Abstract
The threat of predictable and unpredictable aversive events was developed to assess short-duration (fear) and long-duration (anxiety) aversive states in humans. A typical experiment consists of three conditions: a safe condition (neutral (N)), during which participants are safe from aversive stimuli, and two threat conditions—one in which aversive events are administered predictably (P) (i.e., signaled by a threat cue), and one in which aversive stimuli are administered unpredictably (U). During the so-called NPU -threat test, ongoing change in aversive states is measured with the startle reflex. The NPU -threat test has been validated in pharmacological and clinical studies and can be implemented in children and adults. Similar procedures have been applied in animal models, making the NPU -threat test an ideal tool for translational research. The procedure is relatively short (35 min), simple to implement and generates consistent results with large effect sizes.
INTRODUCTION
This paper describes a protocol in humans for investigating fear and anxiety with the goals of developing a more operational way to study these aversive states and of promoting their investigation in the laboratory under controlled conditions. Distinguishing and defining fear and anxiety have been difficult and controversial because of their overlapping nature1,2. However, several lines of research support the notion that fear and anxiety differ in a number of key dimensions. Clinicians in general accept the distinction between fear, a phasic response to an imminent and identifiable danger, and anxiety, a sustained state of apprehension about future threat leading to tension, worry and a feeling of insecurity3,4. This view is supported by psychometric analyses of anxious symptomatology that distinguish symptoms of anxiety (e.g., anxious apprehension and general distress) from those of fear (e.g., fight/flight and arousal)5,6. Also, on the basis of the structure of internalizing disorders, two broad factors have been described: a fear factor which includes phobias and panic; and an anxious misery factor including generalized anxiety disorder, post-traumatic stress disorder, dysphoria and depression7,8.
More generally and from an evolutionary perspective, fear and anxiety can be viewed as basic defensive responses that motivate organisms to detect, react and cope with threat and danger. These defensive responses vary greatly with the nature of the danger; they depend on whether the threat is present and requires immediate action or is temporally uncertain or distant, prompting sustained vigilance9. These responses to threats are well conserved across species and are precursors to human fear and anxiety9,10.
There is now substantial evidence to suggest that fear and anxiety can also be differentiated at the neural level. Specifically, studies based on the startle reflex show that lesions of the central nucleus of the amygdala (CeA) block phasic, cued-induced fear responses without affecting more sustained anxiety states associated with uncertain danger, whereas lesions of the bed nucleus of the stria terminalis (BNST) have the opposite effects11. Recent studies have confirmed these results by using measures of defensive states other than startle12–14, and a similar differentiation between phasic fear and sustained anxiety has been made on the basis of psychopharmacological modulations of the startle reflex10.
In these preclinical studies, fear and anxiety differ in at least two dimensions: their duration (phasic versus sustained) and the nature of the threat (predictable versus unpredictable). We used the balance between these dimensions as an operational definition of fear and anxiety, and this distinction is the logic behind the threat of the predictable and unpredictable aversive events test (NPU-threat test). This procedure has been used in several studies by our group, as well as by other investigators15–22.
NPU-threat test
The NPU-threat test consists of three conditions: (i) no aversive event (N condition); (ii) predictable aversive events (P condition); and (iii) unpredictable aversive events (U condition). In the N condition, subjects are safe from aversive events. By contrast, aversive events are administered predictably in the P condition (i.e., signaled by a short-duration threat cue) and unpredictably in the U condition (i.e., not signaled). The aversive events can be unpleasant shocks5 or developmentally and ethically appropriate stimuli (e.g., screams, blast of air to the neck) to study special populations or children20. Subjects’ fear and anxiety are assessed using the startle reflex, a cross-species measure of aversive state23. In humans, the startle reflex is measured most reliably by an electromyogram (EMG) of the orbicularis oculi, which captures the blink component of the reflex24. Before the beginning of the NPU-threat test, participants are informed about the details of the contingency between the different conditions and the aversive events.
The NPU-threat test evokes a short-duration (fear-potentiated) and a long-duration (anxiety-potentiated) startle potentiation in the P and U conditions, respectively. People with panic disorder or PTSD are selectively more sensitive to anxiety- than to fear-potentiated startle17,19. In addition, anxiolytic drugs decrease anxiety-potentiated startle at doses that do not affect fear-potentiated startle16,18. A key advantage of the NPU-threat test is that very similar procedures have been implemented in animal models, making the NPU-threat test ideal for translational research23. This allows for greater generalizability of results and implications for the underlying neurophysiological processes. The NPU-threat test has also been successfully adapted for use in children and adolescents20, thereby providing a link to developmental research on anxiety, which is key for uncovering risk factors for anxiety disorders as well as pathological mechanisms and potential targets for prevention.
Several other laboratories have adapted the NPU-threat test with minor changes to the original procedure (i.e., counting down to the shock25), or alternative manipulations of predictability (i.e., using only short-duration cues with 100, 60 or 20% shock probability26; manipulation of the predictability of the shock intensity27). Although these adaptations offer interesting additional information, they have not been validated in anxious subjects or with anxiolytics. Another useful modification is the adaptation of the NPU-threat test to examine aversive conditioning. The NPU-threat test uses an instructed threat procedure to inform participants of the association between a cue and the shock. In the conditioning version of the task, participants learn via Pavlovian conditioning to associate the shock with the different contexts and cues28,29. Whereas the former procedure provides a tool for studying the expression of fear and anxiety responses without the confound of learning and memory, the latter can be used to study associative processes involved in fear and anxiety learning.
The following protocol describes the basic NPU-threat test, applying electric shocks as the aversive stimulus, as it has been implemented in studies of healthy and psychiatrically ill adult populations in our laboratory.
MATERIALS
REAGENTS
Human subjects: participants need to be literate and have normal or corrected to normal vision and hearing
 Informed consent should be obtained from all subjects and the study protocol must be approved by the appropriate institutional review board or human subjects committee.
Informed consent should be obtained from all subjects and the study protocol must be approved by the appropriate institutional review board or human subjects committee.
EQUIPMENT
Basic psychophysiological laboratory equipment (a detailed description of the basic setup of a psychophysiological laboratory and a list of vendors for psychophysiological equipment can be found elsewhere30)
Subject’s room
Human EMG acquisition system (e.g., Psychlab, Contact Precision Instruments) and consumable supplies
White noise sound generator for eliciting startle responses (e.g., Psychlab, Contact Precision Instruments)
Constant current stimulator for electric shock application (e.g., Psychlab, Contact Precision Instruments). Additional equipment is required if other types of aversive stimuli are applied (e.g., compressed tank of air, regulator and solenoid for delivering air blasts)
Monitor for stimulus presentation
Headphones
Video camera/webcam for monitoring the subject during the recording
Visual aids (printed copies of the six types of stimuli that are presented on the monitor; these stimuli serve as visual aids to explain the contingency between the various conditions and the administration of the aversive event)
Questionnaires: retrospective anxiety- and pain-rating form (see Supplementary Methods 1)
Investigator’s room
Computer hardware for presentation of stimuli and recording of physiological parameters (e.g., Dell desktop), which is connected to the subject’s monitor and a second monitor for the investigator to control stimulus presentation and observe physiological measurements during the recording. Specific hardware requirements depend on the acquisition system used (parallel ports, etc.)
Stimulus presentation software (e.g., Psychlab, Contact Precision Instruments; Presentation, Neurobehavioral Systems)
 The stimulus presentation software must insert triggers or event codes into the physiological recording in order to relate physiological measures to the stimulus presentation. Without event triggers, data analysis will be impossible.
The stimulus presentation software must insert triggers or event codes into the physiological recording in order to relate physiological measures to the stimulus presentation. Without event triggers, data analysis will be impossible.Hardware and software to display the video feed (optional; dependent on the camera used to monitor the subject)
EQUIPMENT SETUP
Aversive stimulus selection
Aversive stimuli should be carefully selected depending on the population tested. Robust results have been obtained with 100-ms-duration electric shocks (1–5 mA) as aversive stimuli for adult populations and milder aversive stimuli (blast of air to the neck, picture of a scared woman accompanied by a scream) for youth.
Visual stimuli generation
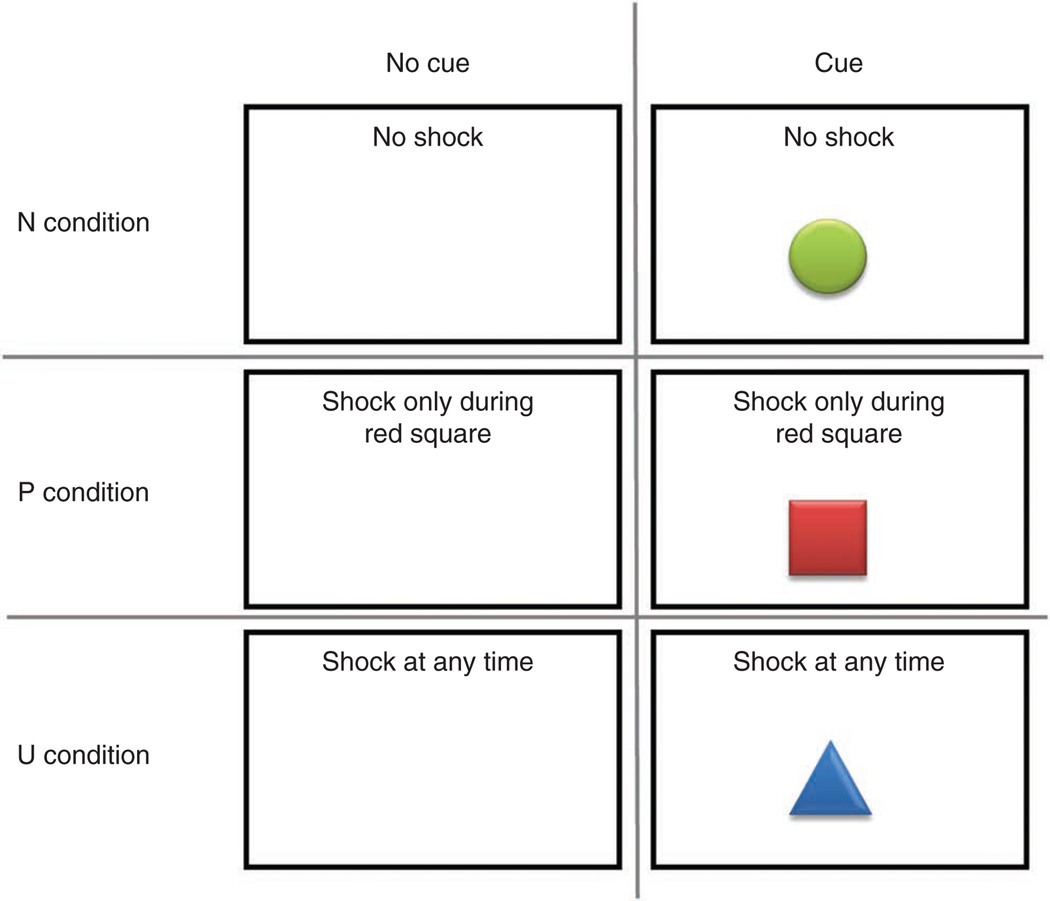
Visual stimuli (e.g., geometric shapes, indication of present condition; Fig. 1) can be generated with any appropriate graphic software. Text should be readable from a 2-m distance without difficulties.
Figure 1.
Visual material presented during the experiment by cue/no cue status and condition. For a given condition, subjects see one of the ‘No cue’ signs (e.g., ‘No shock’ in the N condition). Occasionally, a cue (e.g., a green circle in the N condition) is presented for 8 s.
Stimulus presentation
The NPU-threat test consists of two recording blocks, which are separated by a 5-min break and preceded by a startle habituation phase. The habituation phase consists of nine startle probes (103 dB white noise, 40 ms duration, instantaneous rise time). The startle blink reflex shows a relatively strong habituation especially within the first few trials. This procedure ensures that the initial, very strong habituation does not disproportionally influence the results.
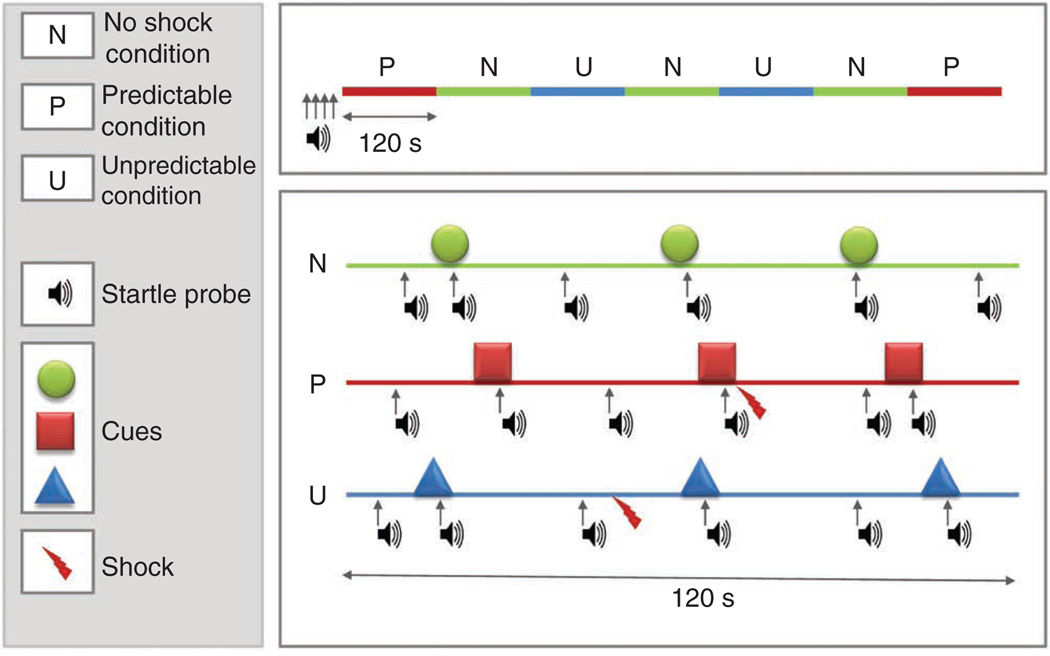
The testing phase consists of two P, two U and three N conditions each, which are presented in one of following orders: P N U N U N P or U N P N P N U. The two orders are used for each subject with half the subjects starting with the P condition first and the other half with the U condition first. Both orders begin with four additional startle probes to further habituate startle. Care should be taken to balance the order of the two blocks within experimental groups when appropriate. Each condition (i.e., N, P, U) lasts ~120 s and includes three short presentations (8 s) of a colored geometric cue (e.g., red square, blue triangle, green circle). The same cue is used consistently within each condition. Startle probes (103 dB white noise, 40 ms duration, instantaneous rise time) are administered once during each cue presentation and once during each interval between cues. Startle probes are separated by a minimum interval of 20 s. See Figure 2 for a schematic representation of the sequence of stimuli.
Figure 2.
Schematic representation of sequences of stimulus presentation during each condition in one block of the NPU-threat test. The upper part of the figure represents a complete block including two P (predictable), two U (unpredictable) and three N (no shock) conditions. The lower part shows examples of each condition, including startle probes, cues (8 s duration) and shocks. Adapted from reference 20.
Each ongoing condition is indicated on the screen at all times (e.g., ‘shock only during red square’, ‘shock at anytime’, ‘no shock’; see Fig. 1). Shocks are applied once or twice per P (0.5 s before the end of the cue) or U condition (randomized during absence of the cue), resulting in 6 shocks per block and 12 shocks overall during the experiment. Note that the reason why shocks are not administered during the cue in the U condition is to prevent the subject from believing that shocks have a higher probability of being administered during the cue compared with the no cue period. Our results show that the absence of shock during the cue in the U condition does not lead to conditioned inhibition (safety learning) to the cue, probably because subjects are exposed to too few shocks. An example of the timing of all stimuli in the first block can be found in the supplementary material (Supplementary Table 1).
PROCEDURE
Prerecording
 15 min
15 min
-
1
Obtain informed consent from the subject.
-
2
Give detailed instructions to the subject including a description of the experiment and all conditions using the visual aids depicting the stimuli that will be presented on the monitor during the experiment (for example, see Supplementary Methods 2): Instruct the subject that there are three different conditions, one in which no shock is administered and two during which shocks are administered either predictably or unpredictably. Next, instruct the subject that in the P condition, shocks can be administered only in the presence of a cue; shocks cannot be administered in the absence of a cue, and that in the U condition shocks can be administered at any time (i.e., in the presence or absence of a cue). The subject will also be informed that each condition is signaled by a short note at the top of the computer screen. ‘No shock’ indicates that no shock can be administered. ‘Shock only during red square’ signals that the subject might receive a shock, but only if the red square is actually on the screen. The subject will never receive a shock during this condition when the red square is absent. ‘Shock at any time’ signifies that the subject can receive a shock when the blue triangle is present or absent. The individual providing the instruction needs to convey a sense of trust. He/she must add that all the instructions are true and that there is no attempt at deception. Add that occasional loud startling sounds will be administered from time to time. Make clear that the subject’s only task is to stay focused on the screen and try to avoid voluntary movements as these can affect the physiological measurement. Explain that the subject will hear several loud, startling sounds before the actual test starts.
-
3
Ask if the subject needs clarification and answer any questions.
-
4
Conduct shock workup: the shock workup procedure consists of the administration of a total of four sample shocks. The procedure entails delivery of a graded series of shocks starting at 2 mA and up to a maximum of 5 mA. Each shock is rated by the subject and shock intensity is subsequently adjusted in order to achieve the desired rating of 4 (quite unpleasant/uncomfortable) on a scale ranging from 1 (barely felt) to 5 (very unpleasant/uncomfortable). The shock intensity is increased in steps of 1 mA until the rating of 4 is reached. If the rating of 4 is reached before the fourth shock, additional shocks of the current intensity are given to reach a total of four shocks. If the rating of 4 is not reached after four shocks, the maximum intensity of 5 mA is used.
-
5
Present the startle probe to the subject and confirm that the subject can hear the white noise (if auditory stimuli are applied as aversive stimuli, make sure the subject can hear these as well).
-
6
Prepare the subject for EMG recording of the orbicularis oculi according to general guidelines (e.g., see ref. 31). In brief, clean the skin underneath the left eye of makeup and dead skin cells (e.g., with soap or alcohol). Attach two Ag/AgCl miniature electrodes filled with high-conductivity electrode gel under the left eyelid; one in line with the pupil at forward gaze and the other electrode 1–2 cm lateral to the first one. An isolated ground electrode should be attached at an electrically inactive site (e.g., the forehead).
-
7
Remind the subject of the opportunity to stop the experiment at any time.
-
8
Test the electrode readings: ask the participant to blink to check the settings of EMG recording (e.g., recording range).
Recording
 35 min
35 min
-
9
Start physiological recording and presentation of the first block.
-
10
Monitor the subject during the procedure and document any violation of instructions (e.g., voluntary movements, closing eyes).
-
11
After the first block of the experiment is completed, give a short break of 5–10 min. During this period, ask the subject to rate his/her anxiety/fear level retrospectively during each of the conditions with the retrospective anxiety-rating form (see Supplementary Methods 1). Give the subject the opportunity to drink some water, to stretch his or her legs, and so on. Briefly reiterate the instructions and remind the subject to refrain from excessive movements.
-
12
Start physiological recording and presentation of the second stimulus set.
-
13
Monitor the subject during the procedure and document any violation of instructions (e.g., voluntary movements, closing eyes).
-
14
After the second block, ask the subject for a second retrospective rating of fear/anxiety. Finally, ask the subject to rate the level of pain evoked by the shocks by using the pain-rating form (see Supplementary Methods 1).
Postrecording
 20 min
20 min
-
15
Clean and disinfect the electrodes according to standard procedures.
-
16
Exclude data sets with invalid data on the basis of monitoring protocols (e.g., if subjects fell asleep during the procedure or did not pay attention to the screen).
-
17
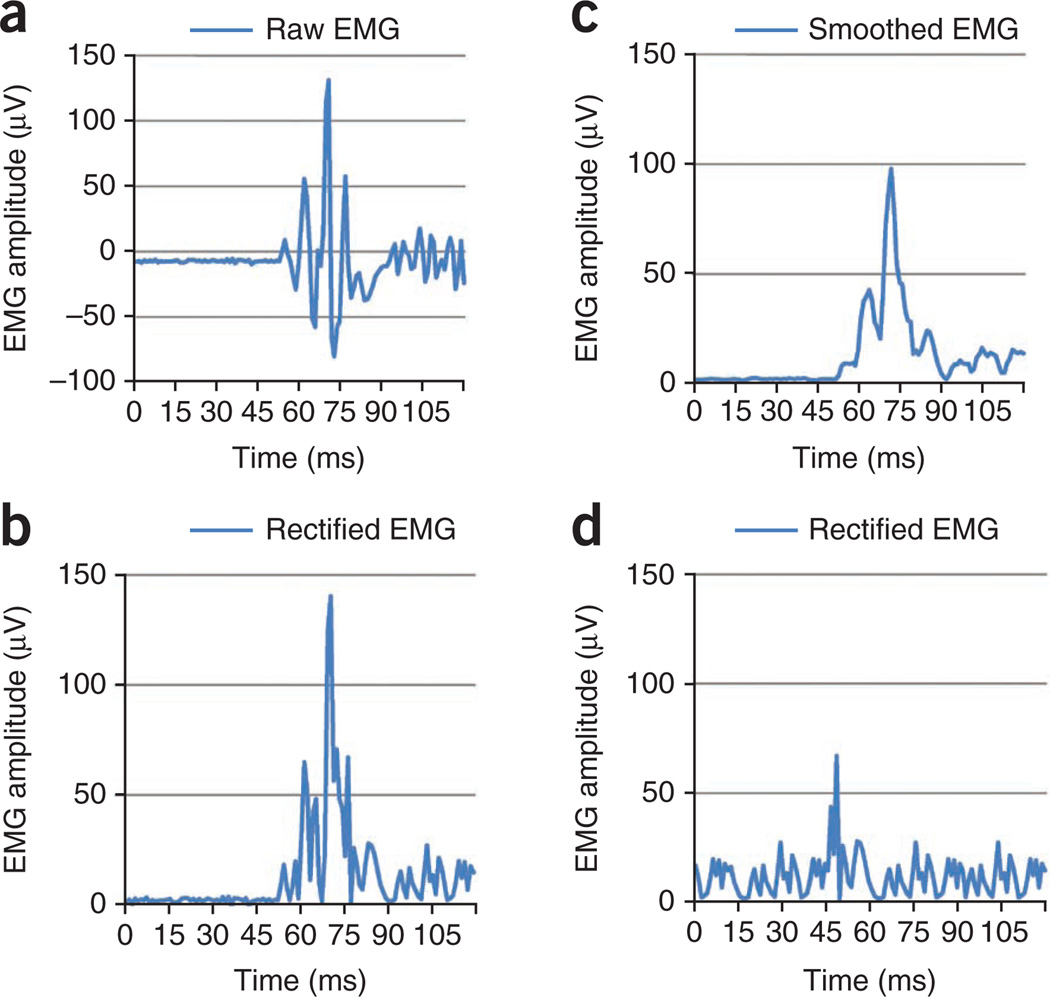
Prepare physiological data for analysis with appropriate software (e.g., Psychlab). First rectify and smooth EMG (see Fig. 3a–c for examples of raw, rectified and smoothed EMG). Next, identify relevant time blocks for analysis (150-ms block after onset of startle probe).
-
18
Reject trials with excessive baseline activity or other contaminations (Fig. 3d). This includes all trials with excessive noise during the baseline period before blink onset, movement artifacts or spontaneous blinks that begin earlier than 21 ms after the startle probe onset (minimal onset latency), which render accurate quantification of the blink magnitude impossible.
-
19
Identify the blink onset and peak, and measure the blink magnitude.
-
20
Calculate the mean magnitude for each trial type (e.g., habituation, no cue N, cue N, no cue P, cue P, no cue U, cue U). Transform the raw magnitude data into t scores and calculate the mean t scores for each trial type. An alternative to using t scores in order to control for differences in baseline startle reactivity is to include the mean baseline startle (e.g., mean startle response during a baseline startle procedure similar to the described habituation phase) as a predictor into the analysis of group differences in fear- and anxiety-potentiated startle (e.g., ref. 32).

Steps 1–8, prerecording: 15 min
Steps 9–14, recording: 35 min
Steps 15–20, postrecording: 20 min
Figure 3.
Blue line represents EMG of the orbicularis oculi. (a–d) Raw EMG (a), rectified EMG (b), smoothed EMG (c), trial with excessive baseline activity (d).
ANTICIPATED RESULTS
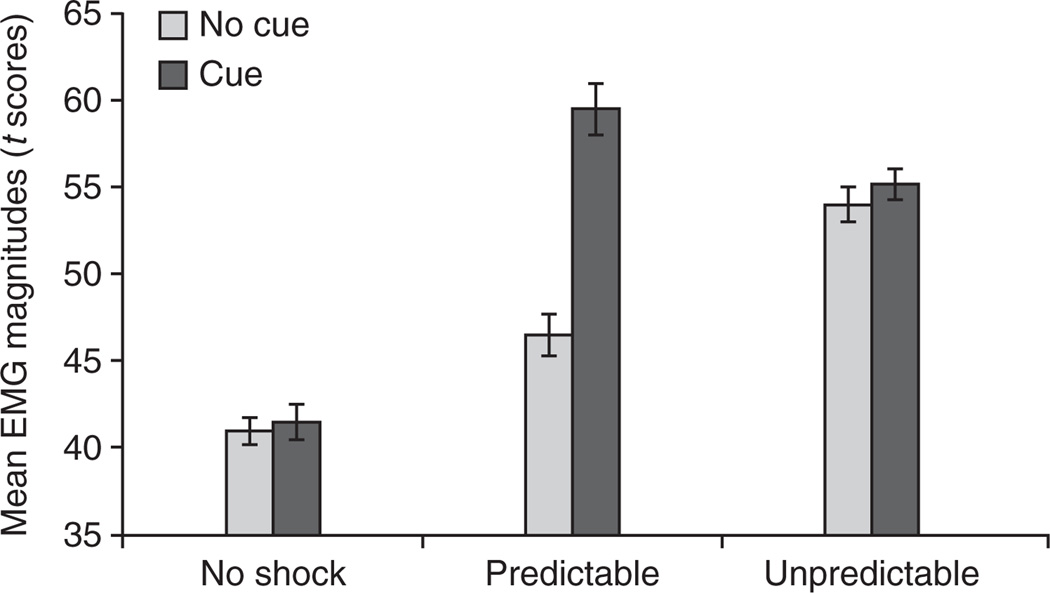
When the aversive event is a shock, the NPU-threat test yields robust fear- and anxiety-potentiated startle in adults in our laboratory15–17,19,22,33. Accordingly, mean startle magnitude during the P condition should be higher during the cue as compared with during the absence of the cue (P cue–no cue P; fear-potentiated startle), and mean startle magnitude during the absence of the cue in the U condition should be higher than during the N condition (no cue U–no cue N; anxiety-potentiated startle). In addition, mean startle magnitude during the P condition in the absence of the cue should be intermediate between no cue N and no cue N. This is because, consistent with findings in rodents34,35, a predictable context is more anxiogenic than a N context, but is less anxiogenic than an unpredictable context. An example of anticipated startle data is displayed in Figure 4. Our previous studies have shown that anxiety-potentiated startle is (i) sensitive to anxiolytic drugs16,18, (ii) a psychophysiological marker for anxiety disorders such as PTSD and panic disorder17,19 and (iii) differs between women and men as well as girls and boys20,36.
Figure 4.
Mean EMG magnitude during the cue and in the absence of the cue in the no shock, predictable and unpredictable conditions. Fear-potentiated startle is the difference score between startle magnitude during the cue, minus startle magnitude in the absence of the cue in the predictable condition. Anxiety-potentiated startle is the difference score between startle magnitude in the absence of the cue in the unpredictable condition minus startle magnitude in the absence of the cue in the no shock condition. Fear- and anxiety-potentiated startle are operational definitions of fear and anxiety, respectively. The error bars represent s.e.m.
Fear and anxiety are ubiquitous. They are generally adaptive responses, but can also be maladaptive in psychiatric and medical disorders. The NPU-test procedure can be used to identify the nature of underlying abnormalities in fear and anxiety. Future applications of the NPU-threat test include a wide range of possibilities, which might help to elucidate the differentiation between fear and anxiety as well as mechanisms underlying these states. Some possible applications are, for example, screening of candidate anxiolytics, discriminating among individuals with mood and anxiety disorders, examining risk factors for these conditions, conducting developmental studies in children and adolescents—including studies during puberty—and neuroimaging studies (e.g., magnetoencephalography) to elucidate the mechanisms underlying fear and anxiety.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NIMH (MH002798 and MH002804-09).
Footnotes
Note: Supplementary information is available via the HTML version of this article.
AUTHOR CONTRIBUTIONS C.G. developed the NPU-threat test and edited the protocol. A.S. drafted the manuscript and contributed to paradigm modifications.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Sarbin T. Anxiety: reification of metaphor. Arch. Gen. Psychiat. 1964;10:630–638. doi: 10.1001/archpsyc.1964.01720240084009. [DOI] [PubMed] [Google Scholar]
- 2.McReynolds WT. Anxiety as fear: a behavioral approach to one emotion. In: Zuckerman M, Spielberger CD, editors. Emotions and Anxiety: New Concepts, Methods, and Applications. Lawrence Erlbaum: 1976. [Google Scholar]
- 3.Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am. Psychol. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- 4.Rachman ST. Anxiety. Psychology Press; 2004. [Google Scholar]
- 5.Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J. Abnorm. Psychol. 1998;107:179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- 6.Zinbarg RE, Barlow DH. Structure of anxiety and the anxiety disorders: a hierarchical model. J. Abnorm. Psychol. 1996;105:181–193. doi: 10.1037//0021-843x.105.2.181. [DOI] [PubMed] [Google Scholar]
- 7.Cox BJ, Clara IP, Enns MW. Posttraumatic stress disorder and the structure of common mental disorders. Depress. Anxiety. 2002;15:168–171. doi: 10.1002/da.10052. [DOI] [PubMed] [Google Scholar]
- 8.Krueger RF. The structure of common mental disorders. Arch. Gen. Psychiat. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard RJ, Yudko EB, John Rodgers R, Caroline Blanchard D. Defense system psychopharmacology: an ethological approach to the pharmacology of fear and anxiety. Behav. Brain Res. 1993;58:155–165. doi: 10.1016/0166-4328(93)90100-5. [DOI] [PubMed] [Google Scholar]
- 10.Miles L, Davis M, Walker D. Phasic and sustained fear are pharmacologically dissociable in rats. Neuropsychopharmacology. 2011;36:1563–1574. doi: 10.1038/npp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 12.Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav. Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- 13.Luyten L, Vansteenwegen D, van Kuyck K, Gabriels L, Nuttin B. Contextual conditioning in rats as an animal model for generalized anxiety disorder. Cogn. Affect. Behav. Neurosci. 2011;11:228–244. doi: 10.3758/s13415-011-0021-6. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan GM, et al. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav. Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 16.Grillon C, et al. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol. Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Grillon C, et al. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am. J. Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillon C, Chavis C, Covington MF, Pine DS. Two-week treatment with the selective serotonin reuptake inhibitor citalopram reduces contextual anxiety but not cued fear in healthy volunteers: a fear-potentiated startle study. Neuropsychopharmacology. 2009;34:964–971. doi: 10.1038/npp.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grillon C, et al. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol. Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz A, et al. Measuring anxious responses to predictable and unpredictable threat in children and adolescents. J. Exp. Child Psychol. 2011;110:159–170. doi: 10.1016/j.jecp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. J. Abnorm. Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grillon C, et al. Acute hydrocortisone treatment increases anxiety but not fear in healthy volunteers: a fear-potentiated startle study. Biol. Psychiatry. 2011;69:549–555. doi: 10.1016/j.biopsych.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landis C, Hunt W. The Startle Pattern. Farrar & Rinehart; 1939. [Google Scholar]
- 25.Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? Int. J. Psychophysiol. 2011;81:107–115. doi: 10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hefner KR, Curtin JJ. Alcohol stress response dampening: selective reduction of anxiety in the face of uncertain threat. J. Psychopharmacol. 2012;26:232–244. doi: 10.1177/0269881111416691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shankman SA, Robison-Andrew EJ, Nelson BD, Altman SE, Campbell ML. Effects of predictability of shock timing and intensity on aversive responses. Int. J. Psychophysiol. 2011;80:112–118. doi: 10.1016/j.ijpsycho.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biol. Psychiatry. 2006;60:752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 29.Vansteenwegen D, Iberico C, Vervliet B, Marescau V, Hermans D. Contextual fear induced by unpredictability in a human fear conditioning preparation is related to the chronic expectation of a threatening US. Biol. Psychol. 2008;77:39–46. doi: 10.1016/j.biopsycho.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Curtin JJ, Lozano DL, Allen JJB. The psychophysiological laboratory. In: Coan JA, Allen JJB, editors. Handbook of Emotion Elicitation and Assessment. New York: Oxford University Press; 2007. pp. 398–425. [Google Scholar]
- 31.Blumenthal T, Hackley S, van Boxtel A, Filion D, Cuthbert B. Guidelines for human startle response measurement. Psychophysiology. 2003;40:S12–S1S. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 32.Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: neuroadaptation in human addiction. Biol. Psychiatry. 2010;68:719–725. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- 34.Odling-Smee FJ. Background stimuli and the inter-stimulus interval during Pavlovian conditioning. Q. J. Exp. Psychol. 1975;27:387–392. doi: 10.1080/14640747508400498. [DOI] [PubMed] [Google Scholar]
- 35.Marlin NA. Contextual associations in trace conditioning. Anim. Learn. Behav. 1981;9:519–523. [Google Scholar]
- 36.Grillon C. Greater sustained anxiety but not phasic fear in women compared to men. Emotion. 2008;8:410–413. doi: 10.1037/1528-3542.8.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.