Abstract
Background: Neuroimaging studies in humans have implicated both dysfunction of the medial temporal lobe (MTL) and the dopamine system in psychosis, but the relationship between them is unclear. We addressed this issue by measuring MTL activation and striatal dopaminergic function in individuals with an At Risk Mental State (ARMS) for psychosis, using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), respectively. Methods: Thirty-four subjects (20 ARMS and 14 Controls), matched for age, gender, digit span performance, and premorbid IQ, were scanned using fMRI, while performing a verbal encoding and recognition task, and using 18F-DOPA PET. All participants were naïve to antipsychotic medication. Results: ARMS subjects showed reduced MTL activation when encoding words and made more false alarm responses for Novel words than controls. The relationship between striatal dopamine function and MTL activation during both verbal encoding and verbal recognition was significantly different in ARMS subjects compared with controls. Conclusion: An altered relationship between MTL function and dopamine storage/synthesis capacity exists in the ARMS and may be related to psychosis vulnerability.
Keywords: psychosis, prodromal, dopamine, medial temporal lobe, schizophrenia, PET, MRI
Introduction
Two of the most robust pathophysiological findings in patients with psychosis are elevated subcortical dopaminergic activity1–4 and medial temporal lobe (MTL) dysfunction.5 More recently, MTL and subcortical dopamine function have been investigated in subjects who are experiencing prodromal signs and symptoms of psychotic illness before the clinical expression of the disorder. As in patients with psychosis, prodromal subjects show alterations in MTL volume,6 resting state perfusion,7 activation during an episodic memory task,8 and elevated dopamine synthesis capacity and dopamine release in the striatum.9 Recently, a number of studies have investigated the relationship between dopamine, MTL function, and episodic memory. Bethus and colleagues10 examined the role of dopaminergic afferents to the MTL in memory processing in experimental animals and reported that activation of dopamine receptors in the hippocampus was critical for the persistence of memories that depended on hippocampal processing at the time of encoding. Studies using dopamine agonists in human subjects also show that dopaminergic modulation can influence episodic memory function.11,12 These findings are broadly consistent with the model proposed by Lisman and Grace13 whereby entry of information into long-term memory is regulated by interactions between the hippocampus and dopaminergic neurons of the brainstem. In schizophrenia, a disruption of interneuronal regulation of the ventral subiculum (part of the anterior hippocampus) is thought to lead to an overdrive of the dopamine system.14 Thus, MTL dysfunction is thought to drive striatal hyperdopaminergia through stimulation of dopaminergic neurons in the midbrain and dopamine release in the striatum.15,16 The relationship between MTL dysfunction and subcortical dopamine activity is thus thought to be critical to the onset of psychosis.15
In the present study, we used functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) to measure MTL activation and striatal dopamine synthesis capacity in subjects with prodromal symptoms of psychosis (an At Risk Mental State; ARMS17). Although these techniques cannot directly measure activity in MTL cells or dopamine release in the striatum in humans in vivo, an abnormal relationship between MTL and striatal dopaminergic function may be detectable via proximal neuroimaging measures. MTL activation was assessed during performance of a verbal episodic memory task, whereas dopamine synthesis capacity was examined using [18F]-DOPA PET. We first tested the hypotheses, based on our previous findings,8,9 that the ARMS would be associated with altered MTL activation during verbal encoding and recognition and with elevated dopamine function in the striatum. We then predicted, on the basis of the Lisman and Grace model, that the relationship between MTL activation and striatal dopamine function in subjects with an ARMS would be different to that in healthy controls. Within the striatum, we focused our analysis on its ventral (limbic) and associative subdivisions, where dopaminergic abnormalities have been identified in human studies of psychosis.9,18,19
Methods
Participants
Thirty-four subjects (14 Healthy Controls and 20 with an ARMS) participated in the study. All were right-handed, spoke English as their first language and had no history of neurological illness, drug, or alcohol dependence. The study had National Health Service UK Research Ethics Committee approval, and all participants gave written informed consent. All participants had an estimated premorbid IQ in the normal range as assessed using the Wide Range Achievement Test-Revised (WRAT reading test20). Working memory maintenance and manipulation were assessed using the Digit Span subtest of the Wechsler Adult Intelligence Scale Third Edition (WAIS-III21). Handedness was assessed using the Lateral Preference Inventory.22 Mean age, estimated premorbid IQ, years of education and WAIS-III Digit Span raw scores are reported in table 1. The present study reports data from 11 ARMS subjects who participated in our previous fMRI study.8 Nine further ARMS were recruited for the present study. None of the control subjects reported here participated in our previous fMRI study. Fourteen of the ARMS and 8 control subjects in the present study also participated in our previous 18F-DOPA PET study.9
Table 1.
Demographic Description of the Sample
| Controls (n = 14) | ARMS, (n = 20) | Analysis | |
| Age in years | 25.69 (4.11) | 26.30 (5.14) | t = −0.35, P = .72 |
| Years of education | 13.61 (2.52) | 13.73 (2.15) | t = 0.11, P = .90 |
| Estimated premorbid IQ | 103.65 (9.21) | 99.10 (15.55) | t = 0.94, P = .35 |
| Gender | 9M:5F | 10M: 10F | X = 0.05, P = .85 |
| WAIS-III | 17.15 (4.52) | 17.65 (4.52) | t = −0.31, P = .76 |
| Digit Span | |||
| Symptoms | |||
| PANSS Total | 43.55 (13.89) | ||
| PANSS Positive | 10.35 (3.63) | ||
| PANSS Negative | 9.60 (3.26) | ||
| PANSS General | 24.87 (7.51) |
Note: ARMS, At Risk Mental State; WAIS-III, Wechsler Adult Intelligence Scale Third Edition; PANSS, Positive and Negative Symptom Scales.
Subjects with an ARMS were recruited via Outreach and Support in South London,23 a clinical service for people at high risk of developing psychosis. Diagnosis of an ARMS was made via a detailed clinical assessment using the Comprehensive Assessment of At Risk Mental States (CAARMS24,25). Subjects met one or more of the following criteria: (a) attenuated psychotic symptoms, (b) brief limited intermittent psychosis (BLIP) (psychotic symptom that last for < 1 week: BLIP), or (c) a recent decline in function, together with either schizotypal personality disorder or a first-degree relative with a psychotic disorder. All ARMS subjects studied were experiencing attenuated psychotic symptoms, 4 subjects had also experienced a BLIP, and 3 had a family history together with a decline in function. The mean Global Assessment of Function (GAF) score of the group at initial assessment was 59 (SD = 9). Their self-reported ethnicity was White British (n = 12), Black (n = 3), and Mixed (n = 3) and Asian (n = 2). Psychopathology on the day of scanning was assessed using the Positive and Negative Symptom Scales,26 and scores are presented in table 1. No ARMS subjects were receiving antipsychotic medication at the time of scanning although 2 were receiving antidepressant medication. Subsequent to both MRI and PET scans, 3 ARMS subjects made a transition to 1st episode psychosis according to criteria outlined in Yung and colleagues.27
Healthy controls were recruited from the same geographical area within London through public advertisement. Participants with a history of medical or psychiatric disorders or who were receiving prescription medications were excluded. Their self-reported ethnicity was White British (n = 7), Black (n = 5), and Asian (n = 1) and mixed (n = 1). The ARMS and Control groups were matched for age, gender, years of education, premorbid IQ, and WAIS-III digit span (see table 1).
Functional Magnetic Resonance Imaging Task and Procedure
The encoding paradigm consisted of alternating epochs of experimental Encoding and baseline Control conditions. During Encoding blocks, subjects were required to read aloud and memorize commonly occurring English nouns presented one at a time on a computer screen. They were instructed to read the words out loud and try to memorize as many as possible, as recognition would be tested later. Sixteen lists were presented with one list per block. Each list contained 10 words semantically related to a critical nonpresented word taken from an established list of semantically related words.28 We chose this task as it is sensitive to the effects of organizational encoding strategies reflecting semantic processes that were of interest in our previous study. A full description of the task is provided in Allen and colleagues.8
Recognition was tested after a delay of 12 min. During recognition testing, the probe words were presented individually on a screen and subjects used a button press to indicate whether the word had been presented before. Participants were instructed to make one of 3 possible responses: ‘Remember’ if they were confident they had seen the word during the encoding phase, ‘Know’ if the word seemed familiar but they were less certain, or ‘New’ if they thought it had not been previously presented. These responses correspond to different recognition memory states of recollection and familiarity.29 The recognition list consisted of 28 Targets (words presented and articulated during the encoding phase), 28 Lures (nonpresented words semantically associated with the Target items), and 28 Novel words (nonpresented words not semantically associated with Targets). All participants were trained on the encoding and recognition task (using a practice word list not used in the fMRI experiment) prior to entering the scanner.
Functional Magnetic Resonance Imaging Data Acquisition
Images were acquired in a 1.5 T Magnet (Signa LX–GE) at the Institute of Psychiatry, London. Echo planar images were acquired using a compressed acquisition sequence30 to allow for overt verbal articulation of the word stimuli in the absence of acoustic scanner noise and to reduce motion artifacts related to movement caused by articulation (overall time repetition = 4000 ms, silent period = 2500 ms). A total of 228 image volumes were acquired in a single functional run. Whole brain coverage was achieved using 16 noncontiguous axial planes parallel to the intercommissural plane, with the following characteristics; time echo = 40 ms, slice thickness = 7 mm, slice skip = 0.7 mm, in-plane resolution = 3 × 3 mm.
[18F]-DOPA Positron Emission Tomography
Image Acquisition.
Images were acquired using a ECAT/EXACT3D PET scanner (Siemens/CTI), with a spatial resolution of 4.8 ± 0.2 mm and a sensitivity of 69 cps/Bq/ml.31 High-resolution images of the whole brain were reconstructed from 95 planes with a slice spacing of 2.425 mm. All subjects received 150 mg carbidopa and 400 mg entacapone orally 1 h prior to scanning to reduce the formation of radiolabeled metabolites.32 A full description of the [18F]-DOPA PET procedure is provided by Howes and colleagues.33 The PET data reported here includes data from 14 subjects included in a larger cohort that has been reported previously.9 Subjects underwent structural MR imaging to exclude intracranial abnormalities.
Analysis
Behavioral Data.
Recognition accuracy data were normally distributed in both groups. Response accuracy was assessed using factorial ANOVA in SPSS version 16. OLD (Remember + Know) and NEW responses for word types (Target, Lure, and Novels) were compared across groups (Controls vs ARMS). To examine recognition states, we also examined the mean number of Remember and Know responses separately. Correlational analysis between behavioral measures and parameter estimates derived from the analysis of functional imaging data analyses were conducted using Spearman’s test (2-tailed).
Functional Magnetic Resonance Imaging Data.
Preprocessing was performed using SPM5 software (http//www.fil.ion.ucl.ac.uk/spm), running in Matlab 7.1 (Mathworks Inc.). Movement correction of MRI scans was performed in SPM5 using the Realign and Unwarp option, with all volumes from each subject realigned to the first scan as a reference and resliced with sinc interpolation. There were no group differences observed in the average absolute interscan movement (Controls: 0.10 mm, 0.11 degrees; ARMS 0.65 mm, 0.07 degrees). Scans were spatially normalized to a standard MNI-305 template using nonlinear-basis functions. Functional data were spatially smoothed with a 6 mm full-width at half maximum isotropic Gaussian kernel, to compensate for residual variability in functional anatomy after spatial normalization.
Statistical Parametric Mapping.
A standard first-level fixed effects statistical analysis of regional responses was performed using SPM 5 software to identify regional activations in each subject independently. To remove low-frequency drifts, the data were high-pass filtered using a set of discrete cosine basis functions with a cutoff period of 128 s. For the encoding phase, blocks containing word lists were modeled independently by convolving the onset times. Word list blocks were then contrasted against the control blocks to create first-level SPMs in each subject. For the recognition phase, events were modeled according to subjects’ behavioral responses. Remember and Know responses were collapsed into an ‘OLD’ (recognition) response to ensure there were sufficient events to provide optimal statistical power in the image analysis. All potential recognition memory states were modeled independently by convolving onset times with a canonical haemodynamic response function: Correct Recognition (Target say OLD), Forgetting (Target say ‘NEW’), False Recognition (Lure say OLD), Correct Rejection of Lure (Lure say NEW), False Alarms (Novel say OLD), and Correct Rejection of Novel words (Novel say NEW). Trials were modeled against a low-level baseline consisting of a visual fixation cross.
Second-level analysis were performed using a nonparametric approach, to remove the requirement for data to conform to a normal distribution, to allow for better sensitivity to detect activations in small sample sizes by testing cluster mass (a combination of spatial extent and statistical value),34 and to allow for stringent correction for multiple comparisons.35 Second-level analyses were analyzed with permutation-based methods with the Cambridge Brain Activation software (CAMBA v2.1.0, http://www-bmu.psychiatry.cam.ac.uk/software/) as described in detail elsewhere.34 Response estimations from the first-level analysis were entered into a second-level general linear model, with group as the between subject factor. For the encoding phase first-level contrasts images of encoding > control blocks were modeled to examine group and interaction effects. For the recognition phase, only Correct Recognition trials (Target say OLD) were modeled to examine group and interaction effects. Effects associated with False Recognition (Lure say OLD) and False Alarms (Novel say OLD) were not examined as a number of subjects had too few trials in these response conditions thus precluding reliable first-level analysis with GLM. CAMBA utilizes nonparametric cluster level statistical analysis that has previously been shown to give good type I error control.34–36 First, a voxelwise test statistic was computed by regressing the model on to the observed data, and only those voxels that exceeded a relatively lenient probability threshold (P < .05) were retained for further analyses. These suprathreshold voxels were clustered, and the ‘mass’ (sum of suprathreshold voxel values) of each 3-dimensional voxel cluster was then tested by permutation (by randomizing group membership) using a one-tailed randomization test against the null hypothesis of no blood oxygen level dependent differences between the groups. Analyses are reported at an adaptive cluster level threshold where the expected number of false-positive clusters is less than one per analysis. To restrict our analyses to predefined regions of interest (ROIs), an anatomical mask was used (bilateral MTL). The MTL ROI, comprising the hippocampus and parahippocampal gyrus bilaterally, was defined in SPM-5 using the Wake Forest University Medical Centre Pickatlas v 2.4 (WFU-Pickatlas: http://fmri.wfubmc.edu/).37 Significant clusters were ascribed the coordinates of the centroid voxel.
Positron Emission Tomography Data
18F-DOPA PET images were processed using fully automated methods as previously described.9 Briefly, images were corrected for head movement during the scan by denoising the nonattenuated dynamic image and realigning individual frames to a single frame acquired 8 min after 18F-DOPA injection and applying these transformation parameters to the corresponding attenuation-corrected images. The realigned frames were combined to create a movement-corrected dynamic image for the analyses. Standardized volume of interest (VOI) in Montreal Neurologic Institute space were defined in the cerebellum (the reference region) and striatum using a probabilistic atlas38 and previously described criteria39 to create a VOI map. An 18F-DOPA template was normalized together with the VOI map to each individual PET summation image using statistical parametric mapping (SPM2; Wellcome Department of Cognitive Neurology, London). Graphical analysis40 was used to calculate 18F-DOPA utilization (k i cer, min−1) relative to cerebellar reference tissue in the bilateral limbic and associative functional subdivisions of the striatum (defined as described by Martinez39). These striatal subdivisions were selected on the basis of studies,41,42 which show that anatomical connectivity between the hippocampus and striatum is restricted to the limbic and associative striatal subdivisions.
Integration of F-DOPA Positron Emission Tomography and Blood Oxygen Level Dependent Response During Encoding and Recognition
To examine the relationship between MTL activation during encoding/recognition and striatal dopaminergic function, limbic and associative striatal 18F-DOPA k i cer values were entered as covariates of interest within a separate second-level analysis to identify significant group interactions. This analysis was also performed using CAMBA, and we restrict our analyses to the predefined hippocampal mask (that comprised the hippocampus and parahippocampal gyrus bilaterally as used in the fMRI analysis). Parameter estimates were extracted from significant clusters, and Pearson’s correlations coefficients were used to examine the direction of interactions. Fisher’s Z transformation test was used to test hypotheses about the value of the population correlations coefficient.
Results
18F-DOPA Uptake
There were no significant group differences in 18F-DOPA ki cer in either the associative (Controls = 0.0139 vs ARMS = 0.0144/min−1; t 33 =1.3, P = .18) or limbic (Controls = 0.0145/min−1 vs ARMS = 0.0146/min−1; t 33 = .93, P = .93) subdivisions of the striatum. Three of the ARMS sample had developed a psychotic disorder subsequent to the neuroimaging assessments. Although we had not planned to examine the data in relation to clinical outcome, in view of the absence of the expected group difference in 18F-DOPA ki cer, and recent data indicating that dopamine dysfunction in the ARMS may be specific to those who later become psychotic,43 we compared 18F-DOPA ki cer in the small subgroup of transition cases with ARMS subjects who had not become psychotic. In the associative subdivision of the striatum 18F-DOPA ki cer for the transition subgroup (n = 3) = 0.0154/min−1 while in the nontransition subgroup (n = 17) 18F-DOPA ki cer = 0.0136/min−1 (P = .025). In the limbic subdivision of the striatum 18F-DOPA ki cer for the transition subgroup = 0.0156/min−1 while in the nontransition subgroup 18F-DOPA ki cer = 0.0144/min−1 (P = .14).
Verbal Recognition Performance
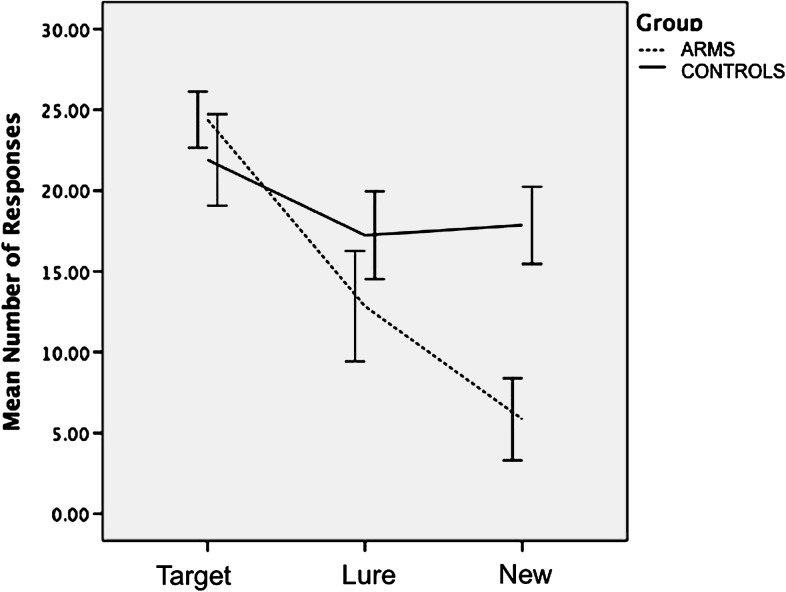
Examination of audio recordings and response files during word encoding revealed that all participants successfully completed the task. The mean number of OLD responses for each Word Type are shown in figure 1. ANOVA revealed a significant main effect for Word Type (F 2,64 = 150.84, P < .001), with all subjects making more OLD responses for Targets than for Lure words (t 33 = 6.12, P < .001) and for Novel words (t 33 = 14.11, P < .001). There was a significant interaction between Word Type and Group (F 2,64 = 5.39, P < .01). Relative to controls, ARMS subjects made significantly more OLD responses for Novel words (ie, False Alarms; t 32 = −2.19, P = .05) but not for Target (t = 1.23, P = .22) or Lure words (t = −1.12, P = .27). There was a significant main effect for Response Type (F 1,32 = 8.3, P < .01), with all subjects making more Remember than Know Responses. The interaction between Response Type and Group was non significant (F 1,32 = 1.45, P = .71).
Fig. 1.
Mean number of OLD responses by group during recognition trials.
Functional Magnetic Resonance Imaging
Main Effect of Group (ARMS vs Controls) During Encoding and Recognition.
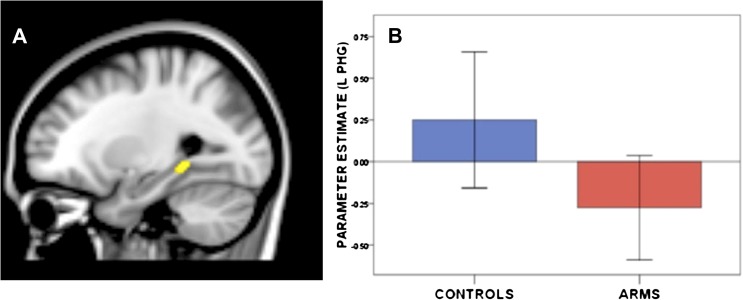
During encoding trials (relative to word repetition), ARMS subjects showed less activation than Controls in the left parahippocampal gyrus (figure 2, table 2). Across all subjects, activation during encoding in this left parahippocampal cluster was positively correlated with the number of Target words correctly identified during the subsequent recognition phase (r = .45, P < .01). Within the ARMS group, GAF scores were positively correlated with parahippocampal activation during encoding (r = .47, P = .04). The main effect of group during recognition trials (Target say OLD) was nonsignificant.
Fig. 2.
Main effect of group on activation during encoding trials (P < 1 false-positive cluster). The At Risk Mental State group showed less activation than controls in the left parahippocampal gyrus.
Table 2.
Spatial Location of Areas Where There Was (a) A Main Effect of Group (ARMS Vs Control Subjects) During Encoding, (b) Main Effect of Group During Correct Recognition, (c) Interaction Effects During Encoding, and (d) Interaction Effects During Recognition
| Region | Side | Cluster | x | y | z |
| (a) Main effect of Group during encoding | |||||
| Controls > ARMS | |||||
| Parahippocampal gyrus | L | 56 | −22 | −44 | −4 |
| Controls < ARMS | |||||
| No significant clusters | |||||
| (b) Main effect of Group during correct recognition | |||||
| Controls > ARMS | |||||
| No significant clusters | |||||
| Controls < ARMS | |||||
| No significant clusters | |||||
| (c) Interaction effect during encoding | |||||
| Group × limbic 18F-DOPA ki cer | |||||
| Hippocampus (subiculum) | L | 231 | −26 | −26 | −8 |
| Group × associative 18F-DOPA ki cer | |||||
| No significant clusters | |||||
| (d) Interaction effect during recognition | |||||
| Group × Limbic ki cer | |||||
| Parahippocampal gyrus/subiculum | R | 138 | 14 | −30 | −8 |
| Parahippocampal gyrus/subiculum | L | 164 | −20 | −30 | −8 |
| Head of hippocampus | L | 95 | −38 | −16 | −16 |
| Hippocampus | R | 47 | 36 | −28 | −8 |
| Group × Associative ki cer | |||||
| No significant clusters |
Encoding Trials—[18F]-DOPA Positron Emission Tomography Uptake × Group Interactions.
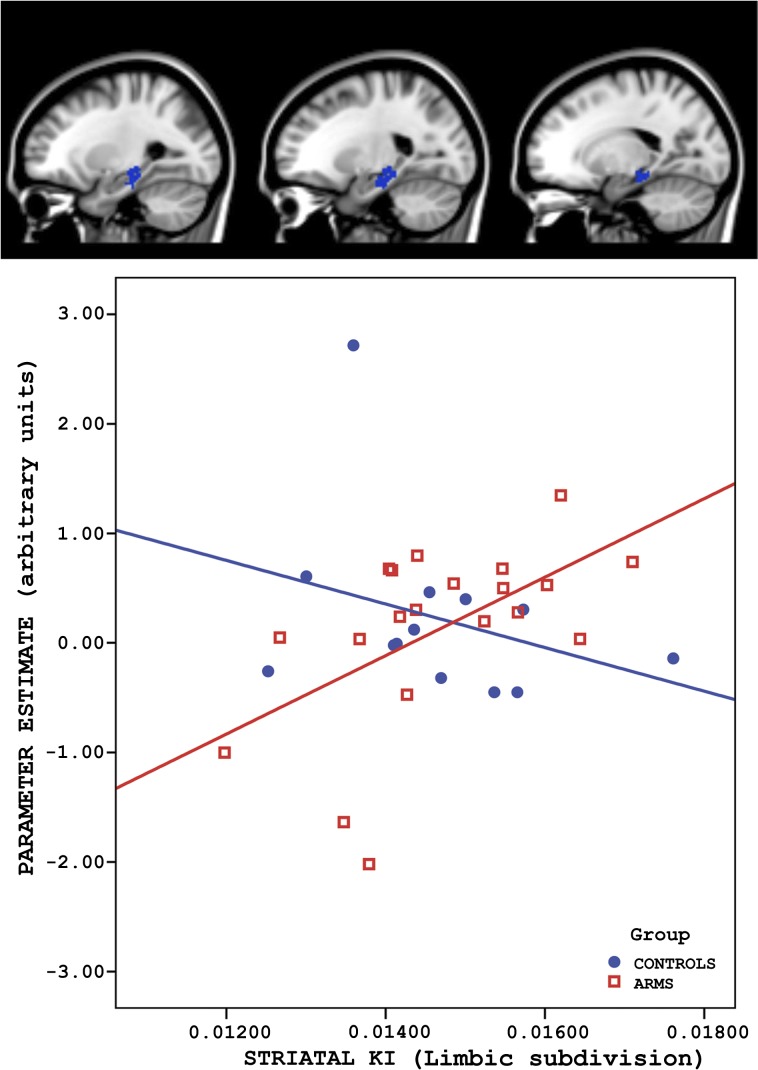
There was a significant group difference in the correlation between activation during encoding in the left hippocampal subiculum and the 18F-DOPA ki cer in the limbic striatum (figure 3 and table 2). This interaction was driven by a positive correlation between subicular activation and the limbic striatal 18F-DOPA ki cer that was evident in the ARMS group (r = .556, P = 0.011) but not controls (r = −0.316; P = 0.293). Fisher’s Z transformation test shows these correlation coefficients are significantly different (P = .01). There was no significant group difference in the correlation between activation within the MTL ROI and the 18F-DOPA ki cer in the associative striatum.
Fig. 3.
Altered relationship between medial temporal lobe activation during encoding and striatal dopamine function. There was a positive correlation between activation in the left hippocampal subiculum and Ki for F-Dopa in the ventral (limbic) striatum in the At Risk Mental State group (red squares) but not in controls (blue dots).
Correct Recognition Trial—[18F]-DOPA Positron Emission Tomography Uptake × Group Interactions.
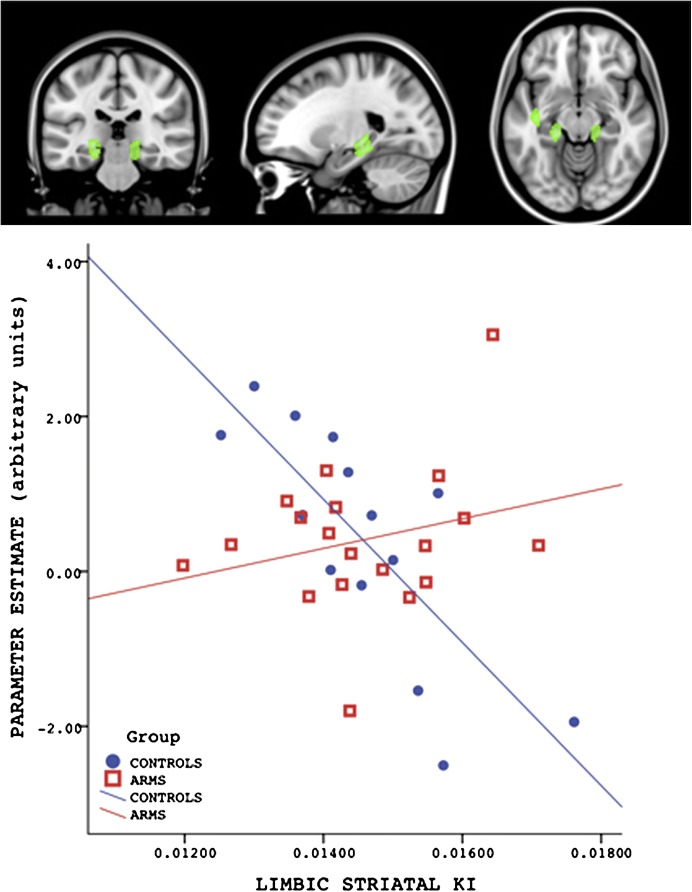
There was a significant group difference in the correlation between activation during correct recognition trials in the subiculum and hippocampus bilaterally and the 18F-DOPA ki cer in the limbic striatum (table 2 and figure 4). In these MTL regions, the interaction with group was driven by a negative correlation between activation (parameter estimates averaged across all MTL clusters) and 18F-DOPA ki cer that was present in the control (r = −.78, P < .01) but not the ARMS group (r = .25, P = .28). Fisher’s Z transformation test shows that these correlation coefficients are significantly different (P < .01). There was no significant group difference in the correlation between activation within the MTL ROI during correct recognition and the 18F-DOPA ki cer in the associative subdivision of the striatum.
Fig. 4.
Altered relationship between MTL activation during correct recognition and striatal dopamine function. The plot shows the averaged parameter estimates across the 4 MTL clusters. There was a negative correlation between activation in the hippocampus and subiculum bilaterally and Ki for F-Dopa in the limbic striatum in the Control group (blue dots) but not in the ARMS group (red squares).
Discussion
By studying the same subjects with a combination of fMRI and [18F]-DOPA PET, we were able to directly examine the relationship between MTL activation and striatal dopaminergic function in subjects at high risk for psychosis.
At the behavioral level, the 2 groups did not differ in the number of word items correctly recognized, but ARMS subjects made more false alarm responses than controls. Higher rates of false alarms during tests of verbal memory are commonly reported in schizophrenia44–46 and may reflect an increased tendency, when the subject is uncertain, to believe that an event has occurred. During encoding, ARMS subjects showed reduced activation in the left parahippocampal gyrus relative to controls. Across all subjects (independent of group), the level of activation in this region was positively correlated with the number of target words correctly recognized, suggesting that its engagement during encoding is important for subsequent recognition performance.47
Contrary to our prediction and in contrast to our previous finding,9 we were unable to show a significant difference in striatal dopamine storage/synthesis between ARMS and control subjects in either the associative or limbic subdivisions, although in absolute terms, it was higher in the ARMS subjects. Several factors may underlie this difference. The present study was designed and powered to investigate MTL-striatal interactions. It had a smaller sample size than the previous study and so the failure to detect a difference may reflect a lack of power. Furthermore, subsequent expansion and clinical follow up of the sample previously reported by Howes et al43 indicates that elevation of dopamine function is specific to the subgroup of ARMS subjects, who later develop psychosis rather than being a generic feature of the group as a whole. Of the ARMS subjects who participated in the present study, only a small proportion (3 of 20 subjects) have since developed psychosis. Examination of the data indicates that 18F-DOPA ki was higher in these 3 ARMS subjects than in the rest of the sample. Thus, while the absence of elevated dopamine function in the ARMS group is not consistent with the original report by Howes et al, it is in line with the most recent data from a larger sample that has been clinically followed up. Moreover, given the complex pattern of cortical-subcortical changes found during the development of psychosis,48,49 studying the interactions between systems, as we have done in this study, can provide data on potentially critical aspects of brain dysfunction in the ARMS that may be missed by the investigation of one process alone.
Our main objective was to examine the relationship between MTL activation and striatal dopamine function in the ARMS. We found that this relationship was significantly different in people with prodromal signs of psychosis and controls, during both verbal encoding and recognition. During encoding, ARMS subjects showed a positive correlation between MTL and limbic striatal dopamine function that was not seen in control subjects. During recognition, a negative correlation was observed in controls but not ARMS subjects (see figures 3 and 4). These results are difficult to interpret but it is possible that the normal relationship between MTL and striatal dopamine is one where decreased striatal limbic dopamine function is associated with greater MTL activation and that this association is perturbed in the ARMS. However, while the data implicates the MTL and limbic striatum and is thus consistent with the model proposed by Lisman and Grace, we cannot say if the direction of the relationship fits with that proposed by that model (ie, MTL driving striatum), or how these areas interact. As such, the present empirical findings do not constrain the specific mechanisms by which such alterations could account for the observed data.
As all the ARMS, subjects were naïve to antipsychotic medication, these findings are not confounded by the effects of previous treatment. In the case of the differential relationship between MTL activation during encoding and dopamine function, the anatomical localization to the subiculum and limbic striatal subregions is consistent with the topography of the anatomical connections between the MTL and the striatum.41,42 The differential relationship between dopamine function and activation during verbal recognition again involved the bilateral subiculum, as well as hippocampus and the limbic striatum. However, we did not find interactions between MTL activation during encoding or recognition trials and dopamine function in the associative striatum. Recent PET studies have identified the associative subregion as the site of elevated dopamine function.9,18 However, other work has also implicated the ventral (limbic) striatum as a site of dopamine dysfunction in psychosis,19 and it is possible that both subregions are relevant to the pathophysiology of the disorder. Although the hippocampus mainly sends projections to the ventral subdivision, this in turn activates dopamine neurons in the midbrain, and these project back to the associative as well as the ventral striatum.50 This may partly explain why psychosis has been associated with hyperdopaminergia in both these striatal subregions.
An altered relationship between the MTL and striatal dopamine function may be involved in the episodic memory and verbal learning impairments reported in subjects with prodromal psychosis.51,52 Recent studies report a link between episodic memory and dopaminergic modulation,11,12 and the present study hypotheses were based on the model of psychosis proposed by Lisman and Grace.15 According to this model, striatal hyperdopaminergia in psychosis is driven by MTL dysfunction. In the present study, consistent with the model, we found altered MTL function in the ARMS. However, we did not find evidence of significantly elevated striatal dopamine function. Clinical follow up indicates that the elevation of dopamine function may be specific to the subgroup of ARMS subjects that go on to develop psychosis.43 Thus, it is possible that the altered relationship between MTL and subcortical dopamine function predicted by the Lisman and Grace model is particularly evident in ARMS subjects that subsequently develop psychosis, as opposed to those that do not. As previously stated, in the present study, only a minority of the sample had become psychotic subsequent to scanning. Moreover, consistent with the notion that the dopamine changes in the ARMS may be specific to later psychosis, dopamine function in the subjects that have since developed psychosis was higher than in the rest of the sample.
Although it is not possible to infer the direction of causality from the findings presented here, to our knowledge, this is the first study to demonstrate a link between altered neurophysiological function in the MTL and striatal dopamine function in human subjects with an elevated risk of developing psychosis. Both MTL7 and dopaminergic abnormalities have also been specifically associated with the later onset of psychosis.43 Presently, it is not possible to examine whether the findings in the present study predict the subsequent onset of illness. To date, 3 ARMS subjects within the sample have made a subsequent transition to first-episode psychosis. Others are still undergoing clinical follow up, and their clinical outcome is still to be defined.
We have previously reported, in 2 separate studies, that the ARMS was associated with altered MTL activation during verbal encoding and recognition8 and with elevated striatal dopamine synthesis.9 The present study extends this work by combining these 2 different neuroimaging paradigms in the same individuals, so that it has been possible, for the first time in humans, to directly examine the relationship between MTL function and subcortical dopamine activity in psychosis. Our data suggest that the alteration in the relationship between these 2 systems predicted by animal models of psychosis is evident in human subjects. This is important because it supports the notion of a whole brain system disorder rather than separate dimensions with separate pathologies as reported previously. Although we did not find elevated storage/synthesis capacity in the ARMS, we did observe altered MTL function in these subjects relative to controls and an altered relationship between MTL and dopamine storage/synthesis capacity in the ARMS. Future prospective and longitudinal studies are required to assess how such an abnormal relationship relates to the transition to first-episode psychosis in these at risk subjects.
Funding
Medical Research Council (UK); National Alliance for Research on Schizophrenia and Depression.
Acknowledgments
We acknowledge the contribution of the volunteers who participated in the study and the clinical professionals at Outreach and Support in South London. The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Reith J, Benkelfat C, Sherwin A, et al. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci U S A. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hietala J, Syvalahti E, Vilkman H, et al. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- 3.Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, et al. Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res. 1997;23:167–174. doi: 10.1016/S0920-9964(96)00102-8. [DOI] [PubMed] [Google Scholar]
- 4.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl. 2007;51:s13–s18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgwardt SJ, Riecher-Rossler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Schobel SA, Lewandowski NM, Corcoran CM, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen P, Seal ML, Valli I, et al. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophr Bull. November 23. 2009 doi: 10.1093/schbul/sbp113. doi:10.1093/schbul/sbp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 10.Bethus I, Tse D, Morris RG. Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J Neurosci. 2010;30:1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montoya A, Lal S, Menear M, et al. Apomorphine effects on episodic memory in young healthy volunteers. Neuropsychologia. 2008;46:292–300. doi: 10.1016/j.neuropsychologia.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Morcom AM, Bullmore ET, Huppert FA, et al. Memory encoding and dopamine in the aging brain: a psychopharmacological neuroimaging study. Cereb Cortex. 2010;20:743–757. doi: 10.1093/cercor/bhp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Grace AA. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010;18:367–376. doi: 10.1007/s12640-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yung AR, Nelson B, Stanford C, et al. Validation of ‘prodromal’ criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–17. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 19.McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch Gen Psychiatry. 2004;61:134–142. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- 20.Jastak S, Wilkinson SG. The Wide Range Achievement Test: Revised Administration Manual. revised ed. Jastak Assoc; 1984. [Google Scholar]
- 21.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 22.Coren S. Measurement of handedness via self-report: the relationship between brief and extended inventories. Percept Mot Skills. 1993;76:1035–1042. doi: 10.2466/pms.1993.76.3.1035. [DOI] [PubMed] [Google Scholar]
- 23.Broome MR, Woolley JB, Tabraham P, et al. What causes the onset of psychosis? Schizophr Res. 2005;79:23–34. doi: 10.1016/j.schres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 25.Phillips LJ, Yung AR, McGorry PD. Identification of young people at risk of psychosis: validation of Personal Assessment and Crisis Evaluation Clinic intake criteria. Aust N Z J Psychiatry. 2000;34(suppl):S164–169. doi: 10.1080/000486700239. [DOI] [PubMed] [Google Scholar]
- 26.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 27.Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (‘prodromal’) group. Schizophr Res. 2003;60:21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 28.Stadler MA, Roediger HL, 3rd, McDermott KB. Norms for word lists that create false memories. Mem Cognit. 1999;27:494–500. doi: 10.3758/bf03211543. [DOI] [PubMed] [Google Scholar]
- 29.Tulving E. Multiple memory systems and consciousness. Hum Neurobiol. 1987;6:67–80. [PubMed] [Google Scholar]
- 30.Amaro E, Jr., Williams SC, Shergill SS, et al. Acoustic noise and functional magnetic resonance imaging: current strategies and future prospects. J Magn Reson Imaging. 2002;16:497–510. doi: 10.1002/jmri.10186. [DOI] [PubMed] [Google Scholar]
- 31.Spinks TJ, Jones T, Bloomfield PM, et al. Physical characteristics of the ECAT EXACT3D positron tomograph. Phys Med Biol. 2000;45:2601–2618. doi: 10.1088/0031-9155/45/9/313. [DOI] [PubMed] [Google Scholar]
- 32.Wahl L, Chirakal R, Firnau G, Garnett ES, Nahmias C. The distribution and kinetics of [18F]6-fluoro-3-O-methyl-L-dopa in the human brain. J Cereb Blood Flow Metab. 1994;14:664–670. doi: 10.1038/jcbfm.1994.83. [DOI] [PubMed] [Google Scholar]
- 33.Wahl L, Chirakal R, Firnau G, Garnett ES, Nahmias C. The distribution and kinetics of [18F]6-fluoro-3-O-methyl-L-dopa in the human brain. J Cereb Blood Flow Metab. 1994;14:664–670. doi: 10.1038/jcbfm.1994.83. [DOI] [PubMed] [Google Scholar]
- 34.Suckling J, Bullmore E. Permutation tests for factorially designed neuroimaging experiments. Hum Brain Mapp. 2004;22:193–205. doi: 10.1002/hbm.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- 36.Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage. 2007;35:105–120. doi: 10.1016/j.neuroimage.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 37.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 38.Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez D, Slifstein M, Broft A, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 40.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 41.Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- 42.Cohen MX, Lombardo MV, Blumenfeld RS. Covariance-based subdivision of the human striatum using T1-weighted MRI. Eur J Neurosci. 2008;27:1534–1546. doi: 10.1111/j.1460-9568.2008.06117.x. [DOI] [PubMed] [Google Scholar]
- 43.Howes O, Bose S, Turkheimer F, et al. Onset of psychosis linked to progressive increase in striatal dopamine synthesis capacity. Mol Psychiatry. doi: 10.1038/mp.2011.20. Mar 1, 2011. PMID: 21358709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 45.Weiss AP, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Biol Psychiatry. 2004;55:668–675. doi: 10.1016/j.biopsych.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Brebion G, David AS, Jones H, Pilowsky LS. Hallucinations, negative symptoms, and response bias in a verbal recognition task in schizophrenia. Neuropsychology. 2005;19:612–617. doi: 10.1037/0894-4105.19.5.612. [DOI] [PubMed] [Google Scholar]
- 47.Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- 48.Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis-A systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Brewer WJ, Francey SM, Wood SJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162:71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- 52.Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkotter J. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr Res. 2007;92:116–125. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]