Abstract
Emotional abnormalities are a critical clinical feature of schizophrenia (SCZ), but complete understanding of their underlying neuropathology is lacking. Numerous studies have examined amygdala activation in response to affective stimuli in SCZ, but no consensus has emerged. However, behavioral studies examining ‘in-the-moment’ processing of affect have suggested intact emotional processing in SCZ. To examine which aspects of emotional processing may be impaired in SCZ, we combined behavior and neuroimaging to investigate effects of aversive stimuli during minimal cognitive engagement, at the level of behavior, amygdala recruitment, and its whole-brain task-based functional connectivity (tb-fcMRI) because impairments may manifest when examining across-region functional integration. Twenty-eight patients and 24 matched controls underwent rapid event-related fMRI at 3 T while performing a simple perceptual decision task with negative or neutral distraction. We examined perceptual decision slowing, amygdala activation, and whole-brain amygdala tb-fcMRI, while ensuring group signal-to-noise profile matching. Following scanning, subjects rated all images for experienced arousal and valence. No significant group differences emerged for negative vs neutral reaction time, emotional ratings across groups, or amygdala activation. However, even in the absence of behavioral or activation differences, SCZ subjects demonstrated significantly weaker amygdala-prefrontal cortical coupling, specifically during negative distraction. Whereas in-the-moment perception, behavioral response, and amygdala recruitment to negative stimuli during minimal cognitive load seem to be intact, there is evidence of aberrant amygdala-prefrontal integration in SCZ subjects. Such abnormalities may prove critical for understanding disturbances in patients’ ability to use affective cues when guiding higher level cognitive processes needed in social interactions.
Keywords: schizophrenia, emotion, amygdala, fMRI, functional connectivity, IAPS, attention
Introduction
Emotional dysfunction is a critical clinical feature of schizophrenia (SCZ).1,2 There is substantial evidence for specific emotional deficits in SCZ; for instance, abnormalities in hedonic pursuits,3–5 affective flattening,6–8 and impaired evaluation of emotional facial cues,9,10 which may be critical for determining clinical trajectory and functional outcome.11–14 However, there is increasing evidence that certain aspects of emotional functioning in SCZ may be intact—namely ‘in-the-moment’ experience of affective stimuli.15,16 At the same time, despite increasing efforts to understand the neurobiological underpinnings of affective disturbances in SCZ,17 the literature on the neural correlates of in-the-moment emotional responsiveness in SCZ has produced mixed findings.17
One focus has been on characterizing amygdala activation in SCZ, given its well-established role in affective processing in healthy adults, particularly the processing of aversive content.18–24 Since the seminal study by Schneider and colleagues25 showing amygdala under-recruitment following mood induction in SCZ, results have varied and no consensus has yet ensued.25–59 In our prior work, a quantitative meta-analysis of amygdala recruitment in SCZ in response to aversive material60 revealed modest amygdala under-recruitment (−0.2 standard deviation [SD]); however, the studies included in this meta-analysis varied in the type of task paradigms used. The majority of investigations used tasks with considerable cognitive engagement, which was found to suppress amygdala responsiveness even in healthy individuals61 and, given well-established cognitive impairments in patients with SCZ,62 may exert an even stronger effect on amygdala activation in this illness. Whereas prior work has investigated affective dysfunction during various implicit and explicit emotional processing tasks, to date, no studies have concurrently investigated effects of aversive affective stimuli on attention and amygdala recruitment in SCZ during minimal cognitive engagement. Given behavioral evidence for intact in-the-moment processing of emotional material in SCZ,16 we predicted that SCZ patients would demonstrate intact behavioral effects of negative distraction on attention as well as intact amygdala signals in response to negative vs neutral stimuli in a paradigm that required limited cognitive engagement.
Above we consider specifically amygdala responsiveness, but it is critical to note that amygdala is a densely interconnected structure,63 which functions as part of a broader circuit involved in processing emotional salience.18 Thus, to fully characterize amygdala dysfunction as part of a distributed interacting system, it may not be sufficient to simply probe amygdala signals in isolation. The first study investigating whole-brain amygdala functional connectivity (fcMRI) in healthy controls64 demonstrated, during resting state, negative correlations between bilateral amygdala and major components of the fronto-parietal network,65 as well as other regions previously implicated in emotional regulation.66 Our work with healthy adults replicated this general pattern during resting state and showed notable differences in amygdala-prefrontal coupling during negative vs neutral distraction and resting state.67
Critically, while amygdala responsiveness to aversive probes may be intact in SCZ when examined separately, its integration with other distributed circuitry during emotional stimulus processing might be disturbed in this illness. Given well-established prefrontal cortical (PFC) abnormalities in SCZ62 and the importance of PFC circuits for affective regulation and reappraisal,68 one possibility is that SCZ patients may show disruptions in amygdala-PFC coupling during negative affective interference even when no overt behavioral abnormalities are evident. Thus, while behavioral and amygdala responses to aversive distraction may be intact in SCZ during minimal cognitive demands, we predicted that amygdala-PFC coupling may still be impaired. Such connectivity impairments could underlie disturbances in higher level emotional appraisal and regulation,66,69 which are compromised in this illness.
There have been several investigations of amygdala fcMRI in SCZ,28,30,58,70–72 all of which have documented abnormalities in SCZ patients. However, these studies either focused on specific regions of interest (ROIs)70,71, used only emotional faces as stimuli28,30,58,71 (which may not evoke reliable emotional self-report in SCZ), examined resting state fcMRI only70, or investigated psychosis-prone but nonclinical populations.72 Here, we probed the dynamics of whole-brain amygdala connectivity in SCZ when processing negative vs neutral stimuli. Furthermore, we used stimuli which have been extensively validated73 and were shown to evoke reliable self-report on emotional experience in SCZ patients.15 Critically, we examined amygdala task-based fcMRI (tb-fcMRI) in SCZ using a paradigm where both behavioral and amygdala responsiveness are hypothesized to be ‘intact’ (as we predicted in the current study), thus also ruling out differential task effects on amygdala functional coupling. Furthermore, to test the clinical relevance of such amygdala-PFC ‘dysconnectivity,’ we also examined to what extent it might predict symptom severity.
To summarize: (1) we predicted no group differences in the effects of negative distraction on behavior during a simple perceptual task with minimal cognitive demand and ratings of emotionally aversive stimuli; (2) we predicted that SCZ patients may exhibit intact amygdala activation in response to negative vs neutral distraction; and (3) we predicted significant group differences in whole-brain amygdala-PFC tb-fcMRI in response to negative vs neutral affective stimuli and its correlation with symptom severity.
Materials and Methods
Subject Recruitment
Twenty-eight subjects meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnostic criteria for SCZ and 24 demographically matched healthy control (CON) subjects were recruited through the Conte Center for Neuroscience of Mental Disorders (CCNMD) at Washington University in St Louis. All subjects were interviewed by a Master's level clinician and underwent the Structured Clinical Interview for DSM-IV-TR,74 as well as symptom ratings using the Scale for Assessment of Positive and Negative Symptoms (SAPS/SANS).75,76 Controls were recruited using local advertisements in the same community as patients but were excluded if they had any lifetime history of Axis I psychiatric disorder or a first-degree relative with a psychotic disorder. Both CON and SCZ subjects were excluded if they: (1) met criteria for DSM-IV substance abuse/dependence within the past 6 months or met criteria for present diagnosis of anxiety or depression; (2) had any severe medical conditions; (3) suffered head injury (past or present) with neurological symptoms or loss of consciousness; or (4) met DSM-IV diagnostic criteria of mental retardation. All SCZ subjects were medicated at the time of the scan and had to be receiving a stable level of medication for a period of at least 2 weeks (but the majority of subjects were on the same dose of medication for 6 weeks or more). Subjects provided informed consent approved by Washington University and were administered the Matrix Reasoning and Vocabulary sections of the Wechsler Adult Intelligence Scale—Third Edition.77 The groups did not differ in terms of handedness, gender, age, parental education, and parental socioeconomic status (table 1). However, patients were impaired relative to controls on standard measures of verbal and nonverbal IQ.77
Table 1.
Clinical Demographics and Characteristics
| Characteristic | Controls | Patients | Significance | |||
| M | SD | M | SD | T Value/Chi-Square | P Value (two-tailed) | |
| Age (in years) | 36.79 | 7.72 | 36.73 | 8.85 | 0.03 | 0.979 |
| Gender (% male) | 0.71 | 0.75 | 0.33 | 0.742 | ||
| Paternal education (in years) | 12.67 | 1.43 | 13.36 | 2.98 | 1.03 | 0.306 |
| Maternal education | 12.46 | 1.50 | 13.52 | 2.94 | 1.59 | 0.118 |
| Paternal SES | 22.17 | 9.15 | 25.77 | 12.14 | 1.16 | 0.253 |
| Maternal SES | 16.96 | 8.49 | 25.19 | 11.62 | 2.79 | 0.007 |
| Participant's education (in years) | 15.33 | 2.10 | 12.96 | 1.97 | 4.19 | 0.000 |
| Handedness (% right) | 100.00 | 82.14 | 1.69 | 0.097 | ||
| IQ verbal | 109.78 | 10.82 | 94.04 | 15.43 | 4.06 | 0.000 |
| IQ performance | 115.43 | 11.37 | 100.77 | 15.98 | 3.64 | 0.001 |
| Medication (CPZ equivalents) | 643.09 | 622.23 | ||||
| Mean SAPS global item score | 0.02 | 0.11 | 2.00 | 1.15 | ||
| Mean SANS global item score | 0.35 | 0.61 | 2.56 | 0.80 | ||
| Disorganization | 0.75 | 1.15 | 5.57 | 2.70 | ||
| Poverty | 1.08 | 2.34 | 10.75 | 3.62 | ||
| Reality distortion | 0.00 | 0.00 | 4.50 | 3.52 | ||
Note: Positive symptoms were the sum of global scores for hallucinations and delusions; negative symptoms were the sum of global scores for alogia, anhedonia, avolition, affective flattening, and attentional impairment; and disorganization symptoms were the sum of global scores for bizarre behavior, positive thought disorder, and inappropriate affect. SAPS, scale for assessment of positive symptoms; SANS, scale for the assessment of negative symptoms; CPZ, chlorpromazine; SES, socioeconomic status.
fMRI Acquisition
All structural and blood oxygenation level–dependent (BOLD) data were acquired using a 3 T Tim TRIO scanner at the Washington University School of Medicine. Functional images were acquired using an asymmetric spin-echo, echo-planar sequence maximally sensitive to BOLD contrast () (repetition time [TR] = 2200 ms, echo time [TE] = 27 ms, field of view = 256 mm, flip = 90°, voxel size = 4 × 4 × 4 mm). Runs lasted 5.68 min and contained 155 sets of oblique axial images (32 slices per volume) acquired parallel to the anterior-posterior commissure. Structural images were acquired using a sagittal magnetization prepared rapidly acquired gradient echo (MP-RAGE) 3D T 1-weighted sequence (TR = 2400 ms, TE = 3.16 ms, flip = 8°; voxel size = 1 × 1 × 1 mm).
Task Design and Stimuli
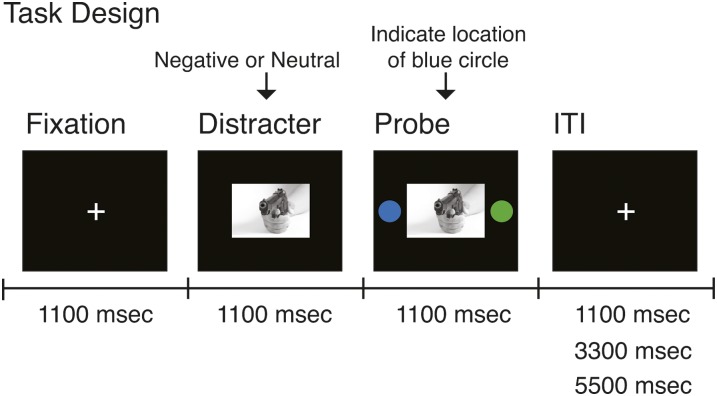
During BOLD acquisition subjects completed a simple perceptual decision task containing: a fixation cross displayed for 1.1 s, followed by an emotionally aversive or neutral image presented centrally on a black screen for 2.2 s that was flanked by 2 isoluminant circles 1.1 s after the picture appeared on screen (figure 1). Using a button response, subjects were asked to indicate the location of the blue circle by pressing their index or middle finger for right vs left side, respectively, while reaction time (RT) data were recorded. The logic of the task was that the negative stimuli should engage attention and slow responses to subsequent probes when compared with neutral stimuli. All the stimuli were presented through an LCD projector to a screen located behind the scanner, which the subjects were able to see through an angled mirror located above the eyes. The intertrial interval was jittered randomly at 1.1, 3.3, and 5.5 s.
Fig. 1.
The overall layout of the task is shown along with different components and their onsets marked along the timeline. During each trial, subjects were presented with a fixation cross for 1100 ms, followed by either a negative or neutral picture presented for a total of 2200 ms. 1100 ms following picture onset, 2 isoluminant circles were presented bilaterally and subjects’ reported, using a button response, on which side they see the blue circle. Each trial was followed by a randomly jittered intertrial interval (ITI). The aim of the task was to minimize cognitive load (via a simple perceptual decision) but still assay the effect of negative distraction on behavior via reaction time of making the perceptual decision.
Distracters were generated from the International Affective Picture System (IAPS)73 and were equated on luminance, contrast, figure-ground relationships, spatial frequency, and color.78–80 The task consisted of 48 trials (24 per condition) pseudorandomized with the criterion that no distracter category could appear for more than 3 consecutive trials to avoid mood induction via negative pictures. Following scanning, subjects indicated experienced arousal and valence for each picture via a computer version of the self-assessment manikin.81 Subjects were allowed as much time as they needed to make the ratings.
Preprocessing
fMRI data preprocessing included: (1) slice-time correction; (2) removing first 5 images from each run to allow BOLD signal to reach steady state; (3) eliminating odd/even slice intensity differences due to interpolated acquisition; (4) rigid body motion correction82; (5) intensity normalization to a whole brain mode value of 1000 without bias or gain field correction; (6) registration of structural images to a template image in the Talairach coordinate system (12-parameter affine transform)83; (7) co-registration of fMRI volumes to the structural image with resampling to 3mm cubic.82,84 To ensure comparable signal-to-noise ratio (SNR) profiles across groups,85,86 SNR was calculated following preprocessing but prior to atlas transformation (ie, in each subject's native space) as done in our prior work.87 Briefly, SNR estimate was computed by obtaining the mean signal and SD for a given slice across the BOLD run, while excluding all nonbrain voxels across all frames. The overall SNR estimate was expressed as mean/SD for a given slice and averaged across all slices. SNR results revealed no significant between-group differences (mean SCZ = 317.04; mean CON = 355.9; [t 49 = 1.4, P = .17, NS]).
fMRI Analyses
A general linear model (GLM) approach was used to estimate subject-specific voxel-wise task-related activity without assuming a hemodynamic response function (HRF).88 Negative and neutral conditions were modeled separately for 7 frames following picture onset. The estimates reflect 7 different time points in 2.2-second increments starting with the picture presentation. The resulting beta estimates of event-related response at each frame were entered into a second-level analysis treating subjects as a random factor. Unassumed modeling allowed maximal sensitivity to any response amplitude and shape differences across groups. We also verified our findings by fitting an assumed HRF89 following picture onset and report these findings to ensure cross-validation using complementary analytic approaches.
At the second level, for the unassumed GLM, we computed a 4-way repeated-measures ANOVA with Distracter Condition (negative vs neutral), Time point (7 estimated frames), and Hemisphere (left vs right) as within-subject factors and Diagnosis (SCZ vs CON) as a between-subject factor. For the assumed GLM, we employed the same approach, but the Time point factor was omitted because we obtained a single estimate of fit across entire trial duration (ie, overall response magnitude).
To isolate task-evoked amygdala signals, we employed a previously validated procedure.67 Briefly, for all subjects, we delineated individual-specific anatomically based amygdala ROI via FreeSurfer.90,91 We combined all the individual amygdala ROIs and down-sampled the resolution to match the functional voxel size (ie, 3 mm3) and identified the region of 50% amygdala voxel overlap across all subjects. We computed all the ANOVAs specifically within this anatomically defined amygdala ROI. Individual-specific anatomically defined amygdala ROIs were also used as seeds in all tb-fcMRI analyses.
tb-fcMRI Analyses
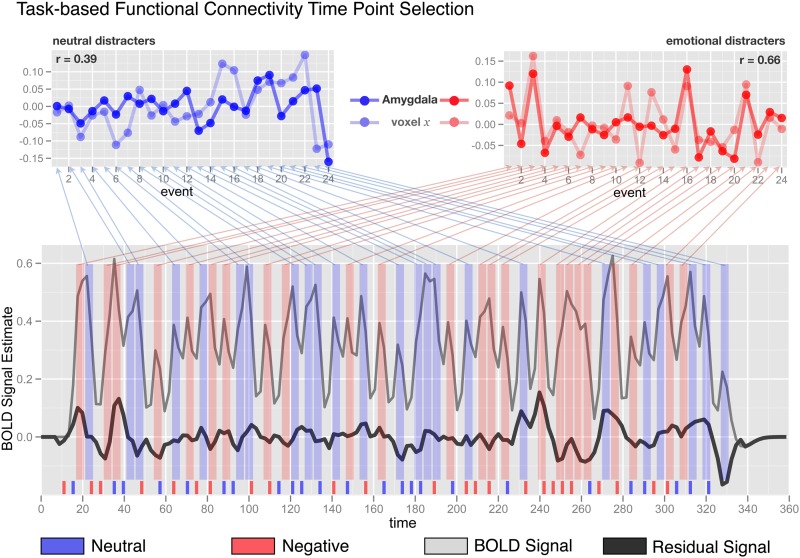
To remove possible sources of spurious correlations,67,92,93 we conducted additional preprocessing before tb-fcMRI analyses: (1) spatial smoothing by 6-mm full width at half maximum Gaussian filter; (2) high-pass filtering (>0.009 Hz) to remove low frequencies and scanner drift; (3) removal of motion correction parameters, ventricle, deep white matter, and global mean signals (GMSs) and their first derivatives using GLM framework; (4) removal of mean task response by modeling task response over 9 frames following trial onset with separate regressors for negative and neutral trials. The removal was based on 9 frames for all trials of a given type across the experiment (ie, not trial by trial). This step was performed concurrently within the same GLM as step 3). This approach, while modeling an overall task response, ignores trial-to-trial variability in BOLD responses (ie, it places it into the residual). In turn, this residual trial-to-trial variability can then be harnessed in subsequent tb-fcMRI steps by analyzing correlation between voxels for BOLD signal time-locked to events of interest (further described in the online supplementary material and shown in figure 2). We conducted all subsequent tb-fcMRI analyses on the residual values, excluding variance accounted for by nuisance signal and mean task response but still capturing trial-to-trial variability in task response. While some studies have suggested that removing GMS may induce negative correlations,94 others have shown that this is a crucial measure to optimize fcMRI specificity.95 Critically, group differences in fcMRI cannot be differentially induced by this step as both groups underwent the same preprocessing.
Fig. 2.
Task-based functional connectivity (tb-fcMRI) time point selection approach used in the present study is shown on a simulated time series example. The bottom panel shows the overall original BOLD time series in light gray. The time series following overall task structure removal is shown in black and is substantially attenuated in overall variability. Negative and neutral events are marked in red and blue, respectively, across the figure. Event onset is shown at the bottom along the x-axis. The average of time points 4 and 5 following each event onset is marked with vertical red or blue bars spanning the y-axis of the bottom figure. The top panels show examples of 2 extracted and concatenated voxel trial-to-trial variability time series for neutral (left) and negative (right) conditions. All tb-fcMRI analyses are performed on these extracted time courses, which reflect variation in peak response following each distracter—as indicated by obtained correlation coefficients shown in corners of each top panel. As noted, this approach, along with removal of task structure, largely circumvents the concern that correlations are being driven by overall task response.
To examine amygdala tb-fcMRI, we followed an approach used in our previously published studies67,92 (see online supplementary material for comprehensive details and discussion of the employed analytic approach). Briefly, we computed the average BOLD signal value following picture onset (average of time points 4 and 5) at each trial for each voxel in the image. These values were then concatenated into two 4D (brain volume × trial) time series separately representing trial-to-trial variability in response to negative and neutral stimuli (figure 2). Extracting only specific time-locked components of the time series, along with removal of mean task response, ensured that the correlations are driven primarily by trial-to-trial variability rather than overall task response and enabled us to obtain separate tb-fcMRI estimates for each task condition (neutral and negative). To further eliminate possible biases due to stimuli sequence, the ordering of task trials was carefully balanced (see online supplementary material for complete details).
Amygdala tb-fcMRI maps were computed by extracting average values across all the voxels in the individual anatomically defined amygdala ROI and computing their correlation with each voxel in the brain. Group-level statistical significance was estimated by converting individual correlation maps to Fisher's z maps and computing a voxel-wise one-sample or independent samples t-tests. All tb-fcMRI analyses were based on the average of both correct and incorrect trials to maximize power given no a priori predictions with regard to connectivity differences as a function of performance or speed. All foci meeting appropriate whole-brain type I error correction are reported in table 2.
Table 2.
Foci Showing Significant Group Differences in Whole-Brain Amygdala Task-Based Functional Connectivity
| X | Y | Z | Hemisphere | Anatomical Landmark | Region Size (mm cubic) | Peak Z-score |
| Bilateral amygdala SCZ vs CON—negative condition | ||||||
| SCZ > CON | ||||||
| 56 | −25 | −14 | Right | Middle temporal gyrus | 3024 | 3.90 |
| −62 | −45 | −10 | Left | Middle temporal gyrus | 1458 | 3.91 |
| −24 | 46 | 26 | Left | Superior frontal gyrus | 351 | 4.54 |
| 23 | 47 | 29 | Right | Superior frontal gyrus | 378 | 4.15 |
| 17 | 28 | 37 | Right | Middle frontal gyrus | 621 | 4.47 |
| −28 | 29 | 39 | Left | Middle frontal gyrus | 378 | 3.70 |
| −19 | 23 | 51 | Left | Superior frontal gyrus | 621 | 4.20 |
| 7 | 16 | 51 | Left | Superior frontal gyrus | 1620 | 4.26 |
| −6 | 39 | 52 | Right | Superior frontal gyrus | 3699 | 3.81 |
| CON > SCZ | ||||||
| 25 | −46 | −27 | Right | Cerebellum | 405 | −3.54 |
| −17 | −84 | 10 | Left | Cuneus | 513 | −3.91 |
| −24 | −84 | 22 | Left | Middle occipital gyrus | 378 | −4.96 |
| Bilateral amygdala SCZ vs CON—negative minus neutral contrast (interaction test) | ||||||
| −27 | −36 | −26 | Left | Cerebellum | 810 | 4.07 |
| 2 | −23 | 18 | Right | Medial dorsal thalamus | 864 | 4.34 |
| 13 | 39 | 23 | Right | Medial frontal gyrus | 432 | 4.26 |
| −22 | −74 | 23 | Left | Precuneus | 432 | 3.24 |
| −27 | 48 | 25 | Left | Superior frontal gyrus | 702 | 4.02 |
| -5 | 33 | 31 | Right | Medial frontal gyrus | 594 | 3.85 |
| 15 | 32 | 35 | Right | Medial frontal gyrus | 891 | 3.93 |
| −11 | 42 | 43 | Left | Superior frontal gyrus | 3537 | 4.23 |
| −20 | 25 | 50 | Left | Superior frontal gyrus | 378 | 3.46 |
| 5 | 23 | 57 | Right | Superior frontal gyrus | 702 | 4.03 |
Note: Top panel shows group differences using random-effects independent samples t-tests for negative condition specifically. Bottom panel shows the results of the interaction test (ie, Controls vs. Patients for Negative Minus Neutral Contrast). All reported foci met appropriate whole-brain type I error correction (z > 3.00, k = 13, Corresponding to P < .05, Monte Carlo Corrected Given Employed Smoothing Level). SCZ, patients with schizophrenia; CON, controls.
To examine whether amygdala tb-fcMRI correlated with negative symptom severity in the patient sample, we also extracted the magnitude of amygdala correlations with a specific a priori ROI of the prefrontal cortex for each subject (fronto-polar cortex corresponding closely to Brodmann's Area 10; x = 37; y = 52; z = 15). An independent region was chosen: (1) to ensure statistical independence96,97 and (2) given that this ROI showed amygdala tb-fcMRI differences between negative and neutral distraction in our prior work.67 Given no a priori predictions regarding specific negative symptom dimensions, we examined all subscales of the SANS76 for both neutral and negative conditions. We employed false discovery rate correction to ensure appropriate type I error correction.98
Effect Size Calculations
For all statistical tests, we calculated Hedge's g or classical η2 effect size statistics as appropriate (See online supplementary material for complete details). Hedge's g (Hg) was calculated for all statistics where there was a difference between means.99 In contrast, classical η2 (not partial) was calculated for all ANOVA statistics.100
Results
Behavior
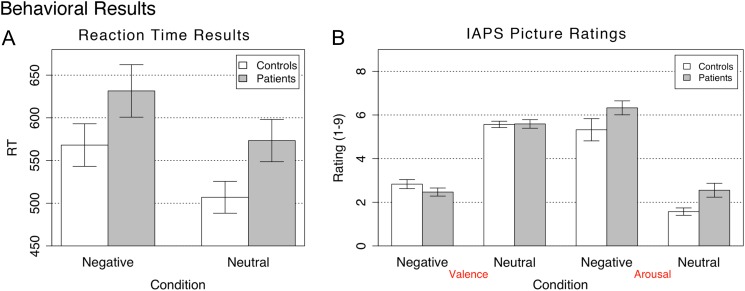
We examined group RT differences for negative vs neutral distraction by computing a mixed model 2-way ANOVA with one between-subject factor (Diagnosis, SCZ vs CON) and one within-subject factor (Distraction Condition, negative vs neutral). There was a trend main effect of Diagnosis (F 1,50 = 3.31, P = .074, η2 = 0.54) suggesting patients were overall slower, a significant main effect of Distraction Type (F 1,50 = 66.31, P < .0001, η2 = 0.46) but no significant Diagnosis × Distraction Type interaction (F 1,50 = 0.04, P = .84, NS, η2 ≈ 0.00). Although SCZ subjects were generally slower, the effect of negative distraction did not differ across groups (figure 3A). Within-group t-tests confirmed that slowing as a function of emotion was significant for both CON (t 23 = 5.68, P < .001) and SCZ (t 27 = 5.83, P < .001) subjects, with similar effect sizes calculated using Hedge’s g (Hg-CON = 0.57; Hg-SCZ = 0.4).99,101 There were no group differences as a function of negative emotion when the RTs were expressed to account for subject-specific slowing—ie, as a percent change from neutral distraction (negative-neutral/neutral) (t 50 = 0.84, P = .4, Hg = 0.23). All subjects performed the task with perfect accuracy, irrespective of the probe laterality, corroborating overall low cognitive load.
Fig. 3.
Behavioral results are shown for (A) probe reaction time during the fMRI task following negative and neutral picture presentation and (B) stimulus ratings across valence and arousal dimensions following scanning sessions. Results for patients and controls are shown in gray and white bars, respectively. Error bars represent ±1 standard error of the mean.
We also examined group differences for arousal and valence ratings (figure 3B), which were obtained in a postscan task. We computed a 2-way mixed model ANOVA with one between-subject factor (Diagnosis, SCZ vs CON) and one within-subject factor (Emotion, negative vs neutral). Consistent with prior findings,15 the valence ANOVA revealed no main effect of Diagnosis (F 1,50 = 0.75, NS, η2 ≈ 0.00), and no Diagnosis × Emotion interaction (F 1,50 = 1.16, NS, η2 ≈ 0.00), but a highly significant main effect of Emotion (F 1,50 = 275.45, P < .0001, η2 = 0.99), indicating that both groups rated experiencing negative pictures as significantly more aversive when compared with neutral pictures. The arousal ANOVA revealed a main effect of Diagnosis (F 1,50 = 6.29, P < .02, η2 = 0.06), indicating that SCZ subjects rated all pictures as generally somewhat more arousing when compared with CON subjects, but no Diagnosis × Emotion interaction (F 1,50 = 0.001, NS, η2 ≈ 0.00). Again, there was a highly significant main effect of Emotion (F 1,50 = 166.23, P < .0001, η2 = 0.94), indicating that both groups rated experiencing negative pictures as significantly more arousing when compared with neutral pictures.
Task-Evoked Amygdala fMRI Results
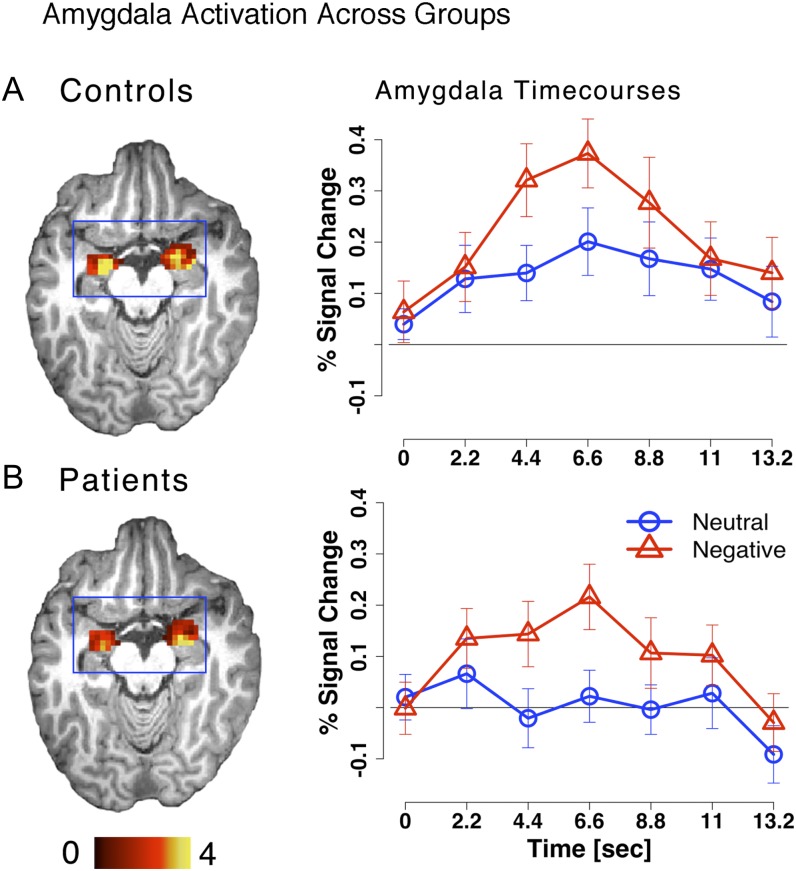
We examined group differences in amygdala activation profiles in response to negative vs neutral stimuli. To test this, we computed a 4-way repeated-measures ANOVA with Distracter Emotion (neutral vs negative), Time point (7 frames), and Hemisphere (left vs right) as within-subject factors and Diagnosis (SCZ vs CON) as a between-subject factor by using the a priori anatomical amygdala ROI (see ‘Materials and Methods’ section).
The ANOVA results revealed no significant main effect of Diagnosis (F 1,49 = 0.14, P = .70, NS, η2 ≈ 0.00), no significant Diagnosis × Distracter Emotion × Time point interaction (F 6,294 = 0.04, P = .99, NS, η2 ≈ 0.00), but a highly significant Distracter Emotion × Time point interaction (F 6,294 = 1466.42, P < .0001, η2 = 0.46), indicating that there was an overall difference in negative vs neutral amygdala response irrespective of group (figure 4). No term involving the Diagnosis factor reached significance, suggesting highly similar group patterns of amygdala response. In fact, both groups showed a highly significant Distracter Emotion × Time point interaction in the a priori amygdala region when examined separately. Furthermore, both groups showed a Distracter Emotion × Time point interaction in the amygdala even in whole-brain exploratory analyses at a whole-brain significance level with the appropriate type I error correction (ie, Z > 3 and k = 13 contiguously active voxels) (figure 4).
Fig. 4.
Amygdala activation is shown for (A) controls and (B) patients with the associated time courses extracted from bilateral amygdala ROIs. Time courses for the negative and neutral conditions are marked in red triangles and blue circles, respectively.
Results did not differ for the HRF model approach—there was no Diagnosis × Distracter Emotion interaction (F 1,49 = 0.15, P = .69, NS, η2 = 0.002) but a highly significant main effect of Distracter Emotion (F 1,49 = 33.17, P < .001, η2 = 0.50). Of note, when using the assumed HRF model, the results revealed a significant main effect of Diagnosis (F 1,49 = 4.54, P < .04, η2 = 0.32), suggesting somewhat lower levels of amygdala signal in SCZ subjects—but this was true irrespective of distracter type. Taken together, these findings suggest that the pattern of amygdala responses as a function of emotion did not differ across groups and argue against sizable group differences in amygdala activation.
We also investigated group differences in distracter responses at the whole-brain level by computing a voxel-wise Diagnosis × Distracter Emotion × Time point interaction using the unassumed model. No significant regions emerged after whole-brain type I error correction. For qualitative inspection, whole-brain Distracter Emotion × Time point maps for both groups can be found in supplementary materials (see online supplementary material figure S1).
Amygdala tb-fcMRI Group Differences
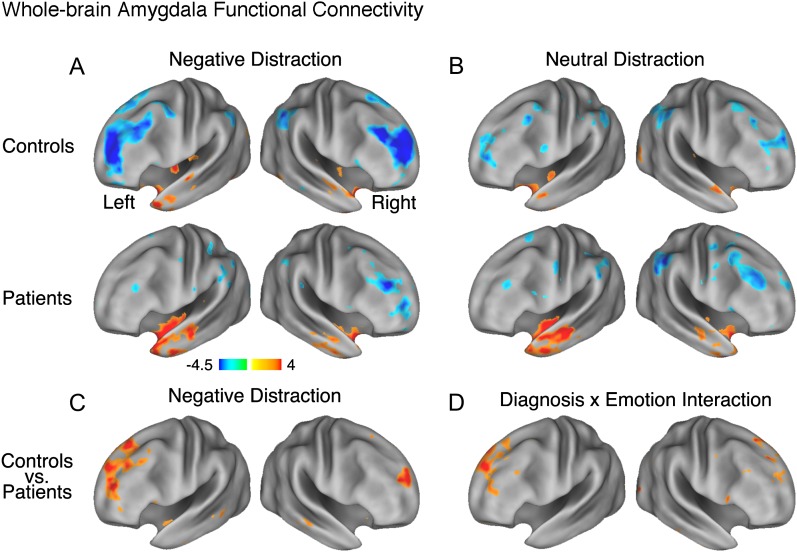
First, we computed voxel-wise amygdala tb-fcMRI in each group to verify that the general pattern of results from prior investigations is well replicated.64,67,102 The overall pattern of tb-fcMRI revealed negative coupling between fronto-parietal cortical regions and amygdala, which was present in the negative, but attenuated in the neutral condition, closely matching our previous work in healthy adults67 (figures 5A and 5B). Next, we computed an independent samples t-test examining group differences in amygdala tb-fcMRI during negative distraction (figure 5C). As shown by the red foci in figure 5C, patients, as compared with controls, exhibited significant reductions in negative amygdala coupling during the aversive emotion distraction condition. This pattern was most prominent for fronto-polar cortex and superior frontal sulcus.
Fig. 5.
Whole-brain amygdala task-based functional connectivity (tb-fcMRI) maps are shown using Z statistics and visualized using the population-average, landmark- and surface-based (PALS) atlas.110 (A) Following negative distraction for both controls (top panel) and patients (bottom panel); (B) Following neutral distraction for both controls (top panel) and patients (bottom panel); (C) Results of an independent samples t-test comparing patients vs controls following negative distraction; (D) Results of a Diagnosis × Distracter Condition interaction (ie, foci where controls show increases in negative coupling between amygdala-PFC as a function of emotion and patients do not). In panels (C) and (D), we show foci using a Z > 2.5 threshold demonstrating that, even with a lower statistical significance threshold, group differences in amygdala tb-fcMRI are centered mainly on the prefrontal cortex and not elsewhere. Brighter colors mark regions showing either more positive or more negative tb-fcMRI with amygdala. The online version of this article shows positive and negative tb-fcMRI with the amygdala in orange–yellow and blue colors, respectively. The complete list of significant foci at appropriate whole-brain type I error correction (z > 3.00, k = 13, corresponding to P < .05) is shown in table 2.
We also examined the Diagnosis × Distracter Emotion interaction for amygdala tb-fcMRI. That is, we examined whether there were regions where controls showed a significant increase in negative coupling for negative vs neutral condition when compared with patients. We computed this by first differencing negative vs neutral maps in each subject and then computing an independent sample t-test between patients and controls (figure 5D). Again, a set of PFC regions showed a significant Diagnosis × Emotion interaction for amygdala-PFC coupling, which was strikingly similar to results found in healthy adults in our prior work.67 When examining individual group maps (figures 5A and 5B), it was evident that the source of the interaction was significantly stronger negative amygdala-PFC correlations following emotionally aversive vs neutral pictures specifically for controls, but not for patients. All foci surviving appropriate whole-brain correction are shown in table 2.
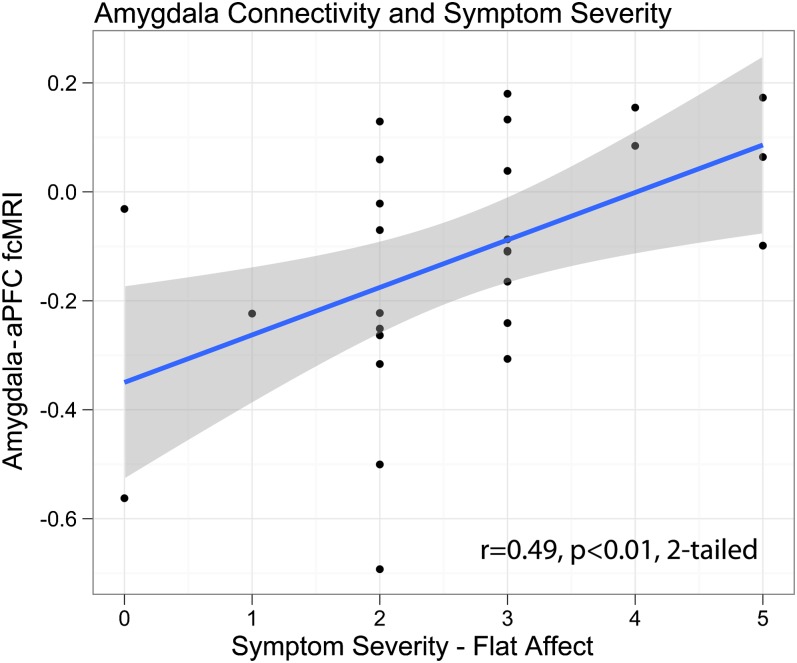
Given between-group differences in amygdala-PFC tb-fcMRI, we examined whether the magnitude of amygdala-PFC coupling correlates with symptom severity. As noted, to avoid region selection bias,97 we selected an independent right lateral PFC ROI identified in our prior work, closely matching the location of maximal tb-fcMRI differences in the present study.67 Only flat affect correlated significantly with anterior PFC-amygdala tb-fcMRI in the neutral distraction condition (figure 6), with less negative coupling between PFC and amygdala associated with higher ratings of flat affect. However, the significance of this correlation would not survive corrections for multiple comparisons.
Fig. 6.
Correlation between negative symptoms (flat affect) measured using SANS and tb-fcMRI between amygdala and fronto-polar cortex (aPFC) during neutral distraction.
Discussion
We advanced our understanding of emotional processing in SCZ by showing that both groups: (1) demonstrated equal amounts of behavioral interference when presented with aversive emotional content while performing a basic perceptual decision task; (2) reported similar levels of emotional experience, closely replicating prior work15; and (3) showed similar amygdala recruitment in response to negative vs neutral stimuli. Despite these similarities, SCZ subjects exhibited significantly weaker amygdala-PFC coupling, especially during negative distraction, which predicted negative symptom severity.
Intact Behavioral Effects of Emotional Salience in SCZ
While there are several domains of affective disturbances in SCZ,17 prior work has suggested that SCZ patients may exhibit largely intact in-the-moment processing of emotional material, either when rating emotional stimuli or their own feelings in response to those stimuli.16 However, few studies have employed tasks that can assay behavioral effects of emotion under conditions of minimal cognitive load. We demonstrated that, while slower overall, patients showed similar effects of aversive emotional interference during a simple perceptual decision task and showed little difference in postscan ratings of affective pictures. Taken together, these findings closely replicate and extend prior work examining basic emotional processing in SCZ.15,16 Moreover, present findings suggest that ‘real-time’ perception of aversive material, as well as processing low-level visual properties of affective stimuli—that momentarily command attention—may be largely intact in SCZ.
Amygdala Responsiveness in SCZ
The present findings suggested no group differences in amygdala responsiveness during minimal cognitive engagement, in line with prior SCZ neuroimaging studies using IAPS stimuli.55 While it is impossible to definitively demonstrate an absence of an effect (ie, to prove a null finding), it is still critical to note that the present results go counter to suggestion of sizeable group differences across conditions.67 Furthermore, we carefully matched groups for SNR profiles, ensuring adequate power to detect, at least, medium effect sizes. Therefore, the lack of group differences in amygdala signals and the presence of significant differences in functional connectivity (discussed below) are unlikely to be due to SNR or power concerns. However, there was a mild overall attenuation of amygdala signals in patients relative to controls irrespective of valence (ie, when averaging across both aversive and neutral conditions). One speculative hypothesis is that this overall attenuation could possibly reflect a deficit in vigilance or orienting to salient stimuli in general,103,104 a hypothesis that remains to be tested in future work.
While the present results provide little evidence for amygdala under-recruitment in response to aversive vs neutral complex visual material (ie, no Diagnosis × Emotion interaction), there are prior studies using different emotional material (eg, facial expressions) that have reported amygdala under-recruitment in this illness in response to aversive vs neutral stimuli.17 Thus, it will be critical to further characterize amygdala responsiveness in SCZ by directly comparing different emotional stimuli in a controlled fashion. Of note, other work from our own laboratory suggests little group difference in amygdala responsiveness when subjects rated facial, verbal, and complex image stimuli for emotional content.4 However, different studies have employed different task designs where some studies examined passive viewing vs explicit rating of emotional content or judgment of facial expressions. Such different task demands may differentially impact amygdala responses to various types of stimuli depending on the level of cognitive load and ability of emotional information to reach awareness.61 Additionally, we examined the entire amygdaloid complex; however, it may be possible that specific amygdala subnuclei exhibit abnormalities in SCZ.105 Future studies with optimized acquisition protocols, able to collect more precise spatial and temporal information on amygdala activation,105,106 may detect abnormalities in amgydala subdivisions that we were not able to assay.
Amygdala tb-fcMRI
While prior work investigated amygdala fcMRI in SCZ, previous work has not examined whole-brain patterns of amygdala coupling in response to well-validated emotional stimuli during a task in which both behavior and brain activation were well matched between groups (which helps to rule out confounds associated with group differences in activation). Our prior work with healthy adults demonstrated stronger negative amygdala-PFC coupling during emotionally aversive vs neutral distraction in healthy adults. This pattern was particularly prominent for components of the fronto-parietal control network107 as well as other regions previously implicated in emotional regulation,108 which was closely replicated in the present study (see figures 4A and 4B). In contrast, SCZ subjects displayed significantly less negative amygdala-PFC tb-fcMRI, specifically during aversive emotional interference, suggesting that—even in the absence of sizable behavioral and task-evoked amygdala differences—the pattern of amygdala-PFC coupling may be abnormal in SCZ subjects. That is, while the amygdala itself may show intact response to emotional inputs, its interaction with other cortical regions may still be compromised in SCZ, particularly in the ability to moderate the strength of that coupling in response to specific task demands.
One speculative possibility is that the strength of amygdala-PFC coupling reflects the degree to which emotional information engages regulatory processes potentially supported by PFC. In other words, when CON subjects are detecting emotional stimuli and engaging with the content of this information, a concomitant recruitment of PFC signals may occur that serves to downregulate amygdala responsiveness,30 particularly when such responsiveness is task irrelevant. If so, then weaker inverse amygdala-PFC coupling in patients may reflect an impaired capacity to downregulate amygdala responses.72 That is, even if both groups respond to emotional stimuli to the same extent, healthy controls may have a spared ability to regulate amygdala reactivity following visual detection and initial processing, whereas patients may lack this capacity due to dysfunction in regions enacting top-down control.62 Such dysfunction may also manifest as a breakdown in conscious appraisal of emotional information,68,69,109 which results in a failure in SCZ to adaptively guide behavior in response to incoming affective cues. This may not be apparent in the present paradigm, as minimal cognitive demand is required and emotional stimuli are not used to guide behavior. However, paradigms where detecting emotional information is critical for optimal task performance or where responses to emotional stimuli may be more disruptive of task-relevant processing may be sensitive to such deficits.
Symptoms
We found that PFC-amygdala fcMRI predicted negative symptom severity (flat affect) in the neutral condition. One speculative possibility is that optimal PFC-amygdala interaction is required for detection and internal appraisal of environmentally salient events, even if they do not carry emotional information. However, these analyses were exploratory in nature (as we did not have specific predictions regarding precise symptom clusters) and the significant correlation would not survive appropriate multiple comparison testing. Thus, present findings in regards to symptom relationships should be treated as provisional and should be followed up on in future studies. Furthermore, while the present investigation was adequately powered to detect, at least, medium effect sizes for most of the proposed analyses, it was not ideally powered for an individual-differences type analyses. Thus, prospective studies with more focal questions and adequate sample size should aim to address whether amygdala responsiveness and/or fcMRI patterns predict symptoms.
Limitations
The present study focused exclusively on negative interference. Thus, further research remains to be done to ascertain whether these effects are present during positively valenced distraction and whether SCZ may demonstrate tb-fcMRI impairment during detection of potentially rewarding stimuli.3,4 Similarly, it would be informative to test whether other emotional material (ie, verbal emotion or facial expressions) result in similar findings to ascertain the generalizability of present results to other affective stimuli. Critically, present stimuli (ie, negative vs neutral) also differed along the arousal dimension, which may have contributed to the observed differences in neural activation. While it is difficult to fully rule out, future work may want to use more carefully arousal-matched positive and negative distracters to verify the specificity of negative distraction found in the present study.
A common concern in imaging studies of clinical populations is the potential influence of medication on results. In the current study, all patients were receiving stable antipsychotic medication. However, the similarity of responsiveness both in terms of behavior and amygdala signals in the emotional capture task suggests that medication status did not impact group differences in emotional processing. Nonetheless, it cannot be ruled out that medication levels may have played a role in tb-fcMRI differences. To fully rule out the possible effects of medication, it will be important to show whether a similar pattern of aberrant tb-fcMRI is present when examining either unmedicated patients, at-risk populations or subjects in the prodromal stages of psychosis who are not yet taking medication.
Conclusions
Present findings support and extend prior work suggesting that in-the-moment experience of affect may be largely spared in SCZ.16 These findings also confirm that well-validated affectively valenced picture stimuli such as the IAPS are a promising tool for further work examining emotional deficits in other contexts in this illness (eg, emotion-cognition interactions). Critically, present findings also suggest that amygdala-PFC coupling may be compromised in SCZ subjects, even in the absence of clear behavioral or task-evoked amygdala deficits. Thus, current work generates potentially compelling cortical targets for future investigations of aberrant amygdala-cortical interactions in SCZ. Further characterizing such connectivity, abnormalities may be critical for understanding deficits in affect regulation and/or other aspects of affective dysfunction in SCZ.
Funding
The McDonnell Center for Systems Neuroscience at Washington University in St. Louis.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We thank three anonymous reviewers for their helpful comments and suggestions. The authors have declared that there are no conflict of intrest in relation to the subject of the study.
References
- 1.Bleuler E. Dementia Praecox, or the Group of Schizophrenias. New York, NY: International Universities Press; 1911. [Google Scholar]
- 2.Kraeplin E. Dementia Praecox and Paraphrenia. New York, NY: International Universities Press, Inc; 1950. [Google Scholar]
- 3.Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67:902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cedro A, Kokoszka A, Popiel A, Narkiewicz-Jodko W. Alexithymia in schizophrenia: an exploratory study. Psychol Rep. 2001;89:95–98. doi: 10.2466/pr0.2001.89.1.95. [DOI] [PubMed] [Google Scholar]
- 7.Iwase M, Yamashita K, Takahashi K, et al. Diminished facial expression despite the existence of pleasant emotional experience in schizophrenia. Methods Find Exp Clin Pharmacol. 1999;21:189–194. doi: 10.1358/mf.1999.21.3.534828. [DOI] [PubMed] [Google Scholar]
- 8.Mattes RM, Schneider F, Heimann H, Birbaumer N. Reduced emotional response of schizophrenic patients in remission during social interaction. Schizophr Res. 1995;17:249–255. doi: 10.1016/0920-9964(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 9.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 10.Habel U, Gur RC, Mandal MK, et al. Emotional processing in schizophrenia across cultures: standardized measures of discrimination and experience. Schizophr Res. 2000;42:57–66. doi: 10.1016/s0920-9964(99)00093-6. [DOI] [PubMed] [Google Scholar]
- 11.Häfner H, Maurer K, Löffler W, et al. Modeling the early course of schizophrenia. Schizophr Bull. 2003;29:325–340. doi: 10.1093/oxfordjournals.schbul.a007008. [DOI] [PubMed] [Google Scholar]
- 12.Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112:41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- 13.Kee KS, Green MF, Mintz J, Brekke JS. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophr Bull. 2003;29:487–497. doi: 10.1093/oxfordjournals.schbul.a007021. [DOI] [PubMed] [Google Scholar]
- 14.Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 15.Herbener ES, Song W, Khine TT, Sweeney JA. What aspects of emotional functioning are impaired in schizophrenia? Schizophr Res. 2008;98:239–246. doi: 10.1016/j.schres.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 18.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 19.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 20.Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectrums. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- 21.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 22.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 24.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 25.Schneider F, Weiss U, Kessler C, et al. Differential amygdala activation in schizophrenia during sadness. Schizophr Res. 1998;34:133–142. doi: 10.1016/s0920-9964(98)00085-1. [DOI] [PubMed] [Google Scholar]
- 26.Blasi G, Popolizio T, Taurisano P, et al. Changes in prefrontal and amygdala activity during olanzapine treatment in schizophrenia. Psychiatry Res. 2009;173:31–38. doi: 10.1016/j.pscychresns.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crespo-Facorro B, Paradiso S, Andreasen NC, et al. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- 28.Das P, Kemp AH, Flynn G, et al. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90:284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Dichter G, Bellion C, Casp M, Belger A. Impaired modulation of attention and emotion in schizophrenia. Schizophr Bull. 2010;36:595–606. doi: 10.1093/schbul/sbn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fakra E, Salgado-Pineda P, Delaveau P, Hariri A, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res. 2008;100:191–205. doi: 10.1016/j.schres.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Egea E, Parellada E, Lomeña F, et al. (18)FDG PET study of amygdalar activity during facial emotion recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260:69–76. doi: 10.1007/s00406-009-0020-6. [DOI] [PubMed] [Google Scholar]
- 32.Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 33.Gur RE, McGrath C, Chan RM, et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- 34.Habel U, Klein M, Shah NJ, et al. Genetic load on amygdala hypofunction during sadness in nonaffected brothers of schizophrenia patients. Am J Psychiatry. 2004;161:1806–1813. doi: 10.1176/ajp.161.10.1806. [DOI] [PubMed] [Google Scholar]
- 35.Hall J, Whalley HC, McKirdy JW, et al. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Hempel A, Hempel E, Schönknecht P, Stippich C, Schröder J. Impairment in basal limbic function in schizophrenia during affect recognition. Psychiatry Res. 2003;122:115–124. doi: 10.1016/s0925-4927(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 37.Holt DJ, Kunkel L, Weiss AP, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Holt DJ, Weiss AP, Rauch SL, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 2005;57:1011–1019. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 39.Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur J Neurosci. 2005;22:1221–1232. doi: 10.1111/j.1460-9568.2005.04294.x. [DOI] [PubMed] [Google Scholar]
- 40.Kang JI, Kim JJ, Seok JH, et al. Abnormal brain response during the auditory emotional processing in schizophrenic patients with chronic auditory hallucinations. Schizophr Res. 2009;107:83–91. doi: 10.1016/j.schres.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Kosaka H, Omori M, Murata T, et al. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr Res. 2002;57:87–95. doi: 10.1016/s0920-9964(01)00324-3. [DOI] [PubMed] [Google Scholar]
- 42.Michalopoulou PG, Surguladze S, Morley LA, et al. Facial fear processing and psychotic symptoms in schizophrenia: functional magnetic resonance imaging study. Br J Psychiatry. 2008;192:191–196. doi: 10.1192/bjp.bp.106.032649. [DOI] [PubMed] [Google Scholar]
- 43.Paradiso S, Andreasen NC, Crespo-Facorro B, et al. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry. 2003;160:1775–1783. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- 44.Pauly K, Seiferth N, Kellermann T, et al. Cerebral dysfunctions of emotion-cognition interactions in adolescent-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008;47:1299–1310. doi: 10.1097/CHI.0b013e318184ff16. [DOI] [PubMed] [Google Scholar]
- 45.Phillips ML, Williams L, Senior C, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res. 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 46.Rădulescu AR, Mujica-Parodi LR. A systems approach to prefrontal-limbic dysregulation in schizophrenia. Neuropsychobiology. 2008;57:206–216. doi: 10.1159/000151731. [DOI] [PubMed] [Google Scholar]
- 47.Rasetti R, Mattay VS, Wiedholz LM, et al. Evidence that altered amygdala activity in schizophrenia is related to clinical state and not genetic risk. Am J Psychiatry. 2009;166:216–225. doi: 10.1176/appi.ajp.2008.08020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reske M, Habel U, Kellermann T, et al. Differential brain activation during facial emotion discrimination in first-episode schizophrenia. J Psychiatr Res. 2009;43:592–599. doi: 10.1016/j.jpsychires.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Reske M, Kellermann T, Habel U, et al. Stability of emotional dysfunctions? A long-term fMRI study in first-episode schizophrenia. J Psychiatr Res. 2007;41:918–927. doi: 10.1016/j.jpsychires.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Russell TA, Reynaud E, Kucharska-Pietura K, et al. Neural responses to dynamic expressions of fear in schizophrenia. Neuropsychologia. 2007;45:107–123. doi: 10.1016/j.neuropsychologia.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 51.Schneider F, Habel U, Reske M, et al. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Res. 2007;155:103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Seiferth N, Pauly K, Kellermann T, et al. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology. 2009;34:477–487. doi: 10.1038/npp.2008.93. [DOI] [PubMed] [Google Scholar]
- 53.Surguladze S, Russell T, Kucharska-Pietura K, et al. A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biol Psychiatry. 2006;60:423–431. doi: 10.1016/j.biopsych.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi H, Koeda M, Oda K, et al. An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 55.Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophr Res. 2002;58:159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 56.Taylor SF, Phan KL, Britton JC, Liberzon I. Neural response to emotional salience in schizophrenia. Neuropsychopharmacology. 2005;30:984–995. doi: 10.1038/sj.npp.1300679. [DOI] [PubMed] [Google Scholar]
- 57.Taylor SF, Welsh RC, Chen AC, Velander AJ, Liberzon I. Medial frontal hyperactivity in reality distortion. Biol Psychiatry. 2007;61:1171–1178. doi: 10.1016/j.biopsych.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 58.Williams LM, Das P, Harris AWF, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- 59.Williams LM, Das P, Liddell BJ, et al. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 2007;155:29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Anticevic A, Van Snellenberg JX, Cohen RE, et al. Amygdala recruitment in schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging studies. Schizophr Bull. December 1, 2010 doi: 10.1093/schbul/sbq131. doi:10.1093/schbul/sbq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Curr Opin Neurobiol. 2005;15:188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 63.Young MP, Scannell JW, Burns GA, Blakemore C. Analysis of connectivity: neural systems in the cerebral cortex. Rev Neurosci. 1994;5:227–250. doi: 10.1515/revneuro.1994.5.3.227. [DOI] [PubMed] [Google Scholar]
- 64.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anticevic A, Repovs G, Barch DM. Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cogn Affect Behav Neurosci. 2010;10:159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage. 2009;45:S210–221. doi: 10.1016/j.neuroimage.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoptman MJ, D'Angelo D, Catalano D, et al. Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophr Bull. 2010;36:1020–1028. doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leitman DI, Loughead J, Wolf DH, et al. Abnormal superior temporal connectivity during fear perception in schizophrenia. Schizophr Bull. 2008;34:673–678. doi: 10.1093/schbul/sbn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Modinos G, Ormel J, Aleman A. Altered activation and functional connectivity of neural systems supporting cognitive control of emotion in psychosis proneness. Schizophr Res. 2010;118:88–97. doi: 10.1016/j.schres.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 73.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville: University of Florida; 1999. [Google Scholar]
- 74.First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 75.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1983. [Google Scholar]
- 76.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- 77.Wechsler D. Wechsler Adult Intelligence Scale—Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 78.Bradley MM, Hamby S, Löw A, Lang PJ. Brain potentials in perception: picture complexity and emotional arousal. Psychophysiology. 2007;44:364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 79.Delplanque S, N'diaye K, Scherer K, Grandjean D. Spatial frequencies or emotional effects? A systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. J Neurosci Methods. 2007;165:144–150. doi: 10.1016/j.jneumeth.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 80.Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 81.Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 82.Ojemann J, Akbudak E, Snyder A, et al. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 83.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme; 1988. [Google Scholar]
- 84.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 85.LaBar KS, Gitelman DR, Mesulam MM, Parrish TB. Impact of signal-to-noise on functional MRI of the human amygdala. Neuroreport. 2001;12:3461–3464. doi: 10.1097/00001756-200111160-00017. [DOI] [PubMed] [Google Scholar]
- 86.Parrish TB, Gitelman DR, LaBar KS, Mesulam MM. Impact of signal-to-noise on functional MRI. Magn Reson Med. 2000;44:925–932. doi: 10.1002/1522-2594(200012)44:6<925::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 87.Anticevic A, Repovs G, Van Snellenberg JX, Csernansky JG, Barch DM. Subcortical alignment precision in patients with schizophrenia. Schizophr Res. 2010 doi: 10.1016/j.schres.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- 89.Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fischl B, Salat DH, Busa E, Albert M, Dieterich M. Whole brain segmentation automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 91.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 92.Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fox M, Zhang D, Snyder A, Raichle M. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009 doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 98.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 99.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press; 1985. [Google Scholar]
- 100.Pierce CA, Block RA, Aguinis H. Cautionary note on reporting eta-squared values from multifactor ANOVA designs. Educ Psychol Meas. 2004;64:916–924. [Google Scholar]
- 101.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 102.Mitchell DG, Luo Q, Mondillo K, et al. The interference of operant task performance by emotional distracters: an antagonistic relationship between the amygdala and frontoparietal cortices. Neuroimage. 2008;40:859–868. doi: 10.1016/j.neuroimage.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nuechterlein KH. Reaction time and attention in schizophrenia: a critical evaluation of the data and theories. Schizophr Bull. 1977;3:373–428. doi: 10.1093/schbul/3.3.373. [DOI] [PubMed] [Google Scholar]
- 104.Nuechterlein KH, Barch DM, Gold JM, et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 105.Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology. 2010;35:239–257. doi: 10.1038/npp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sabatinelli D, Lang PJ, Bradley MM, Costa VD, Keil A. The timing of emotional discrimination in human amygdala and ventral visual cortex. J Neurosci. 2009;29:14864–14868. doi: 10.1523/JNEUROSCI.3278-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 110.Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.