes of compounds continue to form the core of today’s psychopharmacological armamentarium.
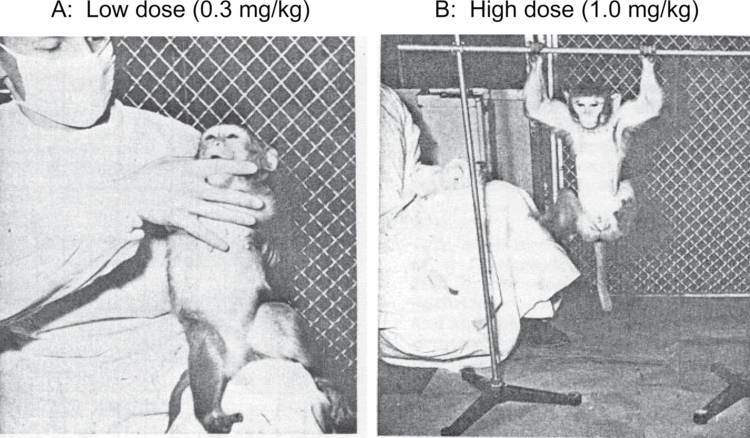
In the midst of this transformational period, initial reports appeared as well for a class of novel sedative agent termed “dissociative anesthetics” exemplified by the molecules phencyclidine (PCP, “angel dust”) and ketamine. In monkeys, these compounds produced behavioral symptoms closely resembling those of schizophrenia, including behavioral withdrawal at low dose and catalepsy at high dose (figure 1). Domino and Luby1 describe the critical steps by which he and his contemporaries verified the unique clinical effects of these compounds in man. The initial characterizations of PCP as causing a centrally mediated sensory deprivation syndrome and producing electroencephalography changes similar to those in schizophrenia were, in retrospect, particularly critical.
Fig. 1.
Effect of phencyclidine (PCP) on behavior in monkey, showing dissociation at low dose (A) and catatonia at high dose (B). From Chen and Weston.12
Although the clinical effects of PCP were well documented by the early 1960s, it took another 20 years to characterize these effects at the molecular level. As described by Coyle,2 key milestones along the way included the pharmacological identification of the PCP receptor in 1979; demonstration of electrophysiological interactions between PCP and N-methyl-D-aspartate-type glutamate receptors (NMDAR) in the early 1980s followed shortly thereafter by pharmacological confirmation; identification of the glycine modulatory site of the NMDAR in 1987; and confirmation of the psychotomimetic effects of ketamine in the mid-1990s. Although researchers still disagree to the paths leading from NMDAR blockade to psychosis, few currently dispute the concept that NMDAR serve as the molecular target of PCP, ketamine, dizocilpine (MK-801), and a host of other clinical psychotomimetic agents.2–4
At their simplest, glutamatergic models predict that compounds stimulating NMDAR function should be therapeutically beneficial in schizophrenia.2 , 4 Potential sites for intervention include the glycine/d-serine and redox sites of the NMDAR, as well as pathways regulating glutamate, glycine/d-serine, and glutathione synthesis/release.4 d-Cycloserine, a partial NMDAR glycine-site agonist, may enhance learning and neural plasticity across a range of disorders, including schizophrenia.5 In addition to providing new drug targets, glutamatergic models provide effective explanation for the hippocampal activation deficits,6 positive and negative symptoms, distributed neurocognitive deficits, and sensory processing abnormalities4 that are critical components of the pathophysiology of schizophrenia.
Since the original description, several variations have been developed with somewhat different treatment predictions. The term “NMDA receptor hypofunction” was originally developed to describe the vacuolization and neurodegeneration seen within specific brain regions following high-dose NMDAR antagonist administration.7 In animal models, neurotoxic effects of PCP were reversed by numerous compounds, including benzodiazepines and α2 adrenergic agonists that ultimately proved ineffective in clinical studies. Nevertheless, this model may explain the pattern of persistent frontotemporal neurocognitive deficits observed in some ketamine abusers.8 Subsequent hyperglutamatergic models focused on the excess glutamate release induced by NMDAR antagonists, particularly in prefrontal cortex, and prompted studies with compounds, such as lamotrigine or metabotropic glutamate receptor (mGluR) 2/3 agonists, that inhibit presynaptic glutamate release.9 GABAergic models focus on NMDAR antagonist-induced downregulation of parvalbumin (PV) expression in interneurons and resultant local circuit level (gamma) dysfunction, and suggest use of subunit selective GABAA receptor modulators.10
More than 50 years after the initial characterization of PCP, and 25 years after the identification of NMDARs as the molecular target of PCP, we still do not know whether the novel pharmacology of dissociative anesthetics can be translated into effective clinical treatments. Encouraging small-scale single site studies have been published with NMDAR agonists, but have not yet been replicated in academic multicenter trials. Encouraging phase 2 results have also recently been reported by Roche with glycine transport inhibitors.4 Nevertheless, phase 3 studies remain ongoing and results cannot be predicted. Additional beneficial effects may be observed in obsessive-compulsive disorder, substance abuse and Parkinsons disease.4 Conversely, NMDAR antagonists, such as ketamine, may be therapeutically beneficial in treatment-resistant depression or autism, suggesting complementary pathology across a range of disorders.11 More than anything else, 50 years of research shows that treatment development in neuropsychiatric disorders is a journey and not a destination, although fortunately one where the end now finally seems in sight.
References
- 1. Domino EF, Luby ED. Phencyclidine/schizophrenia: one view toward the past, the other to the future Schizophr Bull. 2012.. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coyle JT. The NMDA receptor and schizophrenia: a brief history Schizophr Bull. 2012.. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia Am J Psychiatry 1991. 148 1301–1308 [DOI] [PubMed] [Google Scholar]
- 4. Javitt DC. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia.Schizophr Bull In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goff D. D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia.Schizophr Bull In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamminga CA, Southcott S, Sacco C, Gao XM, Ghose S. Glutamate dysfunction in hippocampus: relevance of dentate gyrus and ca3 signaling.Schizophr Bull. 2012.. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia J Psychiatr Res. 1999. 33 523–533 [DOI] [PubMed] [Google Scholar]
- 8. Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study Addiction 2010. 105 121–133 [DOI] [PubMed] [Google Scholar]
- 9.Moghaddam B, Krystal JH. Capturing the angel in angel dust: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans Schizophr Bull. In press. [DOI] [PMC free article] [PubMed]
- 10.Lewis DA, Gonzalez-Burgos G. NMDA receptor hypofunction, parvalbumin-positive neurons and cortical gamma oscillations in schizophrenia. Schizophr Bull In press. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3:102mr102. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen GM, Weston JK. The analgesic and anesthetic effects of 1-(1-phenylcyclohexyl)-piperidine HCl in the monkey Anesth Analg. 1960. 39 132–137 [PubMed] [Google Scholar]