Abstract
It remains unclear whether brain structural abnormalities observed before the onset of psychosis are specific to schizophrenia or are common to all psychotic disorders. This study aimed to measure regional gray matter volume prior to the onset of schizophreniform and of affective psychoses. We investigated 102 subjects at ultrahigh risk (UHR) of developing psychosis recruited from the Personal Assessment and Crisis Evaluation Clinic in Melbourne, Australia. Twenty-eight of these subjects developed psychosis subsequent to scanning: 19 schizophrenia, 7 affective psychoses, and 2 other psychoses. We examined regional gray matter volume using 1.5 mm thick, coronal, 1.5 Tesla magnetic resonance imaging and voxel-based morphometry methods of image analysis. Subjects were scanned at presentation and were followed up clinically for a minimum of 12 months, to detect later transition to psychosis. We found that both groups of subjects who subsequently developed psychosis (schizophrenia and affective psychosis) showed reductions in the frontal cortex relative to UHR subjects who did not develop psychosis. The subgroup that subsequently developed schizophrenia also showed smaller volumes in the parietal cortex and, at trend level, in the temporal cortex, whereas those who developed an affective psychosis had significantly smaller subgenual cingulate volumes. These preliminary findings suggest that volumetric abnormalities in UHR individuals developing schizophrenia vs affective psychoses comprise a combination of features that predate both disorders and others that may be specific to the nature of the subsequent disorder.
Keywords: ultrahigh risk, schizophrenia, affective psychosis, MRI, brain structure, psychosis
Introduction
Neuroimaging studies have consistently shown that schizophrenia, and to a certain extent psychosis in general, is associated with subtle abnormalities of brain structure. Among these, the most replicated are ventricular enlargement, diffuse reduction of gray matter, and volume reduction of frontal (prefrontal cortex) and temporal lobe structures (superior and medial temporal cortex).1 In contrast, psychoses in the affective spectrum have been particularly associated with changes in areas involved in emotional regulation, such as the amygdala and the subgenual cingulate cortex.2–4 Although these abnormalities are already present in first-episode psychoses, it remains to be established whether (a) they precede the first episode, thus representing possible vulnerability markers and (b) if so, whether they are vulnerability markers that are specific to schizophrenia or are generic for all psychoses.
These issues can be addressed by studying nonpsychotic subjects at high risk of developing a psychotic illness. Some studies have attempted this by evaluating subjects at increased risk because of their genetic load for psychosis. These have suggested that a number of neuroanatomical measures in these individuals are intermediate between healthy controls and subjects with established illness.5,6 Another approach, also used by our group, is to identify individuals whose clinical presentation suggests that they are at ultrahigh risk (UHR) of developing psychosis.7,8 With this strategy, a relatively high rate of conversion to psychosis can be expected within a reasonable timeframe. However, studies that have used this approach to date are few in number and small in size. Preliminary data suggest that, in comparison to healthy controls, UHR individuals show some of the brain volume reductions also identified in individuals with established illness.9,10 However, this may not be the case for all brain regions. For example, medial temporal lobe regions did not differ from healthy subjects in the large study of UHR individuals by Velakoulis and colleagues,11 while the same cohort did show reduced volumes of other temporal regions.12 Furthermore, frontal and temporal brain reductions may be particularly marked in those UHR individuals who subsequently go on to develop psychosis (UHR-P) and may progress further with the onset of psychosis.13,14 Relevant to the present study, our data further suggest that the effect is larger in those UHR individuals who subsequently develop schizophreniform rather than affective psychoses.15 This is consistent with data in established psychoses, suggesting that while schizophrenia is associated with reductions in frontal, temporal, and limbic areas, affective psychoses are associated with more subtle and varying volumetric brain reductions and increases, possibly involving parts of the cingulate cortex and the amygdala.2,4
Unfortunately, most UHR studies to date have included only small numbers of subjects who developed psychosis at follow-up, precluding examination of the diagnostic specificity of changes prior to the onset of psychosis. This study had two main aims: (a) investigate if there are brain reductions that differentiate UHR individuals who will develop schizophrenia from individuals who will not develop a psychosis and (b) conduct a preliminary exploratory analysis to investigate if there are brain differences that specifically differentiate those UHR individuals who will develop an affective, rather than schizophrenic psychosis. We studied the largest group of subjects at UHR of psychosis investigated with imaging to date, and these subjects were identified using a combination of clinical characteristics.16 We used magnetic resonance imaging (MRI) to examine the brain structure of these subjects before the onset of their first episode of psychosis and followed them up clinically for at least 12 months to identify those making the transition to psychosis. We used voxel-based morphometry (VBM) because this allows for whole-brain analysis of large samples, and it provides an unbiased estimation of regional differences independently from user-intervention.17
On the basis of previous work, we predicted that individuals who subsequently developed schizophrenia would show, even in the at-risk state (thus before illness onset), reductions in frontal and temporal volumes, to a greater extent than high-risk individuals who later developed affective psychoses. Conversely, we predicted that individuals who subsequently developed an affective psychosis would show relatively more marked regional volume reductions in the subgenual cingulate cortex and amygdala.
Methods
Sample
Individuals at UHR of developing psychosis were recruited from the Personal Assessment and Crisis Evaluation Clinic, Melbourne, Australia, which manages young people at risk of developing a psychotic illness and assessed with a face-to-face interview.18 To be considered as UHR, individuals had to meet at least 1 of 3 sets of operationalized criteria7:
Frequent attenuated subthreshold psychotic symptoms (a number of which are considered under Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, (DSM-IV) schizotypal disorder19): Presence of one or more of the following symptoms: ideas of reference, magical thinking, perceptual disturbance, paranoid ideation, odd thinking, and speech (score of 2–3 on unusual thought content subscale, 1–2 on hallucinations subscale, 3 on suspiciousness subscale, or 1–3 on conceptual disorganization scale of Brief Psychiatric Rating Scale [BPRS]20); Symptoms are held with a reasonable degree of conviction, as defined by a score of 2 on the Comprehensive Assessment of Symptoms and History (CASH)21 rating scale for delusions; Frequency of symptoms is several times per week; The change in mental state has been present for at least 1 week within the past year and not more than 5 years.
Transient psychotic symptoms (brief limited intermittent psychotic symptoms [BLIPS]): Presence of one or more of the following: ideas of reference, magical thinking, perceptual disturbance, paranoid ideation, odd thinking, and speech (score of 4+ on unusual thought content subscale, 3+ on hallucinations subscale, 4+ on suspiciousness subscale [or it is held with strong conviction, as defined by a score of 3 or more on the CASH rating subscale for delusions], or 4+ on conceptual disorganization subscale of BPRS); Duration of episode of less than 1 week; Symptoms resolve spontaneously; The symptoms must have occurred within the past year.
Trait and state risk factors: First-degree relative with a psychotic disorder or schizotypal personality disorder or individual has schizotypal personality disorder; Significant decrease in mental state or functioning—maintained for at least a month (reduction in General Assessment of Function19 Scale of 30 points from premorbid level); Decrease in functioning maintained for at least a month and for not more than 5 years. UHR criteria were confirmed by applying the Comprehensive Assessment of At-Risk Mental States,22 especially developed for this purpose.
Of the 146 UHR individuals recruited and scanned, 44 were excluded because the scans presented image or processing artefacts, or because they were judged of a quality unsuitable for VBM analysis. The remaining 102 individuals included in the study had met the following criteria for the UHR state: 17 Trait and state risk factors; 11 BLIPS; 45 attenuated subthreshold psychotic symptoms; 17 Trait and state risk factors and attenuated subthreshold psychotic symptoms; 9 attenuated subthreshold psychotic symptoms and BLIPS; and 3 all the 3 sets of UHR criteria. Over a follow-up period set at least 12 months, 28 (29%) developed psychosis (UHR-P) (24 within 12 months and a further 4 within 24 months), while 74 remained nonpsychotic (UHR-NP). These subjects had never experienced an episode of frank psychosis and were all antipsychotic-naïve at the time of scanning.
Subjects were assessed with the BPRS, Scale for the Assessment of Negative Symptoms (SANS),23 and National Adult Reading Test (NART)24 at intake, and the diagnosis was ascertained with the Structured Clinical Interview for DSM-IV.25 Handedness was assessed with the Edinburgh Inventory.26
Exclusion criteria were a history of significant head injury, seizures, cerebrovascular disease, other neurological disease, impaired thyroid function, steroid use, or DSM-III-R criteria of alcohol or substance abuse or dependence. Written informed consent was obtained from all subjects, the study was approved by local research and ethics committees and was consistent with the principles outlined in the Declaration of Helsinki.
Clinical and sociodemographic differences between groups were examined using one-way ANOVA, or chi-square test. Statistical analyses of demographic, cognitive, and clinical data were performed with the Statistical Package for the Social Sciences.
Structural MRI
Image Acquisition.
Subjects were scanned on GE Signa 1.5 T scanners at the Royal Melbourne Hospital and at the Royal Children Hospital. The sequences obtained from the scanners were identical. Head movement was minimized by foam padding and velcro straps across the forehead and chin. A 3-dimensional volumetric spoiled gradient recalled echo in the steady state sequence generated 124 contiguous, 1.5 mm thick coronal slices. Imaging parameters were time-to-echo, 3.3 m sec; time-to-repetition, 14.3 m sec; flip angle, 30°; matrix size, 256 × 256; field of view, 24 × 24 cm matrix; voxel dimensions, 0.938 × 0.938 × 1.5 mm. The scanners were calibrated fortnightly using the same proprietary phantom to ensure stability and accuracy of measurements. A code was used to ensure patient confidentiality and blind rating of data. A neuroradiologist reviewed all MRI films.
Image Processing.
MRI data were transferred from digital audio tape to a Silicon Graphics workstation. Structural images were preprocessed using ‘optimized’ VBM implemented with Statistical Parametric Mapping software (SPM5) running under Matlab 7.0. VBM is a whole-brain, unbiased, semiautomated technique for characterizing regional cerebral differences in structural magnetic resonance images.17,27 First, structural images were segmented to extract gray matter and then normalized to an asymmetric T1-weighted template in Montreal Neurological Institute (MNI) stereotactic space, in a recursive fashion. Image segmentation incorporated an intensity nonuniformity correction to account for smooth intensity variations caused by gradient distortions and different positions of cranial structures within the MRI coil.27 A further step was added to ensure that the total amount of gray matter in each voxel was conserved before and after spatial normalization.28 This ‘modulation’ step involved multiplying the spatially normalized gray matter by its relative volume before and after spatial normalization. The resulting gray matter images were finally smoothed with a 12 mm isotropic Gaussian kernel. Smoothing is required to compensate for the inexact nature of spatial normalization and to maximize the chance that regional effects are expressed at a spatial scale where homologies in structural anatomy exist over subjects. After smoothing, each voxel represents the local average amount of gray or white matter in the region, the size of which is defined by the smoothing kernel.
Statistical Analyses.
We performed an ANOVA to compare UHR who converted to a diagnosis of schizophrenia (UHR-SZ), those who converted to a diagnosis of affective psychosis (UHR-AFF), and nonconverters (UHR-NP). We excluded individuals who converted to other types of psychosis. We modeled age, gender, handedness, and scanner site as covariates of no interest, to minimize the potential impact of inter-subject variability. In fact, these variables are known to be associated with neuroanatomical differences28,29; if these differences are not modeled as covariate in the statistical analysis, they would increase the unexplained variance in the data (ie, error noise), thereby decreasing sensitivity. To identify regionally specific reductions that were not confounded by global differences,17 total gray matter was also included as a covariate of no interest. We report effects that reached significance at P < .05 after family-wise error (FWE) correction for multiple comparisons and also any ‘trend’ which reached significance at P < .001 (uncorrected) for completeness. In addition to the whole-brain analysis, we performed some region of interest analyses in the comparison between UHR-SZ and UHR-AFF. These regions of interest (ROIs) included the subgenual part of the cingulate gyrus and the amygdala,4 and they were selected because they have been reported to be specifically affected in established affective psychoses. We used predefined anatomical masks based on the Automatic Anatomical Labeling atlas available in Pickatlas toolbox (http://fmri.wfubmc.edu/cms/software#PickAtlas). MNI coordinates were converted in Talairach coordinates.
Results
Demographic, cognitive, and clinical data are presented in table 1 as individual values, mean ± SD.
Table 1.
Demographic and Clinical Characteristics of the Groups
| Characteristic | All UHRa | UHR-NP | UHR-SZ | UHR-AFF |
| (n = 102) | (n = 74) | (n = 19) | (n = 7) | |
| Age at baseline (mean years, SD) | 20.3 (3.5) | 20.7 (3.5) | 18.5 (3.4) | 20.5 (2.1) |
| Gender (% male) | 52 | 50 | 63 | 43 |
| Handedness (% right) | 86 | 87 | 89 | 100 |
| NART premorbid intelligence quotient (mean, SD) | 95 (13.5)b | 95.3 (14.3) | 93.6 (11.7) | 96.0 (11.6) |
| Time between MRI and onset of psychosis (mean years, SD) | — | — | 0.47 (0.39)c | 0.48 (0.61)d |
| SANS at intake (mean, SD) | 25 (16) | 24 (17) | 30 (17) | 32 (9) |
| BPRS at intake (mean, SD) | 21 (9) | 21 (10) | 22 (9) | 20 (5) |
Note: There was a significant difference in age (P = .04) due to the UHR-SZ being younger than the UHR-NP group. There were no significant between group differences for any of the other variables. BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms; NART, National Adult Reading Test.
The All UHR number of subjects includes 1 subject who developed a psychosis not otherwise specified and 1 subject who developed a brief psychotic episode, and these subjects were excluded from further comparisons.
For 80 people only.
For 17 people only.
For 6 people only.
According to the study protocol, all subjects were followed up for at least 12 months. During this time, 28 UHR subjects developed a psychotic episode (UHR-P) (24 within 12 months and a further 4 within 24 months): schizophrenia (n = 19), brief psychotic episode (n = 1), psychosis not otherwise specified (n = 1), bipolar disorder with psychotic features (n = 2), and major depression with psychotic features (n = 5). The UHR-P subjects were younger than the UHR-NP (F 1.8; P = .021), but they did not differ from the UHR-NP in terms of gender, baseline BPRS, SANS, NART score, or total gray matter volume.
MRI Analyses
Regional gray matter was compared across three groups: UHR who did not develop a psychosis (UHR-NP, n = 74), UHR who developed schizophrenia (UHR-SZ, n = 19), and UHR who developed an affective psychosis (bipolar disorder with psychotic features or major depression with psychotic features, UHR-AFF, n = 7). The 2 UHR individuals who developed other types of psychoses were excluded from further analyses. We report below only the contrasts in which there were significant differences.
Regional Differences in Gray Matter Volume.
UHR-NP vs UHR-SZ.
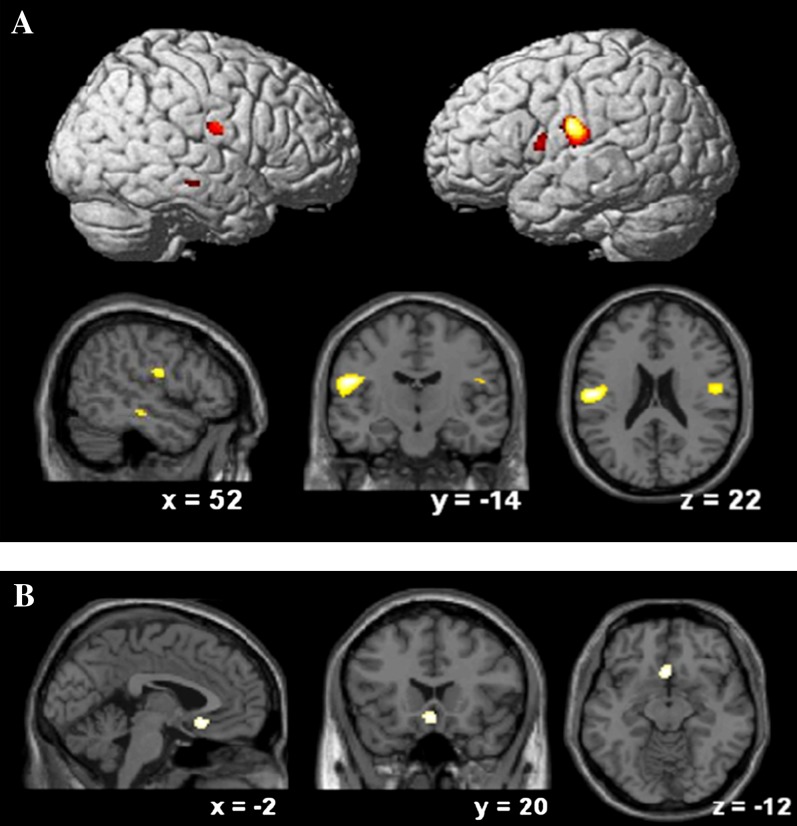
UHR-SZ showed less gray matter than UHR-NP in the left postcentral gyrus (P < .05 after FWE correction) and at trend level on the right postcentral gyrus (P < .001, uncorrected). There were trends for relatively reduced gray matter also in the left pars opercularis (occupying parts of the inferior frontal and precentral gyri) (P < .001, uncorrected) and right middle temporal gyrus (P < .001, uncorrected) (table 2; figure 1).
Table 2.
Talairach Coordinates for the Regions of Significant Gray Matter Volume Differences Between Groups
| Area | Coordinates x, y, z | Z-Score | Significance & Size in Voxels |
| VBM analysis: | |||
| UHR-NP > UHR-SZ | |||
| Left postcentral gyrus | −54, −14, 22 | 5.1 | P < .05 after FWE correction 38 voxels |
| Right postcentral gyrus | 52, −10, 22 | 3.9 | P < .001 (uncorrected)* 73 voxels |
| Right middle temporal gyrus | 52, −22, −14 | 3.5 | P < .001 (uncorrected)* 16 voxels |
| Left pars opercularis (extending into inferior frontal gyrus and precentral gyrus) | −54, 6, 10 | 3.5 | P < .001 (uncorrected)* 38 voxels |
| UHR-NP > UHR-AFF | |||
| Right superior frontal gyrus | 12, 42, 58 | 3.4 | P < .001 (uncorrected)* 23 voxels |
| UHR-SZ > UHR-AFF | |||
| Subcallosal cingulate gyrus | −2, 20, −12 | 3.5 | P < .001 (uncorrected)* 78 voxels |
| Region of interest analysis: | |||
| UHR-SZ > UHR-AFF | |||
| Subgenual cingulate | 0, 20, −10 | 3.5 | P = .039 after FWE correction |
Note: VBM, voxel-based morphometry;UHR-NP, ultrahigh risk who did not develop psychosis; UHR-SZ, ultrahigh risk who developed schizophrenia; UHR-AFF, ultrahigh risk who developed an affective psychosis, FWE, family-wise error.
*Significance shown as uncorrected, therefore at trend level.
Fig. 1.
Comparison across diagnostic subgroups: A, gray matter volume reductions in UHR-SZ in comparison with UHR-NP; B, gray matter volume reductions in UHR-AFF in comparison with UHR-SZ. A threshold of P < .001 (uncorrected) was used to show the trends at uncorrected level reported in the results section.
UHR-NP vs UHR-AFF.
UHR-AFF showed less gray matter than UHR-NP in the right superior frontal gyrus (P < .001, uncorrected) (table 2; figure 1).
UHR-SZ vs UHR-AFF.
UHR-converters who developed an affective psychosis showed less gray matter than UHR-SZ in the subcallosal cingulate gyrus (P < .001, uncorrected) (table 2; figure 1).
We then performed ROI analyses of the amygdala and the subgenual portion of the cingulate cortex, as described earlier and using a statistical threshold of P < .05 with FWE correction for multiple comparisons. We found that UHR-AFF subjects had significantly smaller gray matter volume of the subgenual cingulate (y = 20 z = −10, z-score = 3.5, P = .039 after FWE correction) than the UHR-SZ subjects, but we observed no group differences in the amygdala.
Discussion
This is the first VBM study to conduct an exploratory analysis of the specificity of brain changes in at-risk individuals to later schizophreniform and affective psychoses.
We addressed this issue in the largest sample of UHR individuals from one centre investigated to date. We found that, even before the onset of psychosis, UHR-SZ show significantly smaller volumes of parietal regions (postcentral gyrus). This is an interesting finding because while the parietal lobe has often been neglected in schizophrenia, there is consistent evidence of volume reductions in this area in approximately 60% of ROI MRI studies and in VBM studies and meta-analyses in this disorder.1,30–32 Most importantly, our findings are consistent with evidence, from smaller high-risk populations, that the parietal lobe is affected in at-risk nonpsychotic individuals, particularly in those who subsequently make the transition to psychosis.33–35 We additionally found that UHR-SZ showed reductions in a temporal area (middle temporal gyrus), at trend level, when compared with UHR-NP. This trend is still noteworthy because temporal lobe reductions (superior and medial temporal in particular) are among the most replicated findings in established schizophrenia.1 Interestingly, reductions in the temporal cortex have been particularly associated with subsequent development of psychosis in high-risk nonpsychotic individuals and seem to become more marked in individuals with a first-psychotic episode.11,36 Consistent with evidence that temporal reductions are indeed more prominent in patients with schizophrenia than in patients with affective disorders, in this article we suggest that temporal reductions may be specifically present in those high-risk individuals who will then develop schizophrenia.37
In our study, both UHR-SZ and UHR-AFF already showed, albeit at trend level, smaller volumes in frontal areas (the inferior frontal gyrus—pars opercularis—in schizophrenia and superior frontal gyrus in affective psychosis) than UHR individuals who did not convert to psychosis. Although this finding should be taken as preliminary, it is consistent with evidence of morphological and neurophysiological alterations of the frontal cortex as a core structural and functional alteration of schizophrenia, and with evidence of frontal abnormalities in affective psychoses, already at illness onset,38,39 and possibly even before onset.13,15,36,40 Evidence of a frontal lobe dysfunction preceding psychosis onset is further supported by cognitive data from our sample, showing that although all UHR subjects have worse working memory (a function associated with the frontal cortex, with the pars opercularis particularly involved in retrieving stored information41) than healthy controls, these deficits are more marked in those who develop psychosis.42 Here, we confirm that morphological frontal lobe deficits may characterize those UHR individuals who subsequently develop psychosis, and suggest that this applies to the period prior to both schizophreniform and affective psychoses.
We found some interesting preliminary findings in the investigation of brain abnormalities specific to individuals who subsequently developed an affective psychosis (UHR-AFF), although the small sample size needs to be taken into account when interpreting the results. The whole-brain (at trend level) and the ROI analyses directly comparing UHR-SZ and UHR-AFF showed that UHR-AFF individuals had a smaller gray matter volume in the subgenual cingulate gyrus, compared with UHR-SZ. This is probably not surprising because part of the anterior cingulate is implicated in mood disorders: It has been reported as reduced in volume in individuals with affective disorders, even without psychotic symptoms.43,44 Furthermore, deep brain stimulation of this region induces remission of refractory depression.45 In a previous analysis, our group-investigated thickness of the subgenual cingulate cortex in a larger sample of UHR individuals, matched according to cingulate gyrus morphology. In this analysis, we found no differences in subgenual cingulate thickness between UHR-SZ and those who developed other psychoses, although the UHR-SZ had a thinner cingulate cortex than UHR-NP.46 These discrepancies may be due to the fact that different subjects were included in the two comparisons of thickness (all nonschizophreniform psychoses, including 7 additional subjects) and volume (restricted to individuals with affective psychoses). It is also possible that volume differences appear because of differences in cingulate morphology, when subjects have not been matched to take this anatomical variable into account. Still, it is interesting that the subgenual cingulate has recently been shown to be the only part of the cingulate gyrus that progressively reduces in volume in patients with affective psychosis, following the first-psychotic episode.3
Contrary to our hypothesis, we did not find evidence for a smaller volume of the amygdala. This structure is involved in the regulation of emotions and has been reported as altered in volume and function in individuals with affective disorders.2 This finding would be consistent with our previous analysis, using a different manual volume estimate of this structure, showing no difference in amygdala volume between UHR-P, UHR-NP, and healthy controls.11 Interestingly, in that study, we found that the amygdala was significantly larger in first-episode subjects who had nonschizophreniform psychoses, thus suggesting that changes in this structure possibly appear after illness onset.
Our study has several strengths. First, the strategy we used for the selection of the high-risk population allowed us to have a transition to psychosis in enough subjects to attempt subdiagnostic comparisons. Second, at the time of the MRI scan all our subjects were antipsychotic-naïve. Therefore, our results cannot be attributed to the effect of antipsychotic treatment. Furthermore, this suggests that volume reductions in these brain areas, previously found in patients on treatment with antipsychotics, cannot be fully explained by exposure to these drugs. Third, we used voxel-based analysis that not only permits evaluation of the entire brain, but it is also automated, and therefore free of the intra/inter-operator reliability problems associated with manual tracings. Although there has been some debate on the methodology,47 studies using this approach have produced relatively consistent results in patients with psychosis and the methods have been extensively validated.17
We considered the possibility that some of our findings might have been affected by the fact that we used MRI collected from 2 scanners. However, we believe this is unlikely as the scanners were of identical make, the acquisition protocols were identical, the 2 scanners were calibrated fortnightly, and scanner was included as covariate in the statistical analyses. Furthermore, we have previously shown that differences between the two scanners do not have an effect on neuroanatomical measures such as those included in this study, although it is possible that subcortical structures are more affected.11,48 As recommended by recent evidence on this issue, given the difficulties in recruiting and assessing this type of clinical sample in MRI research, discarding part of a large clinical sample on the sole basis of acquisition on a different scanner, would not be entirely justified. Instead, as we have done, it is recommended that the effect of scanner is included in the statistical analysis.48
It should also be acknowledged that the number of UHR subjects in the diagnostic subgroups, particularly in the affective psychoses subgroup, was small, and this may have affected our statistical power. More specifically, the use of small sample sizes in neuroimaging studies using a random effects approach typically results in reduced sensitivity rather than false positive results. This means that our analysis may not have detected all neuroanatomical differences present between UHR-SZ and UHR-AFF. Indeed, some of our findings are only significant at trend level and as such should be regarded as preliminary. Nevertheless, the fact that we were able to detect a statistically significant difference between UHR-SZ and UHR-AFF in the subcallosal cingulate gyus, indicates that the number of subjects in each diagnostic subgroup was large enough to detect at least some of the differences between them. Furthermore, the fact that the changes identified across diagnostic subgroups are consistent with those identified in the established illness gives one more confidence. Also, we did not include a healthy control group as the UHR nonconverter group seemed the most appropriate to control for the potential influence of the prodromal status on the brain and to differentiate brain abnormalities in UHR-SZ from those of UHR-AFF. In fact, considering the potential for MRI application to the clinical setting, it is more important for MRI to be able to distinguish between prodromal individuals who will subsequently develop psychosis from those who will not, than from healthy individuals, who by definition will not come in contact with clinical services. Moreover, not using healthy individuals as the comparison group underestimates rather than overestimates, the differences identified.
When attempting to explain our findings, it is conceivable that early neurodevelopmental insults to gray matter in patients who are at risk of developing psychosis underlie the reductions we observed.49 There could then be superimposed changes that occur during, or soon after, the onset of frank psychosis.50 Interestingly, we also explored whether the gray matter differences we identified (left poscentral gyrus, left pars opercularis, and right middle temporal gyrus) were particularly present in subjects that could be considered closest to developing psychosis, the ones with the BLIPS. For each of these areas, the comparison (2-tailed t test) between those with and without BLIPS was not significant even at trend level, suggesting that the effects we report are not dependent on the presence or absence of BLIPS, but rather on the development of full-blown psychosis (left postcentral gyrus: F-score: 0.107, P value: .745; left pars opercularis: F-score: 1.314, P value: .259; and right middle temporal gyrus: F-score: 0.020; P value: .887). Indeed, our data would be in accordance with data from studies on individuals at genetic risk of schizophrenia and those at various illness stages, showing that abnormalities in some brain areas, or their progression, are associated with illness onset and establishment.10,15,40,51–53 Our data add to this literature as the subjects evaluated were at increased risk not because of, or not only because of, their family history but also because they were showing state markers (attenuated and fluctuating psychotic symptoms, decreased functioning).
In conclusion, we have shown that reductions in the prefrontal cortex may represent a risk marker of subsequent psychosis and we have provided preliminary evidence that reductions in some brain areas may be specific to the type of psychosis the individuals will eventually develop. Further studies, with larger samples of individuals who convert to different psychoses, will be needed to confirm and help quantify the predictive power of these effects.
Funding
Project grants from the National Health and the Medical Research Council (NHMRC Project grant numbers: 970598, 970391, 981112; NHMRC Program Grant numbers: 350241, 566529); Victorian Health Promotion Foundation; Stanley Foundation; Ian Potter Foundation; Woods Family Trust; Australian Communications and Computing Institute; Percy Baxter Charitable Trust; NARSAD. Also, support from the NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, Kings College London, a Short-Term Travel Grant from the Wellcome Trust; a grant from the University of London; a NARSAD Independent Investigator Award (to Dr. P. Dazzan); a Clinical Career Development Award from the NHMRC and a NARSAD Young Investigator Award (to A/Prof Wood); a NARSAD Independent Investigator Award (to Dr. A. Mechelli). The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Acknowledgments
Part of this work was awarded the 1st Prize for Poster Presentations at the ‘12th Scientific Meeting and Exhibition’ of the International Society for Magnetic Resonance in Medicine (Kyoto, Japan, May 2004). The authors have no conflict of interest and no financial interest to disclose. Dr Dazzan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haldane M, Frangou S. New insights help define the pathophysiology of bipolar affective disorder: neuroimaging and neuropathology findings. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:943–960. doi: 10.1016/j.pnpbp.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry. 2008;65:746–760. doi: 10.1001/archpsyc.65.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Y, Wang F, Xie G, et al. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: a voxel-based morphometric magnetic resonance imaging study. Psychiatry Res. 2007;156:83–86. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Baare WF, van Oel CJ, Hulshoff Pol HE, et al. Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry. 2001;58:33–40. doi: 10.1001/archpsyc.58.1.33. [DOI] [PubMed] [Google Scholar]
- 6.McDonald C, Bullmore ET, Sham PC, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 7.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 8.Demjaha A, Valmaggia L, Stahl D, Byrne M, McGuire P. Disorganization/cognitive and negative symptom dimensions in the at-risk mental state predict subsequent transition to psychosis. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq088. doi:10.1093/schbul/sbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung WH, Kim JS, Jang JH, et al. Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp151. doi:10.1093/schbul/sbp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borgwardt SJ, McGuire PK, Aston J, et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry Suppl. 2007;51:s69–s75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- 11.Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Wood SJ, Yung AR, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 2009;111:94–102. doi: 10.1016/j.schres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 14.Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (‘prodromal’) group. Schizophr Res. 2003;60:21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 17.Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-Based Morphometry of the Human Brain: methods and Applications. Curr Med Imaging Rev. 2005;1:105–113. [Google Scholar]
- 18.Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. Aust N Z J Psychiatry. 1996;30:587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 20.Rhoades HM, Overall JE. The semistructured BPRS interview and rating guide. Psychopharmacol Bull. 1988;24:101–104. [PubMed] [Google Scholar]
- 21.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 22.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 23.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;7:49–58. [PubMed] [Google Scholar]
- 24.Nelson HE, Willison JR. National Adult Reading Test (part II) Test Manual. Windsor, Canada: NFER-NELSON; 1991. [Google Scholar]
- 25.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- 26.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 28.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 29.Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 30.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 31.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanskanen P, Ridler K, Murray GK, et al. Morphometric brain abnormalities in schizophrenia in a population-based sample: relationship to duration of illness. Schizophr Bull. 2010;36:766–777. doi: 10.1093/schbul/sbn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Fusar-Poli P, Crossley N, Woolley J, et al. Gray matter alterations related to P300 abnormalities in subjects at high risk for psychosis: longitudinal MRI-EEG study. Neuroimage. 2011;55:320–328. doi: 10.1016/j.neuroimage.2010.11.075. [DOI] [PubMed] [Google Scholar]
- 35.Shim G, Oh JS, Jung WH, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav Brain Funct. 2010;6:58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meisenzahl EM, Koutsouleris N, Gaser C, et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res. 2008;102:150–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 37.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 38.Seidman LJ, Thermenos HW, Poldrack RA, et al. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr Res. 2006;85:58–72. doi: 10.1016/j.schres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M, Salisbury DF, Hirayasu Y, et al. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62:773–783. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64:1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 41.Henseler I, Falkai P, Gruber O. A systematic fMRI investigation of the brain systems subserving different working memory components in schizophrenia. Eur J Neurosci. 2009;30:693–702. doi: 10.1111/j.1460-9568.2009.06850.x. [DOI] [PubMed] [Google Scholar]
- 42.Wood SJ, Brewer WJ, Koutsouradis P, et al. Cognitive decline following psychosis onset: data from the PACE clinic. Br J Psychiatry Suppl. 2007;51:s52–s57. doi: 10.1192/bjp.191.51.s52. [DOI] [PubMed] [Google Scholar]
- 43.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 44.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Fornito A, Yung AR, Wood SJ, et al. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 2008;64:758–765. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 47.Bookstein FL. ‘Voxel-based morphometry’ should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 48.Suckling J, Barnes A, Job D, et al. Power calculations for multicenter imaging studies controlled by the false discovery rate. Hum Brain Mapp. 2010;31:1183–1195. doi: 10.1002/hbm.20927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dazzan P, Morgan KD, Chitnis X, et al. The structural brain correlates of neurological soft signs in healthy individuals. Cereb Cortex. 2006;16:1225–1231. doi: 10.1093/cercor/bhj063. [DOI] [PubMed] [Google Scholar]
- 50.Lappin JM, Morgan KD, Morgan C, et al. Duration of untreated psychosis and neuropsychological function in first episode psychosis. Schizophr Res. 2007;95:103–110. doi: 10.1016/j.schres.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 51.Tosato S, Ruggeri M, Bonetto C, et al. Association study of dysbindin gene with clinical and outcome measures in a representative cohort of Italian schizophrenic patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:647–659. doi: 10.1002/ajmg.b.30484. [DOI] [PubMed] [Google Scholar]
- 52.Mondelli V, Pariante CM, Navari S, et al. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr Res. 2010;119:75–78. doi: 10.1016/j.schres.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mondelli V, Dazzan P, Gabilondo A, et al. Pituitary volume in unaffected relatives of patients with schizophrenia and bipolar disorder. Psychoneuroendocrinology. 2008;33:1004–1012. doi: 10.1016/j.psyneuen.2008.05.010. [DOI] [PubMed] [Google Scholar]