Abstract
Synaptic glutamate signaling in brain is highly complex and includes multiple interacting receptors, modulating cotransmitters and distinct regional dynamics. Medial temporal lobe (MTL) memory structures receive excitatory inputs from neocortical sensory and associational projections: afferents from neocortex pass to parahippocampal cortex, then to layers II/III of entorhinal cortex, and then onto hippocampal subfields. Principles of Hebbian plasticity govern synaptic encoding of memory signals, and homeostatic plasticity processes influence the activity of the memory system as a whole. Hippocampal imaging studies in schizophrenia have identified 2 alterations in MTL—increases in baseline blood perfusion and decreases in task-related activation. These observations along with converging postsynaptic hippocampal protein changes suggest that homeostatic plasticity mechanisms might be altered in schizophrenia hippocampus. If hippocampal pattern separation is diminished due to partial dentate gyrus failure (resulting in ‘spurious associations’) and also if pattern completion is accelerated and increasingly inaccurate due to increased CA3 associational activity, then it is conceivable that associations could be false and, especially if driven by anxiety or stress, could generate psychotic content, with the mistaken associations being laid down in memory, despite their psychotic content, especially delusions and thought disorder.
Keywords: dentate gyrus, CA3, CA1, metaplasticity, glutamatergic transmission, plasticity
Introduction
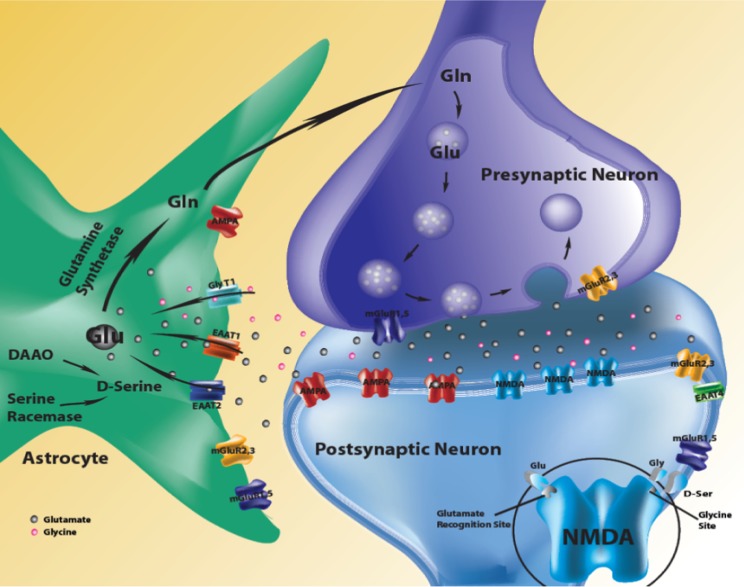
Glutamate signaling impacts a majority of neurons in mammalian brain, supporting activity-dependent signaling, neuronal growth, and synaptic plasticity.1–3 The glutamate system in brain is highly complex and includes multiple interacting receptors, modulating cotransmitters and multisystem synapses (figure 1). Presynaptic, postsynaptic, and astrocytic mechanisms are important to overall excitatory signaling and to unique plasticity mechanisms which are associated with glutamate transmission4–6; the 3 compartments (pre-, postsynaptic, and astrocytic) are all candidate systems for pathology in glutamate-related brain diseases. Regional characteristics of synapse architecture, local circuitry, and specialized signaling mechanisms suggest that important aspects of glutamate regulation are local. Even within anatomic regions, specificity of function occurs on a microcircuit level with the same glutamatergic architecture serving distinct functions.7,8 It is already known that hippocampus and the surrounding medial temporal lobe (MTL) cortex is dependent on glutamate signaling to a greater extent than other neocortical tissue, a feature that underlies its learning and memory functions.9 Moreover, hippocampus is one of the brain regions whose function is altered in schizophrenia, suggesting the potential relevance of glutamate transmission in this structure to psychosis pathophysiology.10 The role of altered glutamate signaling in schizophrenia and the clinical and pharmacological basis for this formulation have already been amply reviewed and widely accepted.10–12 In this review of glutamate mechanisms and schizophrenia, we will maintain a regionally limited focus on glutamate-mediated plasticity mechanisms associated with hippocampally supported aspects of learning and memory (L&M). Hippocampal signaling has been studied for decades because the dramatic demonstration of its essential role in memory function through the case of Henry Moliason,13–15 creating a rich store of knowledge around its role in memory.16–26 We have proposed a speculative and testable model of psychosis, based on evidence of altered hippocampal L&M and suggest that altered glutamate-mediated functions in hippocampal subfields are involved in psychotic and deficient declarative memory manifestations of schizophrenia.10
Fig. 1.
Glutamate synapse showing pre-, postsynaptic and astrocytic systems, each involved in glutamate signaling.
The Anatomy of Hippocampus
The MTL includes the hippocampus proper, dentate gyrus (DG), entorhinal cortex (EC), and subiculum (figure 2). With the perirhinal and parahippocampal cortices, the hippocampal formation is functionally specialized to contribute to declarative memory processing using glutamate-mediated signaling.9,27–32 Different subfields of the hippocampus have distinct memory functions, eg, the DG is thought to function importantly in ‘pattern separation’33–35 and CA3 supports in ‘pattern completion.’21,22,36 The trisynaptic pathway is glutamate-dependent and distinguished by its one-way information flow, from EC to DG, to CA3, to CA1, and onto the subiculum, a microcircuit, which plays a distinctive role in specialized memory functions.18,22,33,37,38
Fig. 2.
Hippocampal trisynaptic pathway (solid lines). EC = entorhinal cortex; DG = dentate gyrus; CA3 = cornu amonius3; CA1 = cornu amonius 1; sub = subiculum. The interrupted line represents the direct projections from EC to CA3 and to CA1.
The MTL memory-related structures receive inputs from neocortical sensory and associational projections: afferents from neocortex pass to parahippocampal cortex, then to layers II/III of EC, and then onto hippocampal subfields. There are 2 prominent glutamate-mediated systems, which project through hippocampus: one is the unidirectional trisynaptic pathway and the other is the system of direct EC projections to individual subfields (figure 2). The trisynaptic pathway starts as the perforant projection from EC to DG and projects in steps, through the mossy fibers (MFs) to CA3, then through the Schaffer collaterals to CA1. Axons from layer II/III EC glutamate neurons project to the proximal dendrites of DG granule cell (GC) neurons. From DG, the MF pathway projects from the DG GCs to the proximal dendrites of CA3 pyramidal neurons (PN). From CA3, the PN run through the Schaffer collaterals and synapse onto the proximal dendrites of CA1 neurons. The CA1 PN project onto the subiculum, which in turn feeds back to EC, layer IV. The EC communicates bidirectionally with its projection targets in other gray matter regions. The anatomy of CA3 is unique and well suited to its function, in that it is comprised of highly organized glutamatergic associational projections that arise from the pyramidal cell axons; the axons send off dense specialized collateral projections onto neighboring pyramidal cells within CA3. The subiculum and EC participate in hippocampal communication circuits back to subcortical structures and neocortex, to the regions originally projecting to EC.
Of particular relevance to CA3 function are the specific features of the MF pathway connecting DG to CA3. Anatomic and physiologic studies demonstrate that one MF axon from a glutamate-containing GC in DG can synapse with 8–15 PN and 18–35 interneurons (ITN) with target-cell–specific probability of release, CA2+ dynamics, and distinct short- and long-term plasticity at individual release sites, all along a common axon.39–41 Each of these MF synapses onto CA3 ITN has distinctive anatomic, electrophysiological, and molecular characteristics, conferring tight control over the functional properties resulting from these innervations. Studies have shown that with basal DG activity, the functional action of DG signaling in CA3 is a broadly distributed feed-forward inhibition. Basal signaling is mediated through 2 distinctive MF-ITN synapses, one containing postsynaptic GluR2-free AMPA receptors (ie, CA2+ permeable) and the other dependent on presynaptic mGluR7 receptors; these 2 innervations serve to inhibit and stabilize neuronal firing in CA3.39 However, with high-frequency stimulation (HFS) by DG GC neurons, the signaling properties of the MF pathway in CA3 change so that the HFS activation not only stimulates CA3 but also can generate long-term depression (LTD) at the MF→ITN synapse (diminishing inhibition) while generating long-term potentiation (LTP) at the MF→PN synapse (facilitating excitation); these changes support extensive facilitation within the CA3 pyramindal neurons and their recurrent collaterals. This specialization within the MF→PN vs MF→INT synapses provides the glutamatergic MF circuit a way of closely modulating the dynamics of excitation and inhibition within CA3 to fit the firing dynamics of the DG GCs. One can speculate that these dynamics are designed to execute the complex memory functions of DG and of CA3 at the fast speeds necessary for successful memory.
Glutamate-Dependent Plasticity Processes in Hippocampus and in Schizophrenia
Glutamate is synthesized from glucose through the tricarboxylic acid cycle, and it is concentrated in presynaptic vesicles at excitatory synapses. Glutamate is released by calcium-triggered vesicle fusion at the presynaptic terminal with depolarizing stimuli. Once in the synapse and after binding to glutamate receptors, the transmitter is rapidly recycled by the excitatory amino-acid transporters into the astrocyte (figure 1).42 Stimulation of glutamate receptors causes activation of postsynaptic signaling cascades led by enhanced activity of protein kinases and cyclic AMP response element binding protein.7,8 These kinases, among their many actions, phosphorylate glutamate receptor subunits of the N-methyl-d-aspartate (NMDA) and the AMPA receptors, which sensitize their response to activity-dependent signaling and increase expression of constitutive and inducible transcription factors.43 These activity signals influence membrane trafficking of glutamate receptor, affect calcium influx, and in turn, modulate postsynaptic sensitivity.
Two ionotropic glutamate receptors are particularly important in learning and memory, NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), defined by their characteristic subunits. NMDA receptors (NMDAR) are heteromers of 2 GluN1 (obligate) subunits and 2 GluN2s (mainly, GluN2a and/or GluN2b in forebrain); they are voltage-gated and routinely pass calcium ions. GluN2a-containing NMDAR predominate in mature tissue, while midbrain region GluN2b-containing NMDAR predominate during development and also pass more CA2+ per stimulus than GluN2a-containing NMDAR. NMDAR activation triggers long-lasting changes in activity-dependent responses in part by modulating trafficking of AMPA receptors into the postsynaptic membrane.44 The NMDA and AMPA receptors mediate the bulk of the depolarizing current responsible for basal glutamatergic transmission and undergo rapid activity-dependent recruitment during synaptic plasticity. Phosphorylation (specifically of Ser831) and dephosphorylation (of Ser845) of AMPA subunits alter the function of AMPA receptors and contribute to the development of LTP and LTD.45 While a full discussion of LTP/LTD is beyond this article, it is important to acknowledge that LTP itself is a local synaptic phenomenon and its influence on cellular function is complexly governed.
Principles of Hebbian plasticity govern synaptic encoding of memory signals. Two mechanisms of synaptic plasticity, thought to contribute to the activity-dependent refinement of neural circuitry during development and with adult learning, are LTP and LTD.46 Rapid adjustments in the strengths of individual synapses are made in response to specific patterns of correlated pre- and postsynaptic activity.45,47 LTP is associated with synaptic strengthening due to a prolonged period of activity. LTD is induced based on a lack of depolarization coincidence. Modulating synaptic plasticity in response to the previous activity state of the synapse, while preserving the Hebbian-directed memory signals, can be explained by various homeostatic plasticity mechanisms, like synaptic scaling, intrinsic plasticity, or metaplasticity.48–51 Metaplasticity is a process which modulates the sensitivity of a population of synapses in response to afferent stimulation and relies on changes in NMDA and AMPA receptors and their trafficking to effect synaptic sensitivity changes.4,6,52–54 This involves changes at the synapse between 2 neurons rather than changes in the electrical properties within a single neuron.55,56 Disruptions in plasticity mechanisms could lead to altered neuronal activation patterns in the hippocampus.
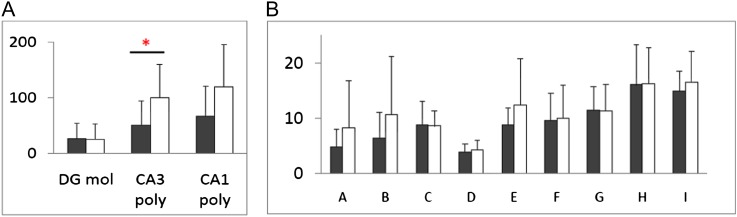
Within the model discussed in ‘Hippocampal Homeostatic Plasticity Model of Psychosis in Schiziophrenia’ section, we would invoke a localized increase in synaptic strength within the synapses of the recurrent collaterals in CA3 extending into CA1 and that this regional synaptic change could underlie the increase in neuronal activity in CA3 that we measure with magnetic resonance perfusion techniques (in ‘Functional Hippocampal Imaging in Schizophrenia’ section). Consistent with this increase in synaptic strength is the regional increase in CA3 brain derived neurotrophic factor (BDNF) levels in hippocampal tissue from schizophrenia (figure 3a), a finding which is important in that BDNF is an activity-dependent marker,57 signaling alterations in plasticity in hippocampus in the illness. Previous experiments from our laboratory and from other groups show reduced GluN1 messenger RNA (mRNA) in hippocampus, particularly in the DG. This reduction in NR1 mRNA is accompanied by a previously unexplained increase in GluN2b in CA3.58–64 Consistent with this observation is our more recent finding that NR1 protein is also reduced, specifically in the DG. We now hypothesize that a reduction in DG signaling (a ‘deprivation’ for CA3) increases the sensitivity of CA3 by increasing the proportion of GluN2b-containing NMDARs selectively in CA3, and thereby generating a greater glutamate signal in CA3 with each depoloarization. This observation is consistent with the model that there is a reduction in glutamate signaling in DG, a reduction extending to the activity of the MF pathway; this formulation is also consistent with anatomic data showing reduced MF insertions in CA3.62,65 Further examination of dendritic branching and spines within CA3, postulating an increase in each, especially spine number, will be important to move this model ahead; previous work has noted reduced dendritic branching in subiculum, a terminal portion of hippocampus.66 Together, the findings implicate altered regionally specific glutamate signaling and activity-dependent synaptic plasticity in schizophrenia.
Fig. 3.
BDNF messenger RNA levels in hippocampal subfields, dentate gyrus molecular layer (DG mol), CA3 polymorphic layer (CA3 poly), and CA1 polymorphic layer (CA1 poly) measured by in situ hybridization in 14 pairs of schizophrenia (open bars) and control (filled bars) cases. Data shown are mean + SD. *Represents P < .05.57 (b) Presynaptic protein concentrations in the anterior hippocampus in 14 pairs of schizophrenia (open bars) and matched controls (filled bars). A = complexin I, B = complexin II, C = synaptotagmin1, D = munc18, E = synaptogyrin, F = Rab3a, G = synaptophysin, H = SNAP25, and I = syntaxin.
Functional Hippocampal Imaging in Schizophrenia
Human brain imaging studies in schizophrenia have been important in identifying brain structures involved in disease manifestations, in understanding genetic contributions to function, and in examining task-specific pathology in brain. Hippocampal imaging studies have identified 2 alterations in measures of brain activity in MTL—baseline blood perfusion and task-related activation—which are paradoxically related in schizophrenia. Regional cerebral blood flow (rCBF) and blood volume (rCBV), perfusion measures of basal (nonstimulated) neuronal activity, are increased in MTL in individuals with schizophrenia, with such increases often being reported as specific to anterior MTL.67–78 By contrast, task-related MTL activation, measured with funtional magnetic resonance imaging (fMRI) blood-oxegen-level dependent (independent of baseline) and representing regional responses to task demands and performance, is reduced in schizophrenia, with reductions observed during processing of novel stimuli, verbal memory encoding, and associative encoding.79–88 We have suggested that the 2 are related—that the blunted activation to task demand is secondary to the increased perfusion at baseline.10 As such, it is the elevated perfusion in schizophrenia that requires a mechanistic explanation; elevated perfusion is taken to represent an increase in excitatory neuronal activity. The increase in hippocampal perfusion could be associated with a subfield, a question we and others have already posed; this would suggest molecular studies pursued within that subfield to mechanistically understand the pathology. Our preliminary data suggest that hippocampal perfusion is increased in drug-free people with schizophrenia in CA3 and CA1. These data suggest that the dynamics of excitation and inhibition in the schizophrenia hippocampus are altered, particularly within CA3 and projecting onto CA1.
Hippocampal Homeostatic Plasticity Model of Psychosis in Schizophrenia
The observations from in vivo imaging studies in schizophrenia (which show increased perfusion in hippocampus67,72,74) and from postmortem schizophrenia tissue studies (which show decreased GluN1 mRNA and protein in hippocampal subfields, especially DG58,89) converge to suggest that homeostatic plasticity mechanisms might be altered in hippocampus in patients.
It is the functional effect that this reduced glutamate signaling in DG has on CA3 that could be of interest for psychosis. CA3 is the projection target of DG in the trisynaptic pathway through the MFs. We currently hypothesize that this decrease in activity-dependent signaling within DG and in the MF pathway sensitizes its target tissue (CA3) to incoming stimuli and generates a lower LTP threshold and increased cellular sensitivity, thus generating higher levels of neuronal activity in CA3, which would be projected onto CA1. We suggest that this results in increases in synaptic strength in CA3, along with increased associational function. The Model is illustrated in figure 4. The ability of homeostatic plasticity phenomena to support this kind of model is included in data generated in the Malenka,65,66 Bear,46–51,53 and Malinow laboratories, among others.49,52,54 It is a speculation that increases in CA3 associational function could generate false associations, some with psychotic content, which could then get laid down in memory as psychotic thoughts and memories. This idea could explain the reduction in glutamate signaling in DG (represented by NR1 protein reduction) as well as the increase in hippocampal perfusion that we detect with rCBF measures10 and the finding that BDNF is increased regionally in CA3 in schizophrenia tissue57 (figure 3a). The dependence of psychotic manifestation on associational dysfunction within CA3 would lead to the assumption that occasional and fleeting associational mistakes in CA3 could be responsible for the fleeting psychotic-like phenomena in normal and in ‘prodromal’ individuals. Persistent and high increases in CA3 activity could instantiate the psychosis and generate manifestations that would resemble schizophrenia.
Fig. 4.
Hippocampal plasticity model for psychosis.
To screen further for a pathological mechanism(s) in glutamate signaling in schizophrenia hippocampus, we hypothesized that levels of presynaptic trafficking proteins could be low, thus reducing excitatory transmission. To examine this further, we measured synaptic proteins using Western blotting in human postmortem hippocampal tissue (N = 12 per group) of high quality.90 In figure 3b, the data show that none of the proteins assessed are altered in schizophrenia, not even those proteins that others had previously found altered, like synapsin91 or synaptophysin.92 Thus, there is no evidence from our data to implicate presynaptic signaling proteins pathology in hippocampal plasticity.
Developing an initial model to account for these findings led us to the simple hypothesis that reduced activity in the MF innervation from DG to CA3, secondary to glutamate signaling reductions in DG, could serve to increase LTP and synaptic strength in CA3 and augment excitatory neuronal function there and in CA1 (reviewed in10). We hypothesized that a reduction in the MF→CA3 afferents onto CA3 neurons would decrease the threshold to LTP in CA3 and increase excitability, especially within the system of CA3 recurrent collateral synapses, a unique feature of CA3 innervation. The literature supports this model, with findings by Koolomeets et al65 showing a reduction in MF synapses in CA3, and a Reif study61 showing reduced neurogenesis in DG, each in schizophrenia tissue. Based on this glutamate-mediated metaplasticity model,10 we predicted an increase in markers of plasticity in CA3, such as an increase in GluN2b-containing NMDARs (augmenting the sensitivity of NMDARs) and increases in BDNF activity in CA3 in schizophrenia (figure 3a). The evidence that DG excitatory activity is reduced in the illness, indicating hypoglutamatergic activity58,89,93,95 coupled with anatomic evidence of reduced MF insertions in CA362,65 and the possibility of reduced neurogenesis61 are observations already made in schizophrenia, consistent with this formulation. The evidence that DISC1 alleles and the NRG Icelandic haplotype are risk genes for schizophrenia and are well known to influence synaptic plasticity (REF), additionally strengthens this hypothesis, given the intimate involvement of these genes in synaptic plasticity especially in hippocampus and the demonstration of alterations in synaptic plasticity with mutations in these genes.95–98
We are pursuing the testing of this model in schizophrenia using both high-resolution brain perfusion measures as well as microdissected hippocampal subfield tissue. The imaging outcomes will inform which subfields show increases in neuronal activity. The molecular studies will identify which, if any, synapse-specific molecular plasticity markers are altered in schizophrenia tissue. An increase in homeostatic plasticity markers would be consistent with regional increases in synaptic strength at the recurrent collateral CA3 synapses, thereby increasing excitation. It is fortunate that basic neuroscience has generated a broad understanding of glutamate-dependent plasticity processes in hippocampus, allowing an identification of protein markers to examine this model further.
How Subfield-Specific Dysfunction in Schizophrenia Could Underlie Psychotic Manifestations
Hippocampal memory functions include ‘pattern separation’ (the process of creating distinct nonoverlapping representations for highly similar events) and ‘pattern completion’ (the process by which prior memories are reinstated from partial cues); these processes are regionally executed to some degree and subserve representational memory function, automatically and at the speed of thought. First, if hippocampal pattern separation is diminished due to partial DG failure (resulting in ‘spurious associations’22) and, second, if pattern completion is accelerated and increasingly inaccurate due to increased CA3 associational activity, then it is conceivable that associations could be false and, especially if driven by anxiety or stress, could generate psychotic content, with the associations then being laid down in memory, despite their psychotic content, including especially delusions and thought disorder.99 The implication of this formulation includes the speculation that many different etiologies, all of which function to reduce activity in the MF pathway to CA3 and simultaneously increase neuronal and associational activity in CA3, can generate psychotic productions. Whether these psychotic productions persist and take over aspects of the memory process (as in schizophrenia), or merely generate individual and mild psychotic thoughts (as in normal people with psychotic experiences), are questions that will need future answers. If this model can be supported, it will make psychosis into a syndromal diagnosis, more like congestive heart failure, where the mental symptoms of psychosis are an end-organ effect of pathological signaling.
The implication of this kind of a formulation for diseases of the brain is that critical alterations in glutamatergic transmission can be local and not be represented in every brain region. Given the highly plastic capacity of hippocampus, it is reasonable to expect learning and memory-like plasticity to be involved in the cognitive manifestations of hippocampal pathology. The finding that glutamate transmission is reduced in DG, can be expected to alter excitatory signaling downstream, in CA3, and then on to CA1. If synaptic alterations already exist, as we can expect in schizophrenia, with risk genes that are known to be involved in synaptic function (NRG1, DISC, dysbindin, BDNF), then normal L&M plasticity characteristics may malfunction, possibly as described here. These ideas are particularly difficult to test in human systems, given the nondynamic aspect of postmortem tissue and the nonmolecular aspect of human brain imaging. But, these methods are sufficient to pose the model for further testing in dynamic systems, and fMRI provides an opportunity to examine whether the balance between pattern separation and pattern completion is altered in schizophrenia hippocampus. Moreover, advances in molecular neuroscience have identified useful markers to evaluate plasticity processes, critical to testing this glutamate-system model further. Specifically, the testing of this model and its falsification are planned in several distinct ways. First, the assessment of markers of plasticity change, like GluN2b-containing NMDA receptors and phosphorylation of the GluA1 subunit of the AMPA receptors, in hippocampal subfields in schizophrenia cases must be demonstrated in CA3. Second, an examination of neuronal anatomy in DG and in CA3 with Golgi and other methods to visualize dendrites and spines would be necessary to demonstrate anatomic changes associated with synaptic strengthening. Then, the possibility of generating an animal model, where reduced GluN1 in DG generates increased homeostatic plasticity in CA3, would provide an addressable animal preparation where LTP itself could be examined. The characteristics of this model, albeit speculative, are all testable using these approaches and can be discarded or modified, as the data suggest.
The glutamate hypothesis of schizophrenia has been highly generative for models of molecular pathology that could underlie the manifestations of schizophrenic illness. Here, we have proposed a novel model of hippocampal dysfunction, which could generate psychosis, and we propose to test this in human and animal systems. Modern techniques to examine human brain glutamate transmission have provided the promise of successful concept testing for manifestations of schizophrenic illness. The next decade should contribute more data to our goal of understanding schizophrenia biology and critically clarify aspects of psychosis pathology. It is the rapid advances in basic neuroscience that will enable sophisticated understanding of the complex mechanisms underlying psychiatric illnesses.
Funding
National Institutes of Mental Health (MH062236 to C.T., PI and MH076932 to A.W., PI), a Conte Project (MH066172 to Eric Nestler, PI), a K Award (MH079253 to S.G., PI); Distinguished Investigator Award from NARSAD (Brain and Behavior Research Foundation to C.T.).2
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Benarroch EE. NMDA receptors: recent insights and clinical correlations. Neurology. 2011;76:1750–1757. doi: 10.1212/WNL.0b013e31821b7cc9. [DOI] [PubMed] [Google Scholar]
- 2.Nikam SS, Kornberg BE. AMPA receptor antagonists. Curr Med Chem. 2001;8:155–170. doi: 10.2174/0929867013373877. [DOI] [PubMed] [Google Scholar]
- 3.Lynch DR, Guttmann RP. NMDA receptor pharmacology: perspectives from molecular biology. Curr Drug Targets. 2001;2:215–231. doi: 10.2174/1389450013348434. [DOI] [PubMed] [Google Scholar]
- 4.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 5.Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 7.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Greer PL, Zieg J, Greenberg ME. Activity-dependent transcription and disorders of human cognition. Am J Psychiatry. 2009;166:14–15. doi: 10.1176/appi.ajp.2008.08111731. [DOI] [PubMed] [Google Scholar]
- 9. Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of the anatomical data. Neuroscience. 1989;31:571–591. [DOI] [PubMed] [Google Scholar]
- 10.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1–16. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 11.Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 12.Joseph TC. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;V26:363–382. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 13.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci. 2000;12:103–113. doi: 10.1176/jnp.12.1.103. [DOI] [PubMed] [Google Scholar]
- 14.Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 15.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corkin S. Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in H.M. Semin Neurol. 1984;4:249–259. [Google Scholar]
- 17.O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- 18.Eichenbaum H. A cortical-hippocampal system for declarative memory. Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 19.Lisman J, Otmakhova N. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the socratic model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- 20.Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Cohen N, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, UK: MIT Press; 1993. [Google Scholar]
- 22.McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 23.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans [published erratum appears in Psychol Rev 1992 Jul;99(3):582] Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 24.Gabrieli JD. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- 25.Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 26. Preston AR, Wagner AD. The Medial Temporal Lobe and Memory. In: Kesner RP and Martinez J. Neurobiology of Learning and Memory. 2nd ed. New York, NY: Elsevier, Inc.; 2007:305–337. [Google Scholar]
- 27.Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]
- 28.Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Duvernoy H. The Human Hippocampus. New York, NY: Springer; 1998. [Google Scholar]
- 30.Insausti R, Juottonen K, Soininen H, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki W, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cytoarchitectonic and chemoarchitectonic organization. J Comp Neurol. 2003;463:67–91. doi: 10.1002/cne.10744. [DOI] [PubMed] [Google Scholar]
- 32.Lavenex P, Banta LP, Amaral DG. Postnatal development of the primate hippocampal formation. Dev Neurosci. 2007;29:179–192. doi: 10.1159/000096222. [DOI] [PubMed] [Google Scholar]
- 33.O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 34.Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- 35.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 37.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;9:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 38.Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation–feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelkey KA, McBain CJ. Target-cell-dependent plasticity within the mossy fibre-CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites. J Physiol. 2008;586:1495–1502. doi: 10.1113/jphysiol.2007.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology. 2011;36:1171–1177. doi: 10.1038/npp.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 44.Biou V, Bhattacharyya S, Malenka RC. Endocytosis and recycling of AMPA receptors lacking GluR2/3. Proc Natl Acad Sci U S A. 2008;105:1038–1043. doi: 10.1073/pnas.0711412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 46.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 47.Sudhof TC, Malenka RC. Understanding synapses: past, present, and future. Neuron. 2008;60:469–476. doi: 10.1016/j.neuron.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iny K, Heynen AJ, Sklar E, Bear MF. Bidirectional modifications of visual acuity induced by monocular deprivation in juvenile and adult rats. J Neurosci. 2006;26:7368–7374. doi: 10.1523/JNEUROSCI.0124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE. 2006;2006:re11. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- 52.Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen WS, Bear MF. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology. 2007;52:200–214. doi: 10.1016/j.neuropharm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Ghose S, Chin R, Lewis-Amezcua K, Frost DO, Roberts R, Tamminga C. Distinct gene expression correlations in schizophrenia between NR1 and GAD67 in the anterior hippocampus. 190.23/NN11. Society of Neuroscience Abstract. 2006. http://www.abstractsonline.com/viewer/viewAbstractPrintFriendly.asp?CKey={F8CCA0FB-FE7B-4A8D-99AD-7F03C79C2115}&SKey={385A8D36-679C-4381-9CE3-DA1A4B276770}&MKey={D1974E76-28AF-4C1C-8AE8-4F73B56247A7}&AKey={3A7DC0B9-D787-44AA-BD08-FA7BB2FE9004} [Google Scholar]
- 58.Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 59.Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- 60.Eastwood SL, McDonald B, Burnet PWJ, Beckwith JP, Kerwin RW, Harrison PJ. Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluR1 and GluR2 in medial temporal lobe neurons in schizophrenia. Mol Brain Res. 1995;29:211–223. doi: 10.1016/0169-328x(94)00247-c. [DOI] [PubMed] [Google Scholar]
- 61.Reif A, Fritzen S, Finger M, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 62.Kolomeets NS, Orlovskaya DD, Uranova NA. Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse. 2007;61:615–621. doi: 10.1002/syn.20405. [DOI] [PubMed] [Google Scholar]
- 63.Porter RH, Eastwood SL, Harrison PJ. Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex, and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res. 1997;751:217–231. doi: 10.1016/s0006-8993(96)01404-7. [DOI] [PubMed] [Google Scholar]
- 64.Lauer M, Beckmann H, Senitz D. Increased frequency of dentate granule cells with basal dendrites in the hippocampal formation of schizophrenics. Psychiatry Res. 2003;122:89–97. doi: 10.1016/s0925-4927(02)00122-1. [DOI] [PubMed] [Google Scholar]
- 65.Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57:47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- 66.Rosoklija G, Toomayan G, Ellis SP, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 67.Schobel SA, Lewandowski NM, Corcoran CM, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- 69.Molina V, Sanz J, Sarramea F, Benito C, Palomo T. Prefrontal atrophy in first episodes of schizophrenia associated with limbic metabolic hyperactivity. J Psychiatr Res. 2005;39:117–127. doi: 10.1016/j.jpsychires.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Ragland JD, Gur RC, Valdez JN, et al. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. Am J Psychiatry. 2005;162:1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malaspina D, Harkavy-Friedman J, Corcoran C, et al. Resting neural activity distinguishes subgroups of schizophrenia patients. Biol Psychiatry. 2004;56:931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 73.Fletcher P. The missing link: a failure of fronto-hippocampal integration in schizophrenia. News Views. 1998;1:266–267. doi: 10.1038/1078. [DOI] [PubMed] [Google Scholar]
- 74.Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 75.Nordahl TE, Kusubov N, Carter C, et al. Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology. 1996;15:541–554. doi: 10.1016/S0893-133X(96)00098-X. [DOI] [PubMed] [Google Scholar]
- 76.Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- 77.Tamminga CA, Thaker GK, Buchanan R, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry. 1992;49:522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- 78.Gur RE. Left hemisphere dysfunction and left hemisphere overactivation in schizophrenia. J Abnorm Psychol. 1978;87:226–238. doi: 10.1037//0021-843x.87.2.226. [DOI] [PubMed] [Google Scholar]
- 79.Achim AM, Bertrand MC, Sutton H, et al. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry. 2007;64:999–1014. doi: 10.1001/archpsyc.64.9.999. [DOI] [PubMed] [Google Scholar]
- 80.Thermenos HW, Seidman LJ, Poldrack RA, et al. Elaborative verbal encoding and altered anterior parahippocampal activation in adolescents and young adults at genetic risk for schizophrenia using FMRI. Biol Psychiatry. 2007;61:564–574. doi: 10.1016/j.biopsych.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 81.Ongur D, Cullen TJ, Wolf DH, et al. The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63:356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- 82.Keri S, Nagy O, Kelemen O, Myers CE, Gluck MA. Dissociation between medial temporal lobe and basal ganglia memory systems in schizophrenia. Schizophr Res. 2005;77:321–328. doi: 10.1016/j.schres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 83.Holt DJ, Weiss AP, Rauch SL, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 84.Heckers S, Zalesak M, Weiss A, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- 85.Jessen F, Scheef L, Germeshausen L, et al. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am J Psychiatry. 2003;160:1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- 86.Leube DT, Rapp A, Buchkremer G, et al. Hippocampal dysfunction during episodic memory encoding in patients with schizophrenia—an fMRI study. Schizophr Res. 2003;64:83–85. doi: 10.1016/s0920-9964(02)00503-0. [DOI] [PubMed] [Google Scholar]
- 87.Eyler-Zorrilla LT, Jeste DV, Paulus MP, Brown GG. Functional abnormalities of medial temporal cortex during novel picture learning among patients with chronic schizophrenia. Schizophr Res. 2002;59:187–198. doi: 10.1016/s0920-9964(01)00340-1. [DOI] [PubMed] [Google Scholar]
- 88.Andreasen NC, O'Leary DS, Flaum M, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- 89.Deicken RF, Pegues M, Amend D. Reduced hippocampal N-acetylaspartate without volume loss in schizophrenia. Schizophr Res. 1999;37:217–223. doi: 10.1016/s0920-9964(98)00173-x. [DOI] [PubMed] [Google Scholar]
- 90.Stan AD, Ghose S, Gao XM, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 92.Eastwood SL, Cairns N, Harrison PJ. Synaptophysin gene expression in schizophrenia. Br J Psychiatry. 2000;176:236–242. doi: 10.1192/bjp.176.3.236. [DOI] [PubMed] [Google Scholar]
- 93.Deakin JF, Simpson MDC. A two-process theory of schizophrenia: evidence from studies in post-mortem brain. J Psychiatr Res. 1997;31:277–295. doi: 10.1016/s0022-3956(96)00042-8. [DOI] [PubMed] [Google Scholar]
- 94.Altar CA, Jurata LW, Charles V, et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. doi: 10.1016/j.biopsych.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 95.Duan X, Chang JH, Ge S, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lichtenstein P, Yip BH, Bjork C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clelland CD, Choi M, Romberg C, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoffman RE, Grasemann U, Gueorguieva R, Quinlan D, Lane D, Miikkulainen R. Using computational patients to evaluate illness mechanisms in schizophrenia. Biol Psychiatry. 2011;69:997–1005. doi: 10.1016/j.biopsych.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]