Abstract
Background: Impairments in facial affect recognition are well documented in individuals suffering from schizophrenia. The aim of the present study was to characterize potential impairments in affect recognition and their electrophysiological correlates in at-risk individuals. Such characterization should add to the question whether the neural processes underlying facial affect recognition deficits might be part of a basic neural dysfunction reflecting a vulnerability factor of schizophrenia.
Methods: To test facial affect recognition, a digitized series of pictures of facial affect, previously used in related studies, was presented to 37 at-risk individuals and 32 healthy controls. Simultaneously, event-related potentials (ERPs) were recorded to investigate electrophysiological activity during the task.
Results: At-risk individuals showed significant impairments in facial affect recognition and reduced amplitudes in the ERP components P100, N170, and N250. Furthermore, prodromal signs in these individuals were associated with a poorer task performance and a diminished N250 amplitude.
Conclusions: The findings suggest that impairments in facial affect recognition precede the onset of the initial psychotic episode. The impairments are associated with neurophysiological abnormalities similar to those observed in manifest schizophrenia and therefore may serve as indicators of vulnerability for developing schizophrenia.
Keywords: schizophrenia, at risk for schizophrenia, facial affect recognition, event-related potentials, N170, N250
Introduction
Individuals with schizophrenia exhibit impaired emotional functioning, including difficulties in recognizing and discriminating facial affect.1 During the past 2 decades, schizophrenia researchers have become increasingly interested in cognitive disturbances, particularly impairments in facial affect recognition. One reason for this increased interest is the robust association between impairments in facial affect recognition and poor social and community functioning.2,3 Moreover, the loss of social cognitive skills, decreased social integration, and social withdrawal associated with the disorder seem to be among the most devastating symptoms.4 If the impairment in facial affect recognition was found to be present in individuals at risk, together with notable abnormalities in neurophysiological correlates, specific early interventions targeting social cognitive processes could be initiated. Another reason for the interest in impairments in facial affect recognition is the assumption that such impairments may have a role in the etiopathogenesis of schizophrenia as a vulnerability factor5 or putative endophenotype.6 Impairments in facial affect recognition have been shown to occur not only in multiple-episode patients but also in first-episode patients7 and to be stable in longitudinal studies independent of the acuity of the psychosis and despite clinically effective treatment.8,9 Beyond its association with schizophrenia and its independence of state, a putative endophenotype needs also to be found at a higher rate in unaffected relatives and at-risk individuals than in the general population.6 There is increasing but inconsistent evidence for impaired processing of emotional faces in individuals at risk for schizophrenia: A few studies found impaired facial affect recognition in unaffected biological relatives5,10 and in individuals in a prodromal state11,12 but another found no noticeable problems in individuals at risk for schizophrenia.13 Thus, more evidence is needed to answer the question whether facial emotion recognition deficits are vulnerability markers for schizophrenia.
Although there is some evidence that facial affect recognition deficits relate to abnormal functioning in the neural network underlying face processing and emotional recognition,3 the pathophysiological processes remain largely unclear. Event-related potentials (ERPs) in the electroencephalogram (EEG) have provided important spatiotemporal information on the information-processing stream.14 ERPs offer the advantage that they disclose mechanisms associated with difficulties in recognizing facial emotions by precisely tracking the time course of cognitive processing with a resolution of milliseconds. Deficits in face processing may be studied based on when an abnormality occurs in the temporal stream of information processing, ie, by distinguishing the relative contributions of early perceptual and later cognitive aspects of a task.
Emotional face stimuli elicit particular ERP components during perception and processing.15 A positive potential about 100 milliseconds after stimulus onset, the P100, is recorded at posterior occipital electrodes and reflects early sensory processing of visual stimuli. This component was shown to be smaller in schizophrenia patients than in controls in studies using several nonface stimuli16,17 or stimuli with emotional content,18 hinting at reduced emotional modulation of early occipitotemporal activity.19 Other studies using facial stimuli found the P100 component to be intact, suggesting that sensory perceptual processes and the successful categorization of stimuli are preserved.20
Looking at faces elicits a ‘face-selective’ negative waveform that peaks approximately 150–180 milliseconds poststimulus and is observed at occipitotemporal sites, reflecting early perceptual processes of structural encoding of the face.21 This component, labeled N170, is thought to arise primarily from the fusiform gyrus and can be differentiated from responses to other classes of stimuli.22 An amplitude reduction of the N170 during face and facial affect recognition in schizophrenia patients is being discussed as indicating deficits in emotion decoding as a consequence of impaired structural encoding of facial features.23
A presumably affect-related negative component is observed at fronto-central sites that peaks at approximately 250 milliseconds (N250). It has been related to the recognition of complex aspects of facial information (such as emotional content and identity) that associate the face with semantic information.20 Previous studies in schizophrenia delivered conflicting results on the N250: while some found intact N170 amplitudes and reduced N250 amplitudes (implying a specific problem in decoding of emotional information),20 others found abnormally small N170 amplitudes but normal N250 responses (suggesting impaired facial feature encoding but unaffected emotion decoding).24
Although facial emotion recognition and its neurophysiological correlates are well documented in acute schizophrenia, to our knowledge no previous study has used ERPs to study individuals at risk for schizophrenia. The present study compared such individuals with healthy controls to investigate whether at-risk individuals show impairments in facial affect recognition and its neurophysiological correlates. It was hypothesized that at-risk individuals show poorer affect recognition performance and abnormalities in ERP components as correlates of impaired encoding of facial features and affect decoding processes. On the one hand, such a pattern of results would add evidence to the assumption that facial affect recognition constitutes a vulnerability factor for schizophrenia and thus might open new windows to the neurobiology of the disorder. On the other hand, the presence of such impairments in affect recognition in at-risk individuals would indicate that respective remediation approaches should be included in the development of indicated prevention strategies.
Methods and Materials
Participants
The present study was a subproject of 2 larger early recognition and early intervention studies25,26 performed within the framework of the German Research Network on Schizophrenia.27
A total of 37 individuals at risk for schizophrenia were recruited from people referred to the Düsseldorf Early Recognition and Intervention Centre (DERIC) at the Department of Psychiatry and Psychotherapy of the University of Düsseldorf, Germany. For comparison, 32 healthy controls matched for age and gender were recruited from the community via advertisement posters placed in the hospital. Exclusion criteria were age below 18 and above 36 years and, for the healthy comparison group, a lifetime diagnosis of a neurological disorder or a disorder affecting the central nervous system.
Inclusion criteria included clinician ratings of early initial prodromal signs (early initial prodromal state, EIPS) or late initial prodromal signs (late initial prodromal state, LIPS).25,26,28,29 Individuals at risk were screened by the Early Recognition Inventory (ERI)28 and then subjected to detailed diagnosis. The EIPS relies on a set of basic symptoms (ie, subtle, subclinical, self-experienced disturbances in thought, speech, and perception processes that are rarely perceivable by an observer) to define an at-risk status,30,31 whereas the LIPS resembles the ‘ultra-high risk’ state, in which the individual experiences attenuated psychotic symptoms or brief limited intermittent psychotic symptoms.26,32 In the present study, EIPS criteria were defined by the presence of thought interferences, preservation, pressure, and blockages; disturbances of receptive language, either heard or read; decreased ability to discriminate between ideas and perception, fantasy, and true memories; unstable ideas of reference; derealization; and visual and acoustic perception disturbances. Basic symptoms had to have appeared continuously, ie, several times a week, in the past 3 months. Alternatively, an early prodromal state was assumed if there was a reduction in the global assessment of functioning score (DSM-IV) of at least 30 points within the past year in combination with at least one of the following risk factors: a first-degree relative with a lifetime diagnosis of schizophrenia, a schizophrenia spectrum disorder, or pre- or perinatal complications in the individual under investigation.
Inclusion criteria for the LIPS group comprised the presence of at least one of the attenuated positive symptoms—ideas of reference, odd beliefs or magical thinking, unusual perceptual experiences, odd thinking and speech, and suspiciousness or paranoid ideation—several times a week for a period of at least 1 week within the past 3 months.
All participants were given the Structured Clinical Interview for DSM-IV.33,34 Persons with any of the following conditions according to DSM-IV criteria were excluded from the study and referred to other specialized services: schizophrenia, schizoaffective disorder, schizophreniform disorder, psychotic mood disorder, dependency on alcohol or drugs, any other mental disorder due to somatic factors, mental retardation, acute suicidal risk, delusional or bipolar disorder, or a present or past diagnosis of a brief psychotic disorder with a duration of 1 week or more. Ratings were performed by specially trained psychiatrists and psychologists.
At the time of the study, the 37 at-risk individuals had a mean age of 23.8 years (SD = 3.3) were neuroleptic naive and were also free of any other psychopharmacological medication. The group of at-risk individuals consisted of 21 early prodromal persons (EIPS; mean age 24.4 ± 5.8 years) and 16 late prodromal persons (LIPS; mean age 25.4 ± 6.5 years). The 32 comparison subjects were 24.3 years old (SD = 3.4). There were no between-group differences in age (F 1,66 = 1.32, P = .27). Furthermore, groups did not differ regarding intelligence (EIPS: IQ = 107.8 ± 11.9; LIPS: IQ = 107.3 ± 14.8; controls: IQ = 108.3 ± 14.3; F 1,65 = 0.19, P = .90). Positive and negative symptoms were measured by the positive and negative syndrome scale (PANSS)35 and were in the very mild range in the group of at-risk individuals (PANSS negative: mean = 11.2 ± 4.0; PANSS positive: mean = 9.2 ± 2.2).
Because there was no significant difference between the EIPS and LIPS subgroups of individuals at risk for schizophrenia regarding either performance (P > .65) or electrophysiological parameters (P > .28), the 2 subgroups were combined to one group of at-risk individuals which was then compared with the healthy controls in the further analyses.
All participants were right handed and had normal or corrected vision. Participants’ characteristics are shown in table 1.
Table 1.
Participants Characteristics
| Healthy Controls (N = 32) | Individuals at Risk for Psychosis | |||
| EIPS + LIPS (N = 37) | EIPS (N = 16) | LIPS (N = 21) | ||
| Gender | 15m, 17f | 20m, 17f | 13m, 8f | 7m, 9f |
| Age | 25.6 (±4.3) | 23.8 (±4.9) | 24.1 (±5.7) | 23.5 (±3.8) |
| IQ (MWT-B) | 108.3 (±14.3) | 107.6 (±13.1) | 107.8 (±11.9) | 107.3 (±14.8) |
| PFA | 24.9 (±2.0) | 21.9 (±3.1) | 22.4 (±2.3) | 21.3 (±4.0) |
| ERI | — | 17.1 (±11.3) | 14.2 (±9.1) | 20.7 (±13.0) |
| PANSS general | — | 8.8 (1.7) | 29.6 (±4.5) | 25.7 (±4.5) |
| PANSS positive | — | 11.2 (±3.7) | 9.2 (±2.0) | 8.2 (±0.9) |
| PANSS negative | — | 28.0 (±4.9) | 21.0 (±4.2) | 10.1 (±2.4) |
| GAF | — | 61.2 (±11.5) | 59 (±12.9) | 64.2 (±9.0) |
Note: EIPS, early initial prodromal signs; ERI, early recognition inventory; f, female; GAF, global assessment of functioning; IQ, intelligence; LIPS, late initial prodromal signs; m, male; PANSS, positive and negative syndrome scale; PFA, pictures of facial affect.
Procedures
After an initial screening, participants were given a comprehensive verbal and written description of all study procedures to ensure that they were able to follow the study procedures and knew the potential risks and benefits of study participation. All participants provided written informed consent. The study was approved by the University of Düsseldorf's institutional ethics committee. EEG recordings were made within 2 days of inclusion in the main intervention studies.
Stimuli and Task
Thirty digitally reworked face photographs selected from the ‘pictures of facial affect’ by Ekman and Friesen36—which we used in our previous investigations37—served as stimuli for studying facial affect recognition. Five faces displayed the 6 basic emotions happiness, fear, anger, surprise, disgust, and sadness, with each face showing each emotion once. Pictures were adjusted for luminance and mounted in the center of the same mid-gray background subtending a visual angle of about 11° to 15° After a fixation cross was presented to indicate the area of the screen that the participants should look at, stimuli were presented in random order for 500 milliseconds. Participants were instructed to respond verbally by selecting the applicable emotion from a multiple-choice list containing the 6 facial emotions. The list appeared on the screen after a delay of 1 second (to avoid confounding motor effects) and was presented for a maximum of 8 seconds. Participants were required to enunciate a selected emotion, which was documented by the experimenter, even in case of uncertainty. A random interval of 1.6–2.2 seconds was set between the subject's response and the onset of the next trial.
EEG Acquisition
EEG data were collected from 28 electrodes, in accordance with the extended 10/20 system and digitized at 500 Hz. The recording reference was Cz. Vertical and horizontal electro-occulograms were recorded from the supra- to suborbit of the right eye and the outer canthus, respectively. Recordings were made with a Syn-Amp controlled through the SCAN software package (Neuroscan) and digitized with a sampling rate of 250 Hz. The impedance was kept below 10 kΩ at all electrode locations.
Event-Related Potentials
Data were analyzed by the software package Vision Analyzer 2.1 (Brain Products). Data were filtered off-line (band-pass 0.1–70 Hz with a 50 Hz notch filter) and baseline corrected to 200 milliseconds prestimulus, and trials with massive artifacts were automatically rejected. An independent component analysis was conducted to eliminate ocular activity (blinks) from the signal.38 Three measurement windows for ERPs of interest were established on the basis of inspection of the waveforms and the windows found in previous studies19,20: P100, a positive peak between 70 and 130 milliseconds at occipital sites (O1/O2); N170, a negative peak between 130 and 200 milliseconds at temporoparietal sites (P7/P8, TP7/TP8); and N250, a negative peak between 200 and 300 milliseconds at fronto-central sites (F3/F4, FC3/FC4). ERP amplitudes were measured as peak-to-baseline values.
After artifact rejection, a mean of 22.9 trials per subject entered statistical analyses. The number of artifact-free trials did not differ between groups (F 2,36 = 0.09, P = .91).
Statistical Analyses
All statistical tests were two-tailed. To evaluate behavioral performance, the percentage of correct responses within the task of affect recognition was analyzed. Effect size was calculated using Cohen's d.39 ERPs were analyzed separately for signal latency and amplitude at the corresponding electrodes and entered into repeated measures ANOVAs, including the Greenhouse-Geisser epsilon correction in case of violation of sphericity. The between-subject variable was group, and the repeated measures within-group variables were electrode position at the occipital site (O1/O2) for the P100 amplitude, at the temporoparietal site (TP7/TP8, P7/P8) for N170, and at the fronto-central site (F3/F4, FC3/FC4) for N250. In case of a significant main effect for group or interaction effects, including group, at the α < .05 level, post hoc tests were calculated using Tukey's correction to account for multiple testing.
Associations between ERP data, task performance, and symptoms were investigated by Spearman's correlation coefficients. In the case of ERP data, amplitude measures at component-specific electrodes (see above) were pooled and correlated as clusters.
In addition to analyses of the complete samples, supplemental analyses were conducted with subgroups of participants to control for potentially confounding effects of poor performance in emotion recognition. For these analyses, groups were matched on the basis of their performance by excluding 25% of poor performing at-risk individuals (n = 9) and 25% of high-scoring healthy controls (n = 8). Thus, subsequent analyses of correct responses and ERP characteristics were conducted with data from 28 at-risk individuals and 24 healthy controls; these analyses are reported separately in the results section.
Results
Behavioral Results
Regarding correct responses, a main effect of group (F 1,67 = 11.04, P < .001) indicated significantly worse performance in recognition of facial expression of emotions in at-risk individuals (mean = 21.9 ± 3.1) than in controls (mean = 24.9 ± 2.0). Calculation of effect size revealed a Cohen's d of 1.15, which is considered a large effect as it indicates that healthy controls outperformed at-risk individuals by more than 1 SD.
The 2 groups of at-risk individuals (EIPS and LIPS) did not differ in emotion recognition performance (t = 1.05, P = .29, d = .35; EIPS: mean = 22.4 ± 2.3; LIPS: mean = 21.3 ± 4.0) and were therefore grouped together for further ERP analyses.
Analysis of the performance-matched subgroups revealed no difference in emotion recognition performance (t = 1.3, P = 0.19) between at-risk individuals (mean = 23.2 ± 2.5) and healthy controls (mean = 23.9 ± 1.2).
EEG Results
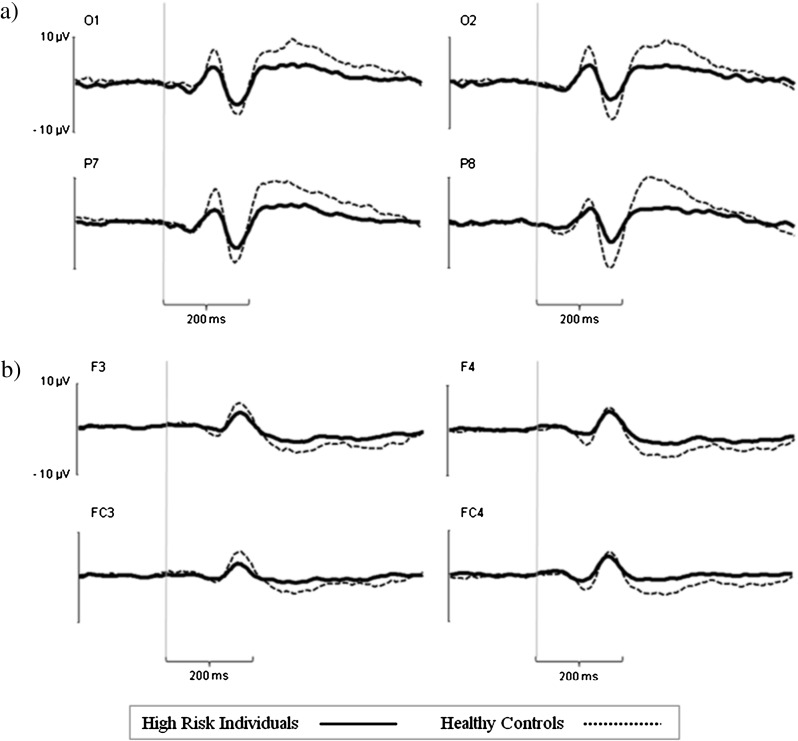
The overall ERP waveforms of at-risk individuals and healthy controls were similar (figure 1). As expected, at occipitotemporal sites a positive peak occurred at about 100 milliseconds (P100) after stimulus onset and a negative peak at about 170 milliseconds (N170). At fronto-central sites, a negative peak occurred at about 250 milliseconds (N250) after stimulus onset.
Fig. 1.
Example of waveforms of event-related potentials at occipito-parietal electrodes (a) and fronto-central electrodes (b) in 37 individuals at risk for schizophrenia and 32 healthy controls during facial emotion recognition.
No significant latency effects were observed when at-risk individuals were compared with healthy controls.
P100.
A repeated measures ANOVA for the P100 amplitude revealed a main effect of group, indicating a smaller amplitude in at-risk individuals than in controls (F 1,67 = 6.51, P = .01) (figure 1a). No other main effects and no interaction proved to be significant.
Results of analyses of subgroups matched for performance in facial affect recognition were similar, ie, a main effect of group was found (F 1,15 = 15.31, P = .01, controls > at-risk). This group effect was particularly obvious at occipital sites, as shown by an interaction of group and electrode (F = 6.35, P = .02).
N170.
Analysis of the N170 amplitude showed a significant main effect of group, indicating smaller amplitudes in at-risk individuals than in controls (F 1,67 = 18.90, P = .0001). Furthermore, there was an interaction of group and electrode site (F 1,67 = 4.4, P = .03), with greater differences between at-risk individuals and controls at parietal sites than at temporoparietal sites (figure 1a). An interaction between hemisphere and electrode site (F 1,67 = 11.8, P = .001) was found at the temporoparietal site—amplitudes were larger at the left electrode (TP7) than at the corresponding right electrode (TP8)—whereas no hemispheric difference in amplitude was observed at parietal sites (P7, P8). This effect was most pronounced in healthy controls, as indicated by a 3-fold interaction (F 1,67 = 4.1, P = .05). The main effect of electrode was insignificant.
Supplemental analyses using subgroups matched for performance confirmed these results: a main effect of group indicated larger N170 amplitudes in healthy controls than in at-risk individuals (F 1,50 = 19.6, P < .001). This difference in amplitude was most pronounced at parietal sites (P7/P8), as indicated by an interaction of group and electrode (F 1,50 = 4.71, P = .03). A 3-fold interaction of group, electrode, and hemisphere located this effect particularly at the right parietal electrode (P8) (F 1,50 = 5.11, P = .02).
N250.
For N250, a significant main effect of group indicated smaller amplitudes in at-risk individuals than in controls (F 1,67 = 6.8, P = .01). An interaction of group and hemisphere indicated larger differences between groups at electrodes on the right hemisphere (F 1,67 = 4.55, P = .03). Post hoc tests indicated that only N250 amplitudes at right fronto-central sites (F4 and FC4) differed between groups (figure 1b). No other main effects and no interaction proved to be significant.
The group effect indicating larger N250 amplitudes in healthy controls than in at-risk individuals was also present within the performance-matched group (F 1,50 = 5.3, P = .02).
Correlation of ERPs and Affect Recognition
There was a trend (r = −.29, P = .07) toward an association between poorer facial affect recognition performance (in terms of percent correct responses) and more pronounced negative symptoms (measured with the PANSS negative scale). Neither general psychopathology (PANSS general) nor positive symptoms (PANSS positive scale) correlated with affect recognition performance. PANSS scores, ie, positive, negative, and general symptoms, were not significantly associated with ERP components. Prodromal signs as assessed by the ERI were negatively correlated with the amplitude of the N250 component in individuals at risk for schizophrenia, ie, higher ERI scores were associated with smaller N250 amplitudes (r = −.35, P = .03). Neither N170 nor P100 amplitudes were associated with the ERI score.
Intercorrelations between ERP components only occurred for P100 and N250 (r = −.52, P < .01) but not for P100 and N170 amplitudes (r = −.20, P = .10). Spearman's correlation coefficients are provided in table 2.
Table 2.
Spearman's Correlation Coefficients for Facial Emotion Recognition Performance, ERP Amplitudes, and Psychopathology
| Total Sample (N = 69) | High Risk Individuals (N = 37) | GAF | P100 | N170 | |||||
| PFA | PANSS Positive | PANSS Negative | PANSS General | ERI | |||||
| PFA | — | r = .07, P = .67 | r = .29, P = .07 | r = −.01, P = .91 | r = −.11, P = .51 | r = .13, P = .42 | |||
| ERP amplitudes | P100 | r = .21, P = .07 | r = .04, P = .77 | r = .01, P = .98 | r = −.07, P = .66 | r = .25, P = .13 | r = −.08, P = .64 | ||
| N170 | r = −.18, P = .12 | r = −.22, P = .19 | r = −.13, P = .43 | r = −.31, P = .06 | r = .20, P = .22 | r = .16, P = .33 | r = −.20, P = .10 | ||
| N250 | r = −.14, P = .24 | r = .09, P = .59 | r = .04, P = .78 | r = .18, p = .27 | r = −.35, *P = .03 | r = −.01, P = .97 | r = −.52, **P < .01 | r = .12, P = .29 | |
Note: Abbreviations are explained in the first footnote to table 1.
*P < .05, **P < .01.
Discussion
To our knowledge, the present study was the first to investigate neurophysiological correlates of facial affect recognition in individuals at risk for schizophrenia. During recognition of emotional faces, at-risk individuals showed significantly worse affect recognition performance and lower ERP amplitudes than healthy controls. The prodromal sample showed ERP abnormalities very similar to those previously reported in manifest schizophrenia. These findings add evidence to the assumption that particular cognitive and emotional dysfunctions precede the onset of initial psychosis. The results of the present study revealed similar deficits in individuals at early and late prodromal phases (EIPS and LIPS), suggesting that analogue characteristics are present in early and late stages of the initial prodrome of schizophrenia.
Our finding that individuals at risk for schizophrenia have an impaired ability to recognize emotional faces is in line with the observations that not only individuals with manifest schizophrenia (for a review, see ref. 40) and first-episode patients7 perform significantly worse than nonpsychiatric controls but that (to a lesser extent) unaffected relatives of schizophrenia patients also show such impairments.5 The present findings show that impaired performance in facial affect recognition already exists in at-risk individuals. Because emotion recognition is an important aspect of social cognition that underlies socially competent behavior, impaired recognition of emotions may be fundamental to an early risk of social decline, such as social withdrawal.41 Although we do not know the exact transition rate to schizophrenia in the present sample, it has to be assumed that this particular deficit constitutes a stable attribute throughout the course of the disorder and therefore could serve as a vulnerability marker for schizophrenia. Together, the observations suggest a potential role for facial affect recognition as a social cognitive endophenotype for schizophrenia.6
Beyond behavioral performance, we found a trend for task performance and prodromal signs to correlate with poorer performance in individuals with higher PANSS negative scores but no association between ERP components and general psychopathology. A correlation of the prodromal signs, as assessed with the ERI, with emotion recognition performance suggests that psychopathology is linked to social cognitive abilities, ie, facial emotion recognition, even before onset of the manifest disorder. Furthermore, ERI scores were negatively correlated with N250 amplitudes, indicating smaller amplitudes and lower performance rates in those individuals presenting more prodromal signs. However, the specific contribution of symptoms to the emotion recognition deficit remains unresolved and needs further investigation within the population of individuals at risk for schizophrenia.
The present study used ERPs as they provide insight into the neural mechanisms associated with the observed behavior. Analyses of performance-matched subgroups were conducted to ensure that the observed neurophysiological findings were not simply a matter of cognitive impairment in the group of schizophrenia patients. The subgroup analyses yielded similar results so that the observed abnormalities in ERP can be assumed to be associated with the at-risk mental state present in the group of individuals at risk for schizophrenia.
Regarding the ‘face-selective’ N170 component, our finding that at-risk individuals showed lower N170 amplitudes than healthy controls confirms observations obtained in patients with schizophrenia.18 As the N170 component is linked to the perceptual integration and structural encoding of faces, it is suggested that the observed reduction in N170 reflects dysfunctions in visual processing of facial structures. This finding seems to be at odds with our group's earlier work in schizophrenia patients, which found the N170 component to be intact. In the present study, the P100 preceding the reduced N170 component was smaller in at-risk individuals than in controls. The P100 is suggested to reflect early visual processing and categorization of stimuli and their physical characteristics (eg, contrast, luminance). The P100 shows larger amplitudes for stimuli presented at attended locations of the visual field than for those presented at nonattended locations.42 Thus, these findings may reflect an impairment in fundamental visual processes in individuals at risk for psychosis. The diminished amplitudes of P100 and N170 indicate that a decrement in these early components may be responsible for the decrement in later face-evoked potentials such as the N25043 and may therefore constitute a decrease of more general visual processing capacities in at-risk individuals. However, the lack of clear correlations between P100 and N170 amplitudes in the present study fails to answer this question as these signals may represent largely independent processes executed at the same time; accordingly, van der Stelt and colleagues44 suggested that ‘… high-level attention-dependent cognitive deficits central to schizophrenia do not originate from potential preceding impairments at lower levels of sensory, perceptual, or cognitive processing …’.
In our previous study, unaltered P100 and N170 components were followed by a reduced N250 amplitude, suggesting deficits in higher order aspects of cognition such as decoding and interpreting the presented facial emotion against the background of intact encoding of facial features.20 A significant correlation between P100 and N250 amplitudes further corroborates the assumption that this deficit may be a secondary flow-on effect of a more general deficit in the perceptual and structural encoding of faces (and other stimuli). Previous studies from other research groups also found prominent abnormalities in early visual processing in schizophrenia, suggesting that both early visual processing and subsequent ‘higher level’ cognitive processing are severely impaired as a result of generally dysfunctional information processing.43,45
The question whether the facial emotion recognition deficits observed in both schizophrenia patients and at-risk individuals are specific to facial emotions or rather general to all facial information remains a matter of debate. The hypothesis that impairments in facial affect recognition are specific to the processing of the affective information is supported by the observations that facial detection of gender seems intact19 and that patients show diminished amplitudes during affect recognition but not during categorization of blurred faces.20 The present finding of reduced P100 and N170 amplitudes supports the assumption that the facial affect recognition deficit is not specific to emotions but may be general to faces. However, as has been recently shown, emotion recognition takes place as early as within the first 100 milliseconds of face processing within a distributed brain network.46 This finding suggests that the interpretation of P100 and N170 as correlates of pure structural encoding processes may be an oversimplification of human information processing that needs to be addressed by future research.
In addition to reduced N170 amplitudes, we found reduced N250 amplitudes in the at-risk individuals, indicating that these individuals may have disturbed cognitive processing in terms of associating the structural representation of the face with semantic and contextual information. Contrary to the present study, earlier studies did not find significantly decreased N250 amplitudes in schizophrenia patients.23,47 This may be explained by the different tasks presented. Herrmann et al47 presented pictures of only 4 different persons, displaying 3 different emotions, with each picture being shown 8 times. After approximately 8 presentations, the subjects had to verbalize the emotional expression of the last face. Turetsky and colleagues23 presented happy, sad, and neutral faces very briefly, thus preventing participants from strategically observing stimulus features and forcing them to spontaneously process elemental facial features indicative of emotional qualities. In our study, 30 different faces (6 basic emotions) were presented and subjects were asked to classify each face. The divergence between our results and those of the studies just mentioned may be explained by the "greater difficulty" of classifying more facial emotions than merely contrasting happy, sad, and neutral faces or classifying only 4 different persons. These methodological differences (eg, facial expression decoding vs face recognition) may mean that these studies cannot be satisfactorily compared and that future studies are therefore needed to address this effect. To investigate the processing of emotional material in individuals at risk, the present study used stimuli showing 6 basic emotions but did not include neutral faces. Because the present study found that poorer performance in general affect recognition in at-risk individuals was associated with abnormalities in the neurophysiological correlates, future studies are needed to extend the earlier findings. Of particular interest are the questions of whether prodromal patients show impairments in recognizing particular emotions and whether these impairments are associated with abnormalities in emotion-specific ERPs.
Conclusions
The reliable discrimination of emotional expressions in faces is essential for adequate social interaction. The results of the present study demonstrate that this ability is impaired in individuals at risk for schizophrenia, ie, before the onset of the manifest disorder. Furthermore, the fact that the EEG abnormalities we found in at-risk individuals in this study are qualitatively similar to those in schizophrenia patients identified in earlier studies suggests that the neural processes underlying facial affect recognition deficits might indeed be part of a basic neural dysfunction reflecting a vulnerability characteristic or an endophenotype of schizophrenia.6 This knowledge in future may facilitate the detection of at-risk individuals in an early stage of developing schizophrenia and may thereby enable targeted early interventions.
Funding
German Federal Ministry for Education and Research (BMBF grant no. 01 GI 9935).
Acknowledgments
This study was part of the German research Network on Schizophrenia and was funded by the German Federal Ministry for Education and Research. The study was mainly initiated by Dr. Marcus Streit, who left a first draft of the manuscript before he was deceased. This paper was finalized in memory of our dear colleague. The authors thank Jacquie Klesing, ELS, for editing assistance with the manuscript. The authors have declared that they have no conflicts of interest in relation to the subject of this study.
References
- 1.Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin Neurosci. 2006;8:59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couture S, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinkham AE, Hopfinger JB, Ruparel K, Penn DL. An investigation of the relationship between activation of a social cognitive neural network and social functioning. Schizophr Bull. 2008;34:688–697. doi: 10.1093/schbul/sbn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns J. The social brain hypothesis of schizophrenia. World Psychiatry. 2006;5:77–81. [PMC free article] [PubMed] [Google Scholar]
- 5.Bediou B, Asri F, Brunelin J, et al. Emotion recognition and genetic vulnerability to schizophrenia. Br J Psychiatry. 2007;191:126–130. doi: 10.1192/bjp.bp.106.028829. [DOI] [PubMed] [Google Scholar]
- 6.Gur RE, Calkins ME, Gur RC, et al. The consortium on the genetics of schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006;85:142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Addington J, Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res. 1998;32:171–181. doi: 10.1016/s0920-9964(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 9.Wölwer W, Streit M, Polzer U, Gaebel W. Facial affect recognition in the course of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1996;246:165–170. doi: 10.1007/BF02189118. [DOI] [PubMed] [Google Scholar]
- 10.Eack SM, Mermon DE, Montrose DM, et al. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophr Bull. 2010;36:1081–1088. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addington J, Penn D, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for psychosis. Br J Psychiatry. 2008;192:67–68. doi: 10.1192/bjp.bp.107.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips LK, Seidman LJ. Emotion processing in persons at risk for schizophrenia. Schizophr Bull. 2008;34:888–903. doi: 10.1093/schbul/sbn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinkham AE, Penn DL, Perkins DO, Graham KA, Siegel M. Emotion perception and social skill over the course of psychosis: a comparison of individuals "at-risk" for psychosis and individuals with early and chronic schizophrenia spectrum illness. Cognit Neuropsychiatry. 2007;12(3):198–212. doi: 10.1080/13546800600985557. [DOI] [PubMed] [Google Scholar]
- 14.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler PD, Martinez A, Foxe JJ, et al. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeap S, Kelly SP, Sehatpour P, et al. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63:1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- 18.Campanella S, Montedoro C, Streel E, Verbanck P, Rosier V. Early visual components (P100, N170) are disrupted in chronic schizophrenic patients: an event-related potentials study. Neurophysiol Clin. 2006;36:71–78. doi: 10.1016/j.neucli.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Bediou B, Henaff MA, Bertrand O, et al. Impaired fronto-temporal processing of emotion in schizophrenia. Neurophysiol Clin. 2007;37:77–87. doi: 10.1016/j.neucli.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Streit M, Wölwer W, Brinkmeyer J, Ihl R, Gaebel W. EEG-correlates of facial affect recognition and categorisation of blurred faces in schizophrenic patients and healthy volunteers. Schizophr Res. 2001;49:145–155. doi: 10.1016/s0920-9964(00)00041-4. [DOI] [PubMed] [Google Scholar]
- 21.Eimer M. The face-specific N170 component reflects late stages in the structural encoding of faces. Neuroreport. 2000;11(10):2319–2324. doi: 10.1097/00001756-200007140-00050. [DOI] [PubMed] [Google Scholar]
- 22.Onitsuka T, Niznikiewicz MA, Spencer KM, et al. Functional and structural deficits in brain regions subserving face perception in schizophrenia. Am J Psychiatry. 2006;163:455–462. doi: 10.1176/appi.ajp.163.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur J Neurosci. 2005;22:1221–1232. doi: 10.1111/j.1460-9568.2005.04294.x. [DOI] [PubMed] [Google Scholar]
- 25.Bechdolf A, Ruhrmann S, Wagner M, et al. Interventions in the initial prodromal states of psychosis in Germany: concept and recruitment. Br J Psychiatry Suppl. 2005;48:s45–s48. doi: 10.1192/bjp.187.48.s45. [DOI] [PubMed] [Google Scholar]
- 26.Ruhrmann S, Schultze-Lutter F, Klosterkotter J. Early detection and intervention in the initial prodromal phase of schizophrenia. Pharmacopsychiatry. 2003;36(suppl 3):162–167. doi: 10.1055/s-2003-45125. [DOI] [PubMed] [Google Scholar]
- 27.Wölwer W, Baumann A, Bechdolf A, et al. The German research network on schizophrenia—impact on the management of schizophrenia. Dialogues Clin Neurosci. 2006;8:115–121. doi: 10.31887/DCNS.2006.8.1/wwoelwer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Häfner H, Maurer K, Ruhrmann S, et al. Early detection and secondary prevention of psychosis: facts and visions. Eur Arch Psychiatry Clin Neurosci. 2004;254:117–128. doi: 10.1007/s00406-004-0508-z. [DOI] [PubMed] [Google Scholar]
- 29.Schultze-Lutter F, Ruhrmann S, Berning J, Maier W, Klosterkötter J. Basic symptoms and ultrahigh risk criteria: symptom development in the initial prodromal state. Schizophr Bull. 2010;36:182–191. doi: 10.1093/schbul/sbn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 31.Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophr Bull. 2009;35:5–8. doi: 10.1093/schbul/sbn139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk ("prodromal") group. Schizophr Res. 2003;60:21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Non-Patient Edition (SCID-I/NP). Version 2. New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P). Version 2. New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 35.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 36.Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- 37.Streit M, Ioannides A, Sinnemann T, et al. Disturbed facial affect recognition in patients with schizophrenia associated with hypoactivity in distributed brain regions: a magnetoencephalographic study. Am J Psychiatry. 2001;158:1429–1436. doi: 10.1176/appi.ajp.158.9.1429. [DOI] [PubMed] [Google Scholar]
- 38.Brown GD, Yamada S, Sejnowski TJ. Independent component analysis at the neural cocktail party. Trends Neurosci. 2001;24:54–63. doi: 10.1016/s0166-2236(00)01683-0. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 40.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22(6):789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 41.Häfner H, Maurer K. Early detection of schizophrenia: current evidence and future perspectives. World Psychiatry. 2006;5:130–138. [PMC free article] [PubMed] [Google Scholar]
- 42.Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining "early" visual processing. Exp Brain Res. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- 43.Foxe JJ, Murray MM, Javitt DC. Filling-in in schizophrenia: a high-density electrical mapping and source-analysis investigation of illusory contour processing. Cereb Cortex. 2005;15:1914–1927. doi: 10.1093/cercor/bhi069. [DOI] [PubMed] [Google Scholar]
- 44.van der Stelt O, Frye J, Lieberman JA, Belger A. Impaired P3 generation reflects high-level and progressive neurocognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2004;61:237–248. doi: 10.1001/archpsyc.61.3.237. [DOI] [PubMed] [Google Scholar]
- 45.Yeap S, Kelly SP, Sehatpour P, et al. Visual sensory processing deficits in Schizophrenia and their relationship to disease state. Eur Arch Psychiatry Clin Neurosci. 2008;258:305–316. doi: 10.1007/s00406-008-0802-2. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Ioannides AA. Emotion separation is completed early and it depends on visual field presentation. PLoS ONE. 2010;5:e9790. doi: 10.1371/journal.pone.0009790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrmann MJ, Reif A, Jabs BE, Jacob C, Fallgatter AJ. Facial affect decoding in schizophrenic disorders: a study using event-related potentials. Psychiatry Res. 2006;141:247–252. doi: 10.1016/j.psychres.2005.09.015. [DOI] [PubMed] [Google Scholar]