Abstract
Cues that predict food can stimulate appetite and feeding independent of physiological hunger. How long such effects might last is currently unknown. Here we began to characterize long-term effects in a rodent model of cue-potentiated feeding. Rats were conditioned to associate a tone with food pellets distinct from their regular laboratory chow, and then were tested along with controls for food consumption following tone presentations. In Experiment 1, rats were tested under sated or food-deprived conditions to determine whether fasting would augment cue-driven feeding. Rats in the control group regulated intake based on physiological state, while conditioned rats consumed similar large amounts of food regardless. Experiment 2 tested the durability of cue-potentiated feeding to repeated testing in sated rats. We observed robust cue-potentiated feeding during the first two tests, while in the third and fourth tests both groups ate similar large amounts of pellets. In both experiments the conditioned tone-cue induced binge-like consumption of the cued food and persistent feeding for the duration of 4-hour tests. Rats then failed to adjust daily chow consumption to account for their increased intake post-cue. In summary, brief cue priming stimulated substantial intake in sated states that was behaviorally uncompensated for by homeostatic mechanisms.
Keywords: Appetite, Conditioning, Learning, Obesity, Obesogenic Environment, Overeating, Palatable food, Priming
Introduction
Food associated cues can stimulate feeding independent of physiological hunger in animals and humans. In experimental settings both discrete cues, such as a tone previously paired with food, and contextual cues, such as a feeding environment, have been shown to potentiate feeding in sated states (Holland, Petrovich, & Gallagher, 2002; Petrovich, Ross, Gallagher, & Holland, 2007; Weingarten, 1983). In these settings food cues drive consumption of the signaled food specifically and selectively, and in humans this is accompanied with a greater reported desire for that food (Delamater & Holland, 2008; Fedoroff, Polivy, & Herman, 1997; Ferriday & Brunstrom, 2008; Galarce, Crombag, & Holland, 2007; Petrovich, Ross, Gallagher, et al., 2007; Petrovich, Ross, Holland, & Gallagher, 2007). However, in some settings if the signaled food is absent, learned cues have also been shown to increase daily consumption of the available food option (Boggiano, Dorsey, Thomas, & Murdaugh, 2009). These circumstances enable an organism to consume a large meal when not hungry (Petrovich, Ross, Gallagher, et al., 2007; Petrovich, Ross, Holland, et al., 2007; Weingarten, 1984).
How long such appetite and feeding might persist is unknown. Non-homeostatic driven feeding is becoming an increasingly important contributor to the regulation of caloric intake and body weight, as overeating and associated obesity are becoming more prevalent in the developed world (World Health Organization, 2011). Indeed, eating dysregulation (e.g., overeating) is believed to drive the obesity epidemic to a much greater extent than metabolic deficiencies (Berthoud, 2011; Hill, Wyatt, Reed, & Peters, 2003; Kessler, 2009; Small, 2009; Volkow & Wise, 2005). To begin to characterize possible long-term effects of cue-potentiated feeding here we tested rats during an extended period following priming with a food-cue. We used a novel behavioral preparation designed to allow for long duration testing with minimal disruptions.
In prior work, cue-potentiated feeding tests were typically carried out in behavioral chambers without access to water (for review see: Holland & Petrovich, 2005; Petrovich, 2011). In the current study all tests were conducted in rats’ home cages with unlimited water access. Thus, this preparation allowed us to monitor intake over extended periods and with minimal disturbance to the rats. Additionally, conducting tests in the home cage enabled us to isolate the effect of the discrete food-cue from any potential conditioned effects of the training context (see Discussion), which alone can influence food intake (Boggiano, et al., 2009; Bouton, 2011; Le Merrer & Stephens, 2006; Petrovich, Ross, Gallagher, et al., 2007; Petrovich, Ross, Holland, et al., 2007).
We trained rats to associate a tone with food pellets that are distinct from their regular laboratory chow. Rats in the conditioned group were repeatedly presented with a tone (conditioned stimulus, CS) immediately prior to food pellet delivery (unconditioned stimulus, US). Rats in the control group were given the same number of tones and food presentations, but randomly arranged. After training we tested rats for food consumption under the influence of the CS.
Food consumption tests consisted of ten CS presentations immediately followed by ad libitum access to the training food pellets and standard laboratory chow. The tests were four hours long, and we additionally monitored post-test daily chow intake. In Experiment 1 we varied physiological hunger state during testing to determine whether fasting would augment cue driven feeding and extend the duration of the effect. Thus, we tested each rat under sated and fasted conditions in a counterbalanced manner. In Experiment 2 we tested sated rats repeatedly to determine the durability of the cue-potentiated feeding effect to repeated testing.
Experiment 1: The Effect of Physiological Hunger State on Cue-Potentiated Feeding
Materials & Methods
Subjects
Sixteen experimentally naïve, male Long–Evans rats approximately 2 months of age (Charles River Laboratories; Raleigh, NC), were individually housed, and maintained on a 12 h light/dark cycle (lights on at 6:00). All training and testing was conducted during the light phase, approximately between 9:00 and 14:00. Upon arrival, subjects were allowed one week to acclimate to the colony room, during which time they had ad libitum access to standard laboratory chow (18% Protein Rodent Diet #2018, Harlan Teklad Global Diets; Madison, WI; 3.1 kcal/g; 20% protein, 18% fat, 58% carbohydrate) and water and were handled daily. All housing and testing procedures were in compliance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals, and approved by the Johns Hopkins University Institutional Animal Care and Use Committee.
Apparatus
Behavioral training was conducted in a set of four identical chambers (30 × 24 × 30 cm; Colbourn Instruments; Allentown, PA), with aluminum top and sides, a transparent Plexiglas back and front, and a grid floor. Each chamber also contained a recessed food cup (3.2 × 4.2 cm). Dim background illumination was provided by two 25 W red bulbs, each placed 1.5 m from the chambers. Masking noise (60 dB) was provided by ventilation fans located outside the conditioning chambers. A tone (1.5 kHz, 75 dB), served as the CS, and 45 mg food pellets (5TUL; Test Diets; Richmond, Indiana; 3.4 kcal/g; 20% protein, 13% fat, 67% carbohydrate) were used as the US. These food pellets have a similar caloric density and macronutrient energy composition to standard laboratory chow, with the exception that the carbohydrates are from starch in the chow and sucrose in the pellets. Video cameras attached to videocassette recorders were placed in the back of the test chambers to record behavior for 10 second periods both before and during stimulus presentation. Stimulus presentation and videocassette recorders were controlled by LabView software (National Instruments; Austin, TX) run on Macintosh computers (Apple Computers; Cupertino, CA).
Behavioral Training Procedure
Before behavioral training, rats were gradually reduced to 85% of their ad libitum weight. After a shaping procedure in which rats learned to eat from the food cup, rats received 10 training sessions (one session per day, excluding weekends) each approximately 32 minutes in length. For half of the rats (conditioned group, Paired), these sessions consisted of eight presentations of the CS, a 10 second tone, immediately followed by delivery of the US, two food pellets, into the food cup. For the other half of the rats (control group, Unpaired), the sessions consisted of the same number of tone and food presentations as the Paired group, but delivered in a non-conditional random order. After the last training session, rats were given ad libitum access to standard laboratory chow for 8–10 days to allow them to reach at least 110% of their pre-training body weight. During this time, rats were habituated to a new testing room and to glass dishes (107 × 87 × 70 mm) that would be used for food presentation during testing.
Rats completed two consumption tests each, which occurred three days apart in a counterbalanced design. For one of the tests, rats were deprived of food for 24-hours prior to testing (“food-deprived condition”) and for the other test rats remained under ad libitum access to standard laboratory chow (“sated condition”). For each test rats were transported to the testing room, and for the sated condition all chow was removed from the cage just prior to transport. Rats remained in their home cages and were given 10 presentations of the CS (10 second tone) over 5 minutes. Rats were then immediately given 20g of chow in one glass dish and 20g of food pellets in a second identical glass dish, and returned to the colony room. After 30 minutes all uneaten chow and pellets were removed and replaced with fresh chow (20g) and food pellets (20g). This process was repeated at 1 hour and 2 hours after the tone test. At the 4-hour time point pellets were removed and only chow was replaced (100g); chow consumption 20 hours later was measured in order to calculate 24 hour post-test chow consumption. For all time points remaining chow and food pellets were weighed and the amount consumed, during the interval as well as cumulative total, was calculated.
Rats were trained in two replications (n=4 per condition for each) that were identical except that the period from the end of training and start of testing was 8 days in the first replication, and 10 days in the second replication.
Behavioral Observations
To confirm that the rats in the Paired group had learned the tone-food association, conditioning was assessed during the last training session (S10). The expression of “food cup behavior” was the primary measure of conditioning, the conditioned response (CR). Food cup behavior included nose pokes into the recessed food cup, and standing in front of and facing the food cup. Observations were made every 1.25 seconds and were paced by auditory signals recorded onto the tapes. Observers were “blind” with respect to the training group of the rats observed. At each observation, only one behavior was recorded (food cup or other). Food cup behavior during the 10 second tone (CS) and for the 10 seconds immediately preceding the CS (Pre-CS) was scored. The percentage of time rats spent expressing food cup behavior during these two periods was calculated by dividing the number of positive observations of food cup behavior by the total number of observations made.
Statistics
Behavioral data were analyzed using appropriate ANOVAs and t-tests in SPSS. In all cases, p<0.05 was considered significant.
Results
Training
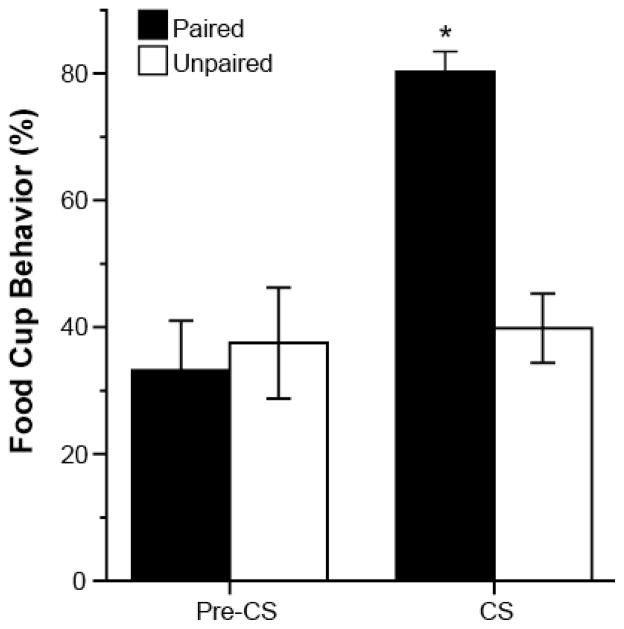
Learning was assessed during the last training session [Figure 1]. Conditioning of the rats in the Paired group was clearly evident from observations of the conditioned responses (CRs) directed toward the food cup during the presentation of the CS (expressed as percentage of total time during observed periods; see Methods). Repeated measures ANOVA showed a significant effect of Pre-CS versus CS time period (F(1,14)=6.81, p<0.001), a significant effect of training group (F(1,14)=18.44, p<0.001), and a significant time period by test group interaction (F(1,14)=23.15, p<0.001).
Figure 1.
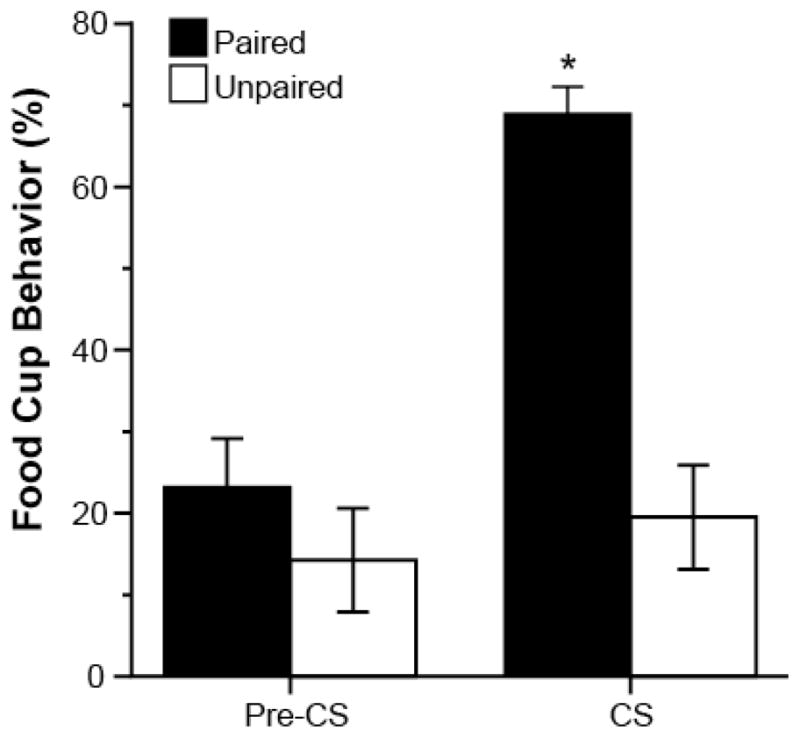
Mean (± SEM) percent of the total time rats expressed food cup behavior in the Pre-CS and CS periods during the last training session in Experiment 1; *p<0.05.
Post-hoc t-Tests confirmed that rats in the Paired group expressed significantly higher levels of CRs during the tone (CS) compared to the behavior of the rats in the Unpaired condition (t(14)=6.85, p<0.001), and compared to their own behavior during the Pre-CS period (t(7)=5.89, p=0.001). The CRs of the rats in the Unpaired condition were low during both periods and comparable to the CRs of the Paired group during the Pre-CS period (p>0.05). Thus, only the rats in the Paired condition learned the tone-food association.
Food Consumption Tests
Food consumption tests for tone-cue potentiated feeding were conducted twice for each rat; one test under sated and the other under food-deprived conditions, and test order was counterbalanced across training groups. For each test rats were primed with CSs in the home cage and then immediately given food pellets and chow; consumption following tone presentation was monitored for 4 hours.
Test under sated conditions
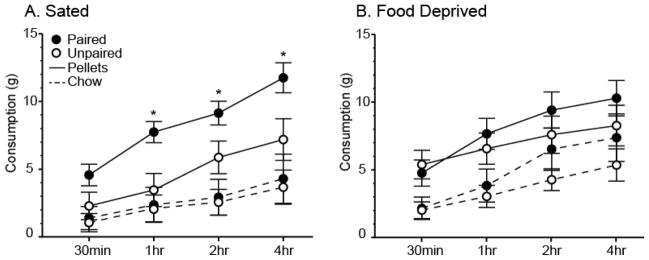
During the sated consumption test rats in the Paired group ate significantly more food pellets than rats in the Unpaired group [Figure 2A; Table 1]. Rats in the Paired group consumed food pellets at a faster rate compared to rats in the Unpaired group during the first hour following CS presentation, and maintained the enhancement in food pellet consumption for the duration of the 4-hour test by continuing to consume at the rate similar to the Unpaired group. During the first hour rats in the Paired group ate more pellets than the rats in the Unpaired group, and the difference in consumption between the two groups was near significance for the 0–30min interval (t(14)>1.77, p<0.10), and statistically significant for the 30min–1hr interval (t(14)=2.21, p<0.05). This led to a difference in cumulative pellet intake between the Paired and Unpaired groups that was statistically significant at the 1hr time point and every time point measured thereafter (t(14)>2.20, p<0.05, all).
Figure 2.
Mean (± SEM) cumulative consumption in grams of food pellets (solid lines) and chow (dashed lines) for rats in the Paired (filled circles) and Unpaired (open circles) groups when tested under sated (A) and food-deprived (B) conditions in Experiment 1. *Indicates a significant difference (p<0.05) in pellet consumption between groups.
Table 1.
Experiment 1 consumption; data is presented as mean ± SEM grams consumed during each of the measured intervals.
| Interval | Group | Sated | Food-Deprived | ||
|---|---|---|---|---|---|
|
| |||||
| Pellets (g) | Chow (g) | Pellets (g) | Chow (g) | ||
| 0–30min | P | 4.57 ± 0.80b | 1.43 ± 0.88b | 4.81 ± 0.97 | 2.17 ± 0.84 |
| U | 2.28 ± 1.01 | 1.08 ± 0.70c | 5.44 ± 1.06 | 2.04 ± 0.62c | |
|
| |||||
| 30min–1hr | P | 3.17 ± 0.71a | 1.00 ± 0.55 | 2.92 ± 0.56a | 1.70 ± 0.65 |
| U | 1.18 0.56a | 1.08 ± 0.58 | 1.12 ± 0.36a | 1.03 ± 0.38 | |
|
| |||||
| 1hr–2hr | P | 1.39 ± 0.40 | 0.58 ±0.49 | 1.77 ± 0.57 | 2.72 ± 1.00 |
| U | 2.40 ± 1.08 | 0.47 ± 0.24 | 1.03 ± 0.37 | 1.25 ± 0.61 | |
|
| |||||
| 2hr–4hr | P | 2.61 ± 0.53 | 1.41 ± 0.61 | 0.89 ± 0.47 | 0.86 ± 0.40 |
| U | 1.32 ± 0.46 | 1.14 ± 0.58 | 0.68 ± 0.26 | 1.01 ± 0.66 | |
|
| |||||
| 4–24hr | P | - | 19.73 ± 1.21 | - | 20.70 ± 2.15 |
| U | - | 22.24 ± 1.61 | - | 23.58 ± 2.82 | |
paired vs. unpaired, p<0.05
pellets vs. chow, p<0.05
sated vs. food-deprived, p<0.05
There were no differences in chow consumption between the groups at any time point under sated conditions (p>0.05, all). Rats in the Paired group showed a strong preference for the pellets, consuming significantly more pellets than chow during the 0–30min and 30min–1hr intervals (t(7)=2.90, p<0.05, both), and cumulatively at every time point throughout the 4-hour test (t(7)>2.90, p<0.05, all). Pellets were preferred by rats in the Unpaired group as well; although they did not eat significantly more pellets than chow during any specific interval (p>0.05, all), they ate cumulatively more pellets than chow by the 2hr time point (t(7)=2.76, p<0.05), and this effect remained significant at the 4hr time point (t(7)=3.08, p<0.05).
Finally, 24-hour chow consumption following the test was similar for the two groups. Rats in the Paired group ate slightly less chow than the rats in the Unpaired group (P: 24.14 ± 2.25g, U: 26.01 ± 1.61g), but this effect was not significant (p<0.05). Thus, the rats in the Paired group did not sufficiently compensate for their increased pellet intake during the test by restricting their daily chow intake.
Test under food-deprived conditions
In contrast to the test under sated conditions, rats in both groups consumed similar amounts of food pellets and chow during the 4-hour consumption test and in daily chow intake when tested under acute food-deprived conditions [Figure 2B; Table 1]. The only exception was in food pellet consumption during the 30min–1hr interval where rats in the Paired group consumed significantly more pellets than rats in the Unpaired group (t(7)=6.85, p<0.05).
Rats in both groups consumed more pellets than chow throughout the duration of the test; however, this difference was not statistically significant in the Paired group at any time point during the test (p>0.05 all). The difference between pellet and chow consumption was significant in the Unpaired group during the first 30 minutes (t(7)=3.15, p<0.05), and this led to a difference in cumulative intake which was maintained through the second hour of the test (t(7)=3.01, p<0.05).
Comparison of consumption between sated and food-deprived conditions
During the first 30 minutes, rats in the Unpaired group consumed about twice as much pellets and chow when tested under the acutely food-deprived condition compared to their consumption under the sated condition. This increase was statistically significant for chow (t(7)=2.45, p<0.05) and nearly significant for pellets (t(7)=2.05, p<0.10). On the other hand, rats in the Paired group consumed similar large amount of pellets regardless of whether they were being tested under sated or under acutely food-deprived conditions (p>0.05). Rats in the Paired group consumed more chow during tests under the food-deprived compared to the sated condition, however the increase was not statistically reliable and only reached near significance at 1–2hr interval and 2hr cumulative intake time point (t(7)>1.98, p<0.10, both). Thus, only the rats in the control group appeared to have regulated their intake according to their physiological state, while the CS drove large consumption for rats in the Paired group regardless of the physiological state.
Body Weights
Rats in the Paired and Unpaired groups had similar body weights prior to the beginning of training (P: 340 ± 5 g, U: 341 ± 5 g), while maintained at 85% body weight during training (P: 286 ± 4 g, U: 288 ± 4 g), and following satiation prior to testing (P: 382 ± 8 g, U: 376 ± 9 g). Independent samples t-Tests confirmed there were no significant differences in body weight between groups at any time point (p>0.05, all).
Discussion
The goal of this first experiment was twofold. The first aim was to characterize when effects of a food-cue on feeding occur during an extended testing period; to identify whether the cue stimulates feeding immediately following cue presentation and then ceases, or whether feeding persists for hours following food-cue presentation. The second aim was to examine how the underlying physiological hunger state might interact with cue-driven feeding and whether fasting prior to test might further enhance cue-potentiated feeding.
We found that cue-potentiated feeding was specific to the cued food (pellets) and occurred during the first hour following food-cue presentation. The enhancement in consumption was maintained throughout the 4-hour test. Therefore, brief food-cue priming resulted in persistent feeding during the test. Conditioned rats continued to eat similar amounts of chow as control rats during the 24hrs post-test, and thus at least during that period did not sufficiently compensate for the increased consumption following cue presentation.
We found robust cue-potentiated feeding when conditioned rats were tested under the sated state. Interestingly, when tested under acute food deprivation consumption was similar between the conditioned and control groups. This was driven by greater consumption by rats in the control condition in the food-deprived state compared to their consumption when sated. Rats in the conditioned group ate similar large amounts of food, specifically food pellets, regardless of their physiological state prior to testing. Thus, it appears that the cue increased feeding in the conditioned rats up to the level that control rats consume under food deprivation. Therefore, additional enhancement during food-deprived conditions might not have been possible under these circumstances. Consequently, our data suggest that only the rats in the control group regulated their intake according to physiological state, while the cue drove substantial consumption for rats in the conditioned group regardless of their physiological state.
All rats preferred food pellets to chow, however priming rats with the cue for pellets further enhanced pellet consumption, and appeared to have changed the strength of the preference and the time at which it emerged. During the first two measured intervals, rats in the conditioned group showed a strong preference for pellets by consuming significantly more pellets than chow. In contrast, rats in the Unpaired group failed to eat significantly more pellets than chow during any measured interval. This immediate preference for pellets by the conditioned group led to significant increased cumulative intake of pellets compared to chow throughout the duration of the test, while rats in the control group did not show a significantly increased cumulative pellet intake until two hours into the test.
It is notable that during the test under acute deprivation all rats consumed substantial amounts of food; rats consumed roughly 30% of their total daily intake in the first hour, and nearly half of their total daily intake by the end of the 4-hour test. We hypothesize that a limit in the amount rats can consume in this setting (“ceiling effect”) is likely why we did not observe food-cue enhancement of eating under acute food deprivation (Bull & Pitts, 1971). Indeed, using different training paradigms prior work has shown that learned cues can contribute to the intake regulation under food deprivation.
Work by Zamble demonstrated that rats maintained on a severely restricted feeding regimen can be trained to rely on cues to eat and in this setting the learned cues worked in concert with physiological signals to regulate feeding (Zamble, 1973). But there are a couple important differences to consider when comparing this result with our findings. While prior work examined only chow consumption, our rats were trained with palatable food pellets (high sucrose content) and had access to both pellets and chow during the food consumption tests. Sweet tastes are innately liked across species as indicated by stereotypical facial expressions (Berridge, 2000). Sweet, calorically-dense foods are typically considered palatable by humans (Pliner & Mann, 2004), and preferred in animal studies (Pecoraro, Reyes, Gomez, Bhargava, & Dallman, 2004; Teegarden & Bale, 2007) as measured by amount consumed compared to that of standard laboratory chow. Additionally, animals have been shown to endure aversive consequences (Oswald, Murdaugh, King, & Boggiano, 2011; Teegarden & Bale, 2007) and work for palatable food pellets while ignoring chow that is freely available (Salamone, et al., 1991). Shifts in food preference have been shown to occur under food deprivation in both human and animal studies (Hoefling & Strack, 2010; Sclafani & Ackroff, 1993), so it is possible that differences in relative consumption may be augmented by physiological hunger state.
Moreover, rats in Zamble’s study were maintained on a 30min/day meal-fed regimen for 25 days during which time substantial weight loss occurred (Zamble, 1973). In that preparation rats learned that the cues predicted the only time food would be available. It contrast, our paradigm used much milder food deprivation procedures during both training and at test, and additionally our rats were allowed ad libitum food access for 8–10 days to regain body weight prior to testing. Under this milder deprivation, the cue failed to augment intake further than was seen under sated conditions.
Another example, Weingarten, also used a meal-fed conditioning paradigm of cue-enhanced feeding; multiple small meals per day were given by pairing presentations of discrete cues with the availability of a palatable (sweet) liquid diet based on an enriched evaporated milk mixture containing ~20% sucrose. During later testing under sated conditions, presentations of the cues elicited rapid and robust feeding in conditioned rats. Similar to our results, rats consumed a significant portion of their total daily intake (~20%) in a brief time period following cue presentation. But contrary to our findings, Weingarten showed that rats would compensate for the initial cue-driven bout of feeding and that total 24-hour intake was similar on days with cues and days without cues (Weingarten, 1983, 1984). The important difference between the two preparations is the availability of food choice. In Weingarten’s study, rats only had access to one food during training and the cue became a signal for the opportunity to feed; the cue was thus integrated with homeostatic regulation and it influenced meal pattern. In our study, the cue was conditioned during training to signal the arrival of an additional food choice; a food choice that the current experiment as well as our prior work has shown is preferred to regular lab chow (Reppucci, 2010).
At test, rats in Weingarten’s study had ad libitum access to the palatable liquid training food (sweetened milk) for 24-hours, and reduced intake following rapid post-cue consumption so that their total daily intake was similar to the amounts consumed on non-cued days. Whether this compensation was due solely to actions of homeostatic regulatory mechanisms or some other cause, such as sensory-specific satiety (Rolls, 1986), is unclear. In contrast, at test we provided rats with standard lab chow in addition to the cued pellets and found that all rats consumed similar amounts of lab chow during the 24 hours post-cue presentation. In this case, conditioned rats did not decrease chow intake during the 20 hours after pellet availability ended, which suggests a lack of compensation for the increased bout of food pellet consumption following the cue.
There are two noteworthy implications from our experiment. First, brief food-cue presentation can induce specific persistent feeding of the cued food in sated rats that lasts for hours. The food-cue appears to act as a primer that jump-starts a feeding bout by eliciting rapid and substantial consumption during the first hour following cue presentation. After this period of robust feeding, the rate of consumption decreases but feeding does not cease. Instead, feeding persists for the next three hours at a rate similar to that of the rats in the control group. The second implication is that cue-driven feeding is somewhat independent of physiological control; rats in the conditioned group ate similar, large amounts of food regardless of whether they were food-deprived or sated prior to testing. Furthermore, the amount of food consumed under the influence of the learned food-cue in the sated condition reached the same high level of consumption seen in the control rats under acute food deprivation.
In this experiment we examined the ability of the conditioned food-cue to potentiate feeding during two tests, which differed in physiological state. In Experiment 2 we examined the durability of the food-cue’s effect on feeding to repeated testing. We used the same conditioning protocol as in Experiment 1 and then tested sated rats in four consumption tests.
Experiment 2: Durability of Cue-Potentiated Feeding to Repeated Testing
Materials & Methods
Subjects
Sixteen experimentally naïve, male Long–Evans rats approximately 2 months of age (Charles River Laboratories; Raleigh, NC), were individually housed, and maintained on a 12 h light/dark cycle (lights on at 6:00). All training and testing was conducted during the light phase, approximately between 10:00 and 15:00. Upon arrival, subjects were allowed one week to acclimate to the colony room, during which time they had ad libitum access to standard laboratory chow (LabDiet 5P00, Prolab RMH 3000; Saint Louis, MO; 3.2 kcal/g; 26% protein, 14% fat, 60% carbohydrate) and water and were handled daily. All housing and testing procedures were in compliance with the National Institutes of Health Guidelines for Care and Use of Laboratory Animals, and approved by the Boston College Institutional Animal Care and Use Committee.
Apparatus
The behavioral training was conducted in a set of eight identical chambers (30 × 28 × 30 cm; Coulbourn Instruments; Allentown, PA) located in a behavioral testing room that was different from the colony housing room. The chambers had aluminum top and sides, a transparent Plexiglas back and front, and a black Plexiglas panel placed on top of the grid floor. Each chamber contained a recessed food cup (3.2 × 4.2 cm), and a 4 W “house light” that was illuminated during training sessions. Each chamber was enclosed within an isolation cubicle (79 × 53 × 53 cm; Coulbourn Instruments; Allentown, PA) composed of monolithic rigid foam walls, which isolate from ambient sound and light. A ventilation fan, located on the back of each isolation cubicle, provided masking noise (55 dB). A tone (2 kHz, 75 dB), served as the conditioned stimulus (CS), and 45 mg food pellets (5TUL; Test Diets; Richmond, Indiana) were used as the unconditioned stimulus (US). Stimulus presentation was controlled by GraphicState 3.0 software (Coulbourn Instruments; Allentown, PA). Video cameras controlled by Digital Video Security System Digital Video Recorder software program (Coulbourn Instruments; Allentown, PA) were mounted on the back of the isolation cubicle and recorded behavior during training and testing.
Behavioral Training Procedure
Behavioral training and testing were identical to the procedures described for the Experiment 1 except for the following. After training, rats had ad libitum access to chow for 15 days prior to testing, and rats were given four consumption tests under sated conditions (ad libitum access to standard laboratory chow) over the course of the following two weeks. Food pellets were given in glass dishes as in Experiment 1, while chow was presented in the wire cage top. As in Experiment 1, during Test 1 all uneaten chow and food pellets were removed and replaced with fresh at 30 minutes, 1 hour, and 2 hours following the tone test; at 4 hours pellets were removed and only chow was replaced. For Tests 2–4 chow and food pellets were replaced after 1 hour and at 4 hours only chow was given.
Behavioral Observations
Food cup behavior during the last training session (S10) was assessed as described in Experiment 1. Observations were made every 1.25 seconds and were paced by a metronome during behavioral scoring.
Statistics
Behavioral data were analyzed using appropriate ANOVAs and t-Tests in SPSS. In all cases, p<0.05 was considered significant.
Results
Training
As in Experiment 1, at the completion of training the percentage of time rats spent exhibiting conditioned responses (CRs) directed toward the food cup was used as a measure of learning. As expected, rats in the Paired groups showed strong evidence of learning the tone-food association [Figure 3]. Repeated measures ANOVA showed a significant effect Pre-CS versus CS time period (F(1,14)=34.26, p<0.001), a nearly significant effect of training group (F(1,14)=4.6, p=0.05), and a significant time period by test group interaction (F(1,14)=28.07, p<0.001). Post-hoc t-Tests confirmed that rats in the Paired group showed significantly elevated CRs during the presentation of the tone (CS) compared to rats in the Unpaired group (t(14)>6.41, p<0.001), and compared to their own behavior during the time period immediately preceding the onset of the tone (t(14)>6.54, p<0.001), which confirms they learned the tone-food association. Rats in the Unpaired group showed similar low CRs during the Pre-CS and CS time periods (p>0.05).
Figure 3.
Mean (± SEM) percent of the total time rats expressed food cup behavior in the Pre-CS and CS periods during the last training session in Experiment 2; *p<0.05.
Food Consumption Tests
After training, which was conducted in a food-restricted state (rats maintained at 85% of initial body weight), rats were allowed ad libitum access to standard laboratory chow for 15 days. Four food consumption tests were then performed in the sated condition over the course of the following two weeks to test the durability of tone-cue potentiated eating following repeated testing.
Analysis of consumption within tests
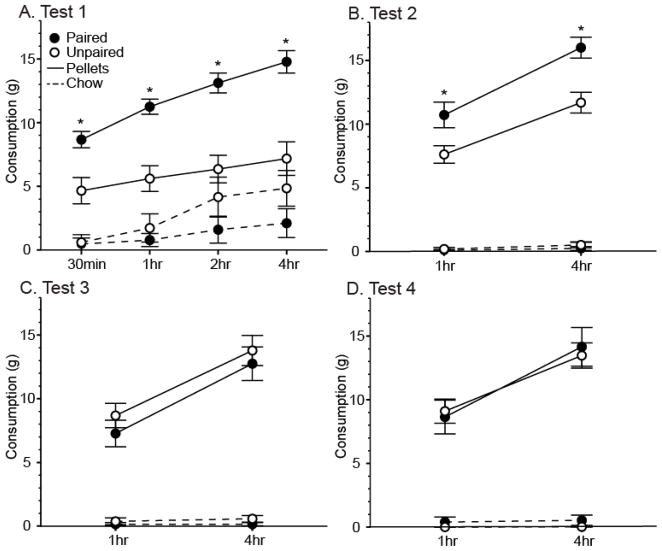
Test 1 replicated the findings from the test in the sated condition in Experiment 1; rats in the Paired group ate significantly more food pellets than rats in the Unpaired group during the 4-hour consumption test [Figure 4A; Table 2]. The difference in consumption between the rats in the Paired and Unpaired groups was statistically significant after the first 30 minutes (t(14)=3.29, p<0.05), and this difference was maintained at every cumulative time point measured thereafter (t(14)>4.84, p<0.05 all). This effect was driven by Paired rats eating significantly more pellets than Unpaired rats in the 0–30min, 30min–1hr, and 1hr–2hr intervals (t(14)>2.36, p<0.05, all). There were no differences in chow consumption between groups at any time point during Test 1 (p>0.05, all).
Figure 4.
Mean (± SEM) cumulative consumption in grams of food pellets (solid lines) and chow (dashed lines) for rats in the Paired (filled circles) and Unpaired (open circles) groups during each of the four consumption tests over the course of two weeks during Experiment 2. *Indicates a significant difference (p<0.05) in pellet consumption between groups.
Table 2.
Experiment 2 (Test 1) consumption; data is presented as mean ± SEM grams consumed during each of the measured intervals.
| Interval | Group | Test 1
|
|
|---|---|---|---|
| Pellets (g) | Chow (g) | ||
|
| |||
| 0–30min | P | 8.66 ± 0.65ab | 0.47 ± 0.47b |
| U | 4.66 ± 1.03ab | 0.60 ± 0.60b | |
|
| |||
| 30min–1hr | P | 2.59 ± 0.24ab | 0.31 ± 0.28b |
| U | 0.95 ± 0.29a | 1.12 ± 0.59 | |
|
| |||
| 1hr–2hr | P | 1.87 ± 0.42a | 0.82 ± 0.58 |
| U | 0.75 ± 0.22a | 2.43 ± 0.92 | |
|
| |||
| 2hr–4hr | P | 1.66 ± 0.34b | 0.51 ± 0.51b |
| U | 0.82 ± 0.41 | 0.69 ± 0.41 | |
|
| |||
| 4–24hr | P | - | 17.81 ± 2.4 |
| U | - | 15.52 ± 1.43 | |
paired vs. unpaired: p<0.05
pellets vs. chow: p<0.05
Both groups preferred pellets to chow during Test 1. Rats in the Paired group consumed significantly more pellets than chow during the 0–30min, 30min–1hr, and 2hr–4hr intervals (t(7)>2.79, p<0.05, all), and at every cumulative time point throughout the 4-hour test (t(7)>7.85, p<0.001, all). Rats in the Unpaired group ate significantly more pellets than chow only during the first 30 minutes (t(7)=3.53, p<0.05). As in Experiment 1, we found that rats in both groups consumed similar amounts of chow during every time point (p>0.05, all), including during the 24 hours following the test (P: 19.92 ± 2.58g, U: 20.37 ± 1.91g). Thus, rats in the Paired group did not compensate for their increased food intake during the test by restricting their subsequent daily chow intake.
Tone-cue potentiated eating persisted in Test 2 [Figure 4B; Table 3], where rats in the Paired group again ate significantly more food pellets than rats in the Unpaired group by the end of the 4-hour test (t(14)=3.72, p<0.05). This effect was driven by rats in the Paired group eating significantly more pellets than rats in the Unpaired group during the first hour (t(14)=2.53, p<0.05), and this trend continued during the 1hr–4hr interval at a near significant level (t(14)=2.00, p<0.10). As in Test 1, there were no differences in chow consumption between groups at any time point (p>0.05, all). Rats in both groups showed a strong preference for pellets, consuming significantly more pellets than chow during both intervals and at the end of the test (t(7)>9.23, p<0.001, all).
Table 3.
Experiment 2 (Tests 2–4) consumption; data is presented as mean ± SEM grams consumed during each of the measured intervals.
| Interval | Group | Test 2 | Test 3 | Test 4 | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Pellets (g) | Chow (g) | Pellets (g) | Chow (g) | Pellets (g) | Chow (g) | ||
| 0–1hr | P | 10.71 ± 1.01ab | 0.08 ± 0.07b | 7.27 ± 1.05b | 0.15 ± 0.12b | 8.64 ± 1.32b | 0.40 ± 0.40b |
| U | 7.6 ± 5.97ab | 0.18 ± 0.13b | 8.68 ± 6.41b | 0.37 ± 0.27b | 9.1 ± 0.96b | 0.01 ± 0.01b | |
|
| |||||||
| 1hr–4hr | P | 5.29 ± 0.55b | 0.15 ± 0.14b | 5.48 ± 0.98b | 0.00 ± 0.00b | 5.52 ± 1.01b | 0.14 ± 0.13b |
| U | 4.07 ± 0.25b | 0.32 ± 0.14b | 5.11 ± 3.92b | 0.21 ± 0.13b | 4.37 ± 0.81b | 0.02 ± 0.01b | |
|
| |||||||
| 4–24hr | P | - | 18.97 ± 1.85 | 22.03 ± 2.21 | 18.71 ± 1.89 | ||
| U | - | 20.01 ± 1.51 | 19.51 ± 2.07 | 18.21 ± 1.23 | |||
paired vs. unpaired: p<0.05
pellets vs. chow: p<0.05
We did not observe differences in food consumption between the Paired and Unpaired groups during Test 3 or Test 4 [Figures 4C & 4D; Table 3]. Rats in both groups ate similar substantial amounts of food pellets, and similar small amounts of chow throughout each of the tests (p>0.05, all). The strong preference for pellets remained, and rats in both groups consumed significantly more pellets compared to their consumption of chow throughout both tests (t(7)>4.97, p<0.01, all).
Analysis of consumption across tests
To additionally evaluate the durability of tone-cue potentiated eating we directly compared the amount of food pellets consumed across the four tests. We chose to focus on consumption of food pellets, since chow intake was consistently low following cue presentation, and there were no significant differences in chow consumption between groups at any time.
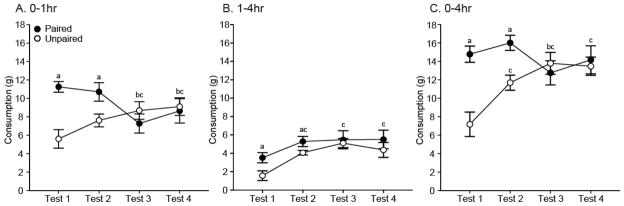
First, we examined differences in cumulative food pellet consumption for the duration of the entire test period (0–4hr) across the four tests [Figure 5C]. Rats in the Paired group consumed similar amounts of pellets across tests except for a decrease in consumption during Test 3, this was also the only test where they did not consume more pellets than rats in the Unpaired group. In contrast, rats in the Unpaired group showed a substantial increase in consumption across tests, nearly doubling their intake between Test 1 and Test 4. Statistical analyses supported these observations. A repeated measures ANOVA on 0–4hr pellet consumption showed significant within-subjects effects of Test Day (F(3,42)=5.88, p<0.01) and a Test Day by Group interaction (F(3,42)=11.80, p<0.001), as well a significant between-subjects effect of Group (F(1,14)=5.14, p<0.05). Post-hoc paired t-Tests showed that rats in the Paired group consumed similar amounts during Tests 1, 2, and 4 (p>0.05, all), but Test 3 consumption was significantly lower than during Test 1 or Test 2 (t(7)>2.52, p<0.05, both). Additional post-hoc paired t-Tests on consumption for rats in the Unpaired group showed significantly increased consumption on Tests 2, 3, and 4 compared to Test 1 (t(7)>3.73, p<0.01, all).
Figure 5.
A comparison of the mean (± SEM) consumption of food pellets (g) across the four tests during the first hour of testing (A), during the 1–4hr interval (B), and during the entire 4hr-testing period (C) in Experiment 2. aSignificant difference between groups (p<0.05); bSignificant decrease from Test 1 in Paired (p<0.05); cSignificant increase from Test 1 in Unpaired.
Next, we examined food pellet consumption during the first hour of the test, the time period where the cue modulated feeding the most reliably. Here we saw a similar pattern to 0–4hr consumption; there was an increase in pellet consumption across tests for rats in the Unpaired group, and a slight decrease in pellet consumption with repeated testing for rats in the Paired group [Figure 5A]. Again, statistical analyses supported these observations. A repeated measures ANOVA on 0–1hr food pellet consumption showed a significant Test Day by Group interaction (F(3,42)=12.05, p<0.001). Post-hoc paired t-Tests showed that rats in the Paired group ate significantly fewer pellets during Test 3 and Test 4 compared to Test 1, and significantly fewer pellets on Test 3 compared to Test 2 (t(7)>2.64, p<0.05, all). Additional paired t-Tests showed that rats in the Unpaired group ate significantly more pellets during Test 3 and Test 4 compared to Test 1 (t(7)>3.56, p<0.05, both).
Finally, we compared food pellet consumption during remainder of the testing period (1hr–4hr interval), to examine the time period temporally distant from cue presentation [Figure 5B]. Rats in the Paired group consumed slightly more than rats in the Unpaired group during this interval. Interestingly, consumption was lower during Test 1 than any other test for both groups. Following this initial increase, within-group consumption was similar across Tests 2, 3, and 4. A repeated measures ANOVA showed a significant within-subjects effect of Test Day (F(3,42)=6.87, p<0.001), and a between-subjects effect of Group (F(1,14)=4.67, p<0.05). Post-hoc paired t-Tests showed that the consumption was sustained across tests for rats in the Paired group (p>0.05, all), while rats in the Unpaired group showed significantly increased consumption during Tests 2, 3, and 4 compared to Test 1 (t(7)>2.66, p<0.05, all).
Body Weights
Rats in the Paired and Unpaired groups had similar body weights prior to the beginning of training (P: 332 ± 7 g, U: 343 ± 8 g), while maintained at 85% body weight during training (P: 281 ± 5 g, U: 291 ± 6 g), and following satiation prior to testing (P: 467 ± 12 g, U: 477 ± 9 g). Independent samples t-Tests confirmed there were no significant differences in body weight between groups at any time point (p>0.05, all).
Discussion
The goal of this experiment was to examine food-cue potentiated feeding in repeated tests to determine its durability. We found that conditioned rats exhibited robust food-cue potentiated feeding during each of the first two tests. These findings replicated the results from Experiment 1 (from the test under sated conditions) and validated our testing procedure. Rats in the control group steadily increased consumption of food pellets across tests, and as a result rats in both groups ate similar large amounts of food during the third and fourth test. Therefore, although the conditioned rats maintained high levels of consumption across all four tests, we did not observe a difference in consumption between the conditioned and control groups during the last two tests.
As in Experiment 1, all rats preferred food pellets to chow. Importantly, conditioned rats showed an enhanced preference for pellets throughout the duration of Test 1 compared to the control group, in agreement with the cue’s specific enhancement of the training food (pellet) consumption (see Discussion for Experiment 1). By Test 2, rats in both groups consumed very little chow (less than 0.5g on average) during the 4-hour testing period and this pattern continued during Tests 3 and 4. Despite high levels of consumption during tests, there were no differences in body weights between the Paired and Unpaired groups at any time point likely due to the intermittent testing schedule. This is consistent with the results from other rat models of binge eating which have previously reported that rats’ weights remain stable with periodic versus chronic regimens of palatable food intake (Corwin, Avena, & Boggiano, 2011; Corwin, et al., 1998).
Interestingly, our analyses showed that rats in the control group increased, while rats in the conditioned group maintained their total (0–4hr) food pellet consumption across tests (except Test 3), despite decreased consumption during the first hour with repeated testing. Thus, there seem to be two opposing factors contributing to the pattern we observed across the four consumption tests: a reduction in the potency of the food-cue to elicit a binge-like burst of feeding in conditioned rats, and an increase in the control group possibly due to a new learning.
The substantial increase in control group pellet consumption across tests deserves further discussion. It is possible that the food consumption tests served as conditioning sessions for these rats, where repeated presentations of a tone-cue came to reliably predict the arrival of a dish of food pellets (Pearce & Bouton, 2001). Alternatively, the sensory properties of the food itself could have come to serve as cues predicting the availability of this palatable food for a few hours. Therefore, the increase in consumption by the control group is conceivably a result of associative learning. It is possible that that new associative learning and fast acquisition was enabled by prior experience with and strong preference for the food pellets. Indeed, multiple theories of associative learning take US properties into account in their calculations of the associative strength of stimuli (Pearce & Bouton, 2001; Rescorla & Wagner, 1972); where greater value or salience of the US increases the learning rate parameter and leads to a larger change in the associative strength of the CS-US relationship (Mackintosh, 1975; Rescorla & Wagner, 1972).
Additionally, it has been well-established that rats will increase their intake of a palatable food over time if their access to it is limited, and this increase is inversely related to the degree of restriction (Corwin, et al., 1998). The resulting binge-like consumption of the palatable food while on a limited access regimen is not dependent on the physiological hunger state (Corwin, 2004). This prior work is consistent with our finding of increased consumption with repeated testing in the control group. Regardless of the exact mechanism that led to it, the control group’s consumption pattern illustrates how easily and rapidly organisms might utilize food predictors, and other means, to adapt to large meals (Woods, 1991; Woods & Ramsay, 2000).
It is also important to emphasize that the learned cue modulated food intake most reliably during the first hour, and this is where we found a decrease across the four tests in the conditioned group. However, the slight decrease with repeated testing during this period should not discount the effects food-cues can have on consumption. Indeed, total 4-hour pellet consumption was consistently high across the tests. Furthermore, consumption during the 1–4hr interval was increased, not decreased, between Test 1 and Test 2 for both the conditioned and control groups and was maintained at these elevated levels across the remaining tests. Thus, the total pellet consumption of the conditioned group remained high across the tests, with the exception of Test 3.
Our findings show that food-cues stimulate and enable consumption of enlarged meals, which are not properly accounted for; we did not a find a decrease in daily consumption following the test. The implications of these findings are relevant to the control of human eating behavior. Humans are highly susceptible to external cues for food intake (Levitsky, 2005; Schachter, 1968; Schachter, Goldman, & Gordon, 1968) and in the developed world we are exposed to multitudes of food-cues that could influence our eating patterns (Berthoud, 2011; Hill, et al., 2003; Kessler, 2009; Small, 2009; Volkow & Wise, 2005). Constant bombardment of food-cues and failure to adjust for even small increases in intake could lead to surplus calorie accumulation over time, and ultimately weight gain and associated health complications.
General discussion
Here we showed in two experiments that a learned food-cue can stimulate persistent feeding in sated rats during 4-hour long tests. We used a novel behavioral design to characterize consumption of rats previously conditioned to associate a tone with food pellets, and consumption of rats in a control group that experienced the same number of tones and food pellets but presented randomly. The preparation was novel in that is was designed to allow for an analysis of the effects that brief priming with a conditioned food-cue has on subsequent feeding in a setting that minimizes disturbances to the rats (home cage testing, unlimited water access). Additionally, the preparation was designed to isolate the effects of the food-cue from any possible conditioned effects of the training context; training was conducted in behavioral chambers and tests were conducted in home cages. This is an important consideration because the environment in which food is consumed during training acquires motivational properties and can alone module subsequent consumption (Boggiano, et al., 2009; Bouton, 2011; Le Merrer & Stephens, 2006; Petrovich, Ross, Gallagher, et al., 2007; Petrovich, Ross, Holland, et al., 2007), and because contextual cues were likely encoded differently across the two groups. The tone (CS) was the best predictor of food pellet availability for the rats in the conditioned group. However, for the rats in the control group, which received unpaired tone and food pellet presentations during training, the contextual cues associated with the training chamber were the best predictor for food pellets.
We found that in response to food-cue priming rats in the conditioned group rapidly consumed more food pellets compared to the control group, typically during the first hour post-cue presentation. Importantly, rats in the conditioned group continued to consume pellets, and ate at a rate that was similar to the controls for the remainder of the four-hour test; we did not observe a compensatory cessation or reduction in consumption of pellets after the initial rapid cue-driven bout in the conditioned group compared to the control group. Similarly, there was no compensatory reduction in daily intake post-test; the daily laboratory chow intake post-test was similar across the two groups. Thus, cue priming resulted in a rapid and substantial intake that was not compensated for behaviorally during the following 24 hours. However, metabolic compensations (Schwartz, Woods, Porte, Seeley, & Baskin, 2000) could have occurred, but were not measured here. Additionally, chow reduction during 24–48 hours post-binge has been shown previously (Corwin, et al., 1998), and similar compensations could have occurred here but were not measured.
All rats preferred the training food pellets to their regular chow, and consumed considerably large amounts of pellets during the tests. Thus, the food-cue enhanced already substantial consumption. Furthermore, this setting enabled rats to consume large amounts of food despite two opposing physiological drives: circadian and satiety signals. Rats were tested under sated conditions (ad libitum access to chow) and all testing was during the light phase of the light/dark cycle, a part of the day when rats typically do not consume significant amounts of food (Siegel, 1961; Siegel & Stuckey, 1947).
Similar previous work has also shown that cues can stimulate rats to eat large amounts of the cued food in a short period of time, in a binge-type manner (Boggiano, et al., 2009; Petrovich, Ross, Gallagher, et al., 2007; Petrovich, Ross, Holland, et al., 2007). The design of the current study is also similar to prior preparations in humans where subjects were briefly primed with food-cues prior to consumption, and our results corroborate their findings as well. In those studies subjects reported increased craving and desire for the cued food (Fedoroff, et al., 1997; Ferriday & Brunstrom, 2008) when primed with food cues compared to the non-cued condition. In both cases this was then followed by increased consumption of the cued food (Fedoroff, et al., 1997; Ferriday & Brunstrom, 2008). In agreement, here we found that the learned food-cue drove selective consumption of the cued food (pellets) but not the other food option (chow). Once caveat is that under sated conditions rats had prior ad libitum access to chow, so it is possible that sensory-specific satiety is why the cue did not drive consumption of the chow. However, prior work has shown that rats that were conditioned to associate two distinct cues with two different foods, at testing selectively increased consumption of only the food previously associated with the particular cue presented (Delamater & Holland, 2008; Galarce, et al., 2007). Furthermore, we saw no differences in consumption of chow between our conditioned and control groups regardless of whether they had prior access to chow (sated test) or not (food-deprived test); the only differences between groups was in the amount of training food pellets consumed.
In accordance with our results, prior work in rodents has shown that consumption of small amounts of palatable food was not accounted for in subsequent daily chow intake. This failure to compensate occurred after rats were placed in a context previously paired with the palatable food, but not following placement into a context normally associated with chow (Boggiano, et al., 2009). Additionally, work in humans has also demonstrated that small increases in intake are not followed by a change in future meal sizes suggesting that they might not be well accounted for by homeostatic mechanisms. In a study where subjects were either given or denied snacks, there was no difference in later meal size; at the end of the day those that had consumed snacks consumed significantly more calories than those who did not (Levitsky, 2005). These studies suggest that in some circumstances, particularly in the case of small caloric gains, homeostatic systems might fail to compensate accurately. It was hypothesized that these types of small increments not being accounted for could compound and result in substantial gain over time (Levitsky, 2005). This lack of compensation in later meals clearly parallels our findings that increased consumption in one meal (pellets) was not compensated for in subsequent meals over the course of the day (chow). Hence, our model might have similarities with what humans experience today in contemporary societies: eating when not hungry, and consuming large amounts of palatable foods.
Our results suggest that satiety signals might be easier to override than hunger signals. This is in accordance with the concept of “thrifty genes” which has been extensively studied in populations of Pima Indians (NIDDK, 1996). There is a strong evolutionary argument for the utility of “thrifty genes” and for plasticity in their expression such that homeostatic mechanisms might function differently when under conditions of food deprivation compared to conditions of satiety (Wells, 2009). These mechanisms may work more efficiently in an attempt to prevent starvation in instances where food is scarce. It is likely that evolutionary pressures aimed at preventing starvation led to the emergence of these “thrifty genes”. In contrast, there has likely been little evolutionary pressure aimed at prevention of obesity until modern times (Zheng & Berthoud, 2007). It has not been until the most recent 20–30 years that overeating, especially of calorically dense foods, has emerged a growing problem and public health concern (World Health Organization, 2011). Together, the evidence we presented here and other work clearly suggest that the ease in which our satiety signals can be overridden when combined with our failure to properly compensate for increased bouts of food intake could be disastrous in an environment full of high calorie palatable foods options and a constant bombardment of food-predictive cues.
Our behavioral preparation was designed to test long-term feeding following priming with a food-cue. Rats were tested in their home cages with unlimited access to water, which allowed us to monitor intake over extended time with minimal disturbance to the rats. This design also allowed us to selectively isolate food-cue effects from the training context. Additionally, our preparation separates cue presentation from the actual consumption, which makes it suitable for future brain analysis studies investigating the neural basis of food-cue elicited feeding. In the studies presented here, using this novel behavioral model, we provided evidence that cues can have long-term effects on food intake. In two experiments we showed that learned food-cues stimulate a rapid and substantial intake in sated rats, and this intake was specific to the food conditioned with this particular cue. Additionally, consumption of the cued food continued after this first bout and persisted for the duration of the four-hour test. Importantly, rats failed to show a compensatory decrease in daily intake post-test. Future studies are needed for further characterization of the long-term effects of cues, and whether repeated priming with the food-cue could lead to dysregulation sufficient for weight gain.
Highlights.
Assessed long-term effects of a food-cue in a model of cue-potentiated feeding.
Rats conditioned to associate tone with food then tone-driven consumption tested
Conditioned rats show binge-like consumption immediately following the cue.
Conditioned rats consume large amounts of food regardless of physiological state.
Increased consumption during testing is uncompensated for in daily chow intake.
Acknowledgments
We thank Pari Mody and Katherine Gildersleeve for technical assistance, and Professor Michela Gallagher for generous space and equipment support for the first experiment. This research was supported in part by NIH grants MH67252 and DK085721 to G.D.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24(2):173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano MM, Dorsey JR, Thomas JM, Murdaugh DL. The Pavlovian power of palatable food: lessons for weight-loss adherence from a new rodent model of cue-induced overeating. Int J Obes (Lond) 2009;33(6):693–701. doi: 10.1038/ijo.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Learning and the persistence of appetite: extinction and the motivation to eat and overeat. Physiol Behav. 2011;103(1):51–58. doi: 10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Bull LS, Pitts GC. Gastric capacity and energy absorption in the force-fed rat. J Nutr. 1971;101(5):593–596. doi: 10.1093/jn/101.5.593. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42(2):139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104(1):87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65(3):545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Delamater AR, Holland PC. The influence of CS-US interval on several different indices of learning in appetitive conditioning. J Exp Psychol Anim Behav Process. 2008;34(2):202–222. doi: 10.1037/0097-7403.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff IC, Polivy J, Herman CP. The effect of pre-exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite. 1997;28(1):33–47. doi: 10.1006/appe.1996.0057. [DOI] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. How does food-cue exposure lead to larger meal sizes? Br J Nutr. 2008;100(6):1325–1332. doi: 10.1017/S0007114508978296. [DOI] [PubMed] [Google Scholar]
- Galarce EM, Crombag HS, Holland PC. Reinforcer-specificity of appetitive and consummatory behavior of rats after Pavlovian conditioning with food reinforcers. Physiol Behav. 2007;91(1):95–105. doi: 10.1016/j.physbeh.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Hoefling A, Strack F. Hunger induced changes in food choice. When beggars cannot be choosers even if they are allowed to choose. Appetite. 2010;54(3):603–606. doi: 10.1016/j.appet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86(5):747–761. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76(1):117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Kessler DA. The end of overeating: Taking control of the insatiable American appetite. New York: Rodale; 2009. [Google Scholar]
- Le Merrer J, Stephens DN. Food-induced behavioral sensitization, its cross-sensitization to cocaine and morphine, pharmacological blockade, and effect on food intake. J Neurosci. 2006;26(27):7163–7171. doi: 10.1523/JNEUROSCI.5345-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky DA. The non-regulation of food intake in humans: hope for reversing the epidemic of obesity. Physiol Behav. 2005;86(5):623–632. doi: 10.1016/j.physbeh.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82(4):276–298. [Google Scholar]
- NIDDK. The Pima Indians: pathfinders for health. Bethesda, Md: National Institute of Diabetes and Digestive and Kidney Diseases, Dept. of Health and Human Services, Public Health Service, National Institutes of Health; 1996. [Google Scholar]
- Oswald KD, Murdaugh DL, King VL, Boggiano MM. Motivation for palatable food despite consequences in an animal model of binge eating. Int J Eat Disord. 2011;44(3):203–211. doi: 10.1002/eat.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Bouton ME. Theories of associative learning in animals. Annu Rev Psychol. 2001;52:111–139. doi: 10.1146/annurev.psych.52.1.111. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145(8):3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Petrovich GD. Learning and the motivation to eat: forebrain circuitry. Physiol Behav. 2011;104(4):582–589. doi: 10.1016/j.physbeh.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiol Behav. 2007;90(2–3):362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci. 2007;27(24):6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliner P, Mann N. Influence of social norms and palatability on amount consumed and food choice. Appetite. 2004;42(2):227–237. doi: 10.1016/j.appet.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ. Unpublished master’s thesis. Boston College; 2010. Chronic stress, food consumption, and emotional behavior in rats. [Google Scholar]
- Rescorla RA, Wagner AR. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Rolls BJ. Sensory-specific satiety. Nutr Rev. 1986;44(3):93–101. doi: 10.1111/j.1753-4887.1986.tb07593.x. [DOI] [PubMed] [Google Scholar]
- Salamone J, Steinpreis R, McCullough L, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104(4):515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Schachter S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science. 1968;161(843):751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- Schachter S, Goldman R, Gordon A. Effects of fear, food deprivation, and obesity on eating. J Pers Soc Psychol. 1968;10(2):91–97. doi: 10.1037/h0026284. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Deprivation alters rats’ flavor preferences for carbohydrates and fats. Physiol Behav. 1993;53(6):1091–1099. doi: 10.1016/0031-9384(93)90364-l. [DOI] [PubMed] [Google Scholar]
- Siegel PS. Food Intake in Rat in Relation to Dark-Light Cycle. Journal of comparative and physiological Psychology. 1961;54(3):294. [Google Scholar]
- Siegel PS, Stuckey HL. The diurnal course of water and food intake in the normal mature rat. J Comp Physiol Psychol. 1947;40(5):365–370. doi: 10.1037/h0062185. [DOI] [PubMed] [Google Scholar]
- Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes (Lond) 2009;33(Suppl 2):S44–48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61(9):1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220(4595):431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Meal initiation controlled by learned cues: basic behavioral properties. Appetite. 1984;5(2):147–158. doi: 10.1016/s0195-6663(84)80035-5. [DOI] [PubMed] [Google Scholar]
- Wells JC. Thrift: a guide to thrifty genes, thrifty phenotypes and thrifty norms. Int J Obes (Lond) 2009;33(12):1331–1338. doi: 10.1038/ijo.2009.175. [DOI] [PubMed] [Google Scholar]
- Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98(4):488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000;110(1–2):175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Obesity and Overweight, Fact Sheet No 311. 2011 Mar; from http://www.who.int/mediacentre/factsheets/fs311/en/
- Zamble E. Augmentation of eating following a signal for feeding in rats. Learning and Motivation. 1973;4:138–147. [Google Scholar]
- Zheng H, Berthoud HR. Eating for pleasure or calories. Curr Opin Pharmacol. 2007;7(6):607–612. doi: 10.1016/j.coph.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]