Abstract
Background
Therapeutic vaccines for cancer are an attractive alternative to conventional therapies, since the later result in serious adverse effects and in most cases are not effective against advanced disease. Human papillomavirus (HPV) is responsible for several malignancies such as cervical carcinoma. Vaccines targeting oncogenic viral proteins like HPV16-E6 and HPV16-E7 are ideal candidates to elicit strong immune responses without generating autoimmunity because: (1) these products are not expressed in normal cells and (2) their expression is required to maintain the malignant phenotype. Our group has developed peptide vaccination strategy called TriVax, which is effective in generating vast numbers of antigen-specific T cells in mice capable of persisting for long time periods.
Materials and methods
We have used two HPV-induced mouse cancer models (TC-1 and C3.43) to evaluate the immunogenicity and therapeutic efficacy of TriVax prepared with the immunodominant CD8 T-cell epitope HPV16-E749-57, mixed with poly-IC adjuvant and costimulatory anti-CD40 antibodies.
Results
TriVax using HPV16-E749-57 induced large and persistent T-cell responses that were therapeutically effective against established HPV16-E7 expressing tumors. In most cases, TriVax was successful in attaining complete rejections of 6–11-day established tumors. In addition, TriVax induced long-term immunological memory, which prevented tumor recurrences. The anti-tumor effects of TriVax were independent of NK and CD4 T cells and, surprisingly, did not rely to a great extent on type-I or type-II interferon.
Conclusions
These findings indicate that the TriVax strategy is an appealing immunotherapeutic approach for the treatment of established viral-induced tumors. We believe that these studies may help to launch more effective and less invasive therapeutic vaccines for HPV-mediated malignancies.
Keywords: Peptide vaccines, HPV, Cervical cancer, CD8 T cells, PIVAC 11
Introduction
Cervical cancer is the second most prevalent cancer among women. Human papillomavirus (HPV) causes 99 % of cervical cancer (CC), and the HPV16 and HPV18 genotypes account for 70 % these cases [1]. Worldwide, CC is responsible for ~250 000 deaths per year and for causing a huge economic burden in related health care costs [2, 3]. The current approved prophylactic vaccines for HPV are not useful for treating established malignant disease, nor can protect already infected individuals from developing cancer [4–6]. Most importantly, a significant proportion of women, especially in the third world, will not receive the prophylactic vaccines and will continue to be at high risk of developing CC [7]. Because, conventional therapies for CC are usually devastating, invasive, toxic, and associated with 10 % recurrence [8], there is an urgent need for developing alternative treatments such as immunotherapy and, more specifically, therapeutic vaccines.
CD8 T lymphocytes are the most effective components of the adaptive immune system capable of recognizing and destroying viral-infected and transformed malignant cells [9–11]. The antigens recognized by CD8 T cells on their target cells are small peptides derived from viral or tumor-associated antigens (TAAs) that associate with cell surface class I products of the major histocompatibility complex (MHC-I). In the case of cells transformed by HPV, peptide sequences derived from the oncogenic E6 and E7 viral proteins have been shown to represent suitable TAAs for CC and are considered as ideal candidates for developing therapeutic vaccines [12–18]. Synthetic peptides representing these TAAs have been tested in numerous ways in patients and mouse cancer models for their ability to generate anti-tumor T-cell responses capable of exhibiting anti-tumor effects [12, 16, 17, 19–21]. However, in most instances, only modest T-cell responses capable of dealing with very early disease stages were obtained, indicating that improved peptide-based immunization strategies need to be developed to have a significant impact against established and advanced disease stages.
Our laboratory has recently described an improved peptide vaccination strategy capable of generating in mice vast numbers of CD8 T cells capable of persisting for long time periods [22, 23]. This vaccine called TriVax consists of a synthetic peptide corresponding to the minimal T-cell epitope, poly-IC adjuvant, and costimulatory monoclonal anti-CD40 antibodies (αCD40 mAb), which are mixed together and administered intravenously. After two sequential TriVax immunizations (prime/boost) with the well-known Ova257-264 T-cell epitope, up to 80 % of all the CD8 T cells in blood were antigen specific, and more than half of these cells persisted for at least 60 days [23]. The goal of the present study was to evaluate the efficacy of TriVax in an HPV cancer mouse model. The results demonstrate that TriVax using peptide HPV16-E749-57 induced large and persistent T-cell responses that were effective against two different tumors expressing HPV16-E7. Interestingly, the anti-tumor effects of TriVax in this tumor model appeared to be independent of NK and CD4 T cells and did not rely to a great extent on either type-I or -II interferons. We believe that the results from these studies may help to develop more effective therapeutic vaccines for CC.
Materials and methods
Mice
Six- to nine-week-old female C57BL/6 (B6) mice were obtained from the National Cancer Institute/Charles River program (Wilmington, MA). Interferon-gamma (IFNγ) knockout (KO) mice in the B6 background were purchased from Jackson Laboratories (Bar Harbor, ME). IFNαβ receptor KO (IFNαβR KO) mice also in the B6 background were obtained from Dr. Philippa Marrack (National Jewish Medical and Research Center, Denver, CO). All animal care and experiments were conducted according to our institutional animal care and use committee guidelines.
Cell lines
TC-1 tumor cells, obtained from Dr. T-C Wu (Johns Hopkins University, Baltimore, MD), were derived from primary lung epithelial cells of B6 mice and express HPV16-E6 and E7 proteins [24]. The C3.43 tumor cell line obtained from Dr. W. M. Kast (University of Southern California, Los Angeles, CA) is an aggressive derivative of the C3 line (B6 background), which was transformed using a pRSVneo-derived plasmid containing the complete HPV16 genome [19]. The EL4 cell line was purchased from the American Type Culture Collection (Manassas, VA). All cells were maintained in tissue culture following the recommendations of the providers.
Peptides, MHC-I tetramer, and antibodies
The synthetic peptide RAHYNIVTF from HPV16-E7 (E749-57), defined as an immunodominant H-2Db-restricted CD8 T-cell epitope [19, 25], and control peptide NAYVFKGL from chicken ovalbumin (Ova176-183) were purchased as >95 % pure from A&A Labs (San Diego, CA). Rat anti-mouse CD40 (FGK45.5) anti-NK1.1 (PK136), anti-CD4 (GK1.5), and anti-CD8 (2.43) monoclonal antibodies were prepared from hybridoma culture supernatants. The E749-57/H-2Db tetramers labeled with Alexa 647 were provided by the National Institute of Allergy and Infectious Disease Tetramer Facility at the Emory University (Atlanta, GA from NIH). Fluorochrome-labeled antibodies specific for mouse CD8a (53-6.7) and MHC-II (M5/114.15.2) were from eBioscience, Inc (San Diego, CA).
Immunizations
Mice were usually vaccinated via the i.v. route (unless otherwise noted). TriVax consisted of a mixture of 30 μg of the E749-57 peptide, 100 μg αCD40 mAb, and 50 μg of poly-IC (Poly-ICLC, Oncovir, Inc.). BiVax contained only the peptide and poly-IC at the same amounts. In all cases, mice are given two sequential vaccinations 13 days apart (prime and boost). In some cases, mice received peptide alone or peptide with αCD40 mAb.
Immunological assays
For tetramer staining, either peripheral blood samples (~3–5 drops) taken from the submandibular vein or splenocytes were stained with a mixture of antibodies to MHC-II, CD8a (eBioscience; San Diego, CA), and tetramer for 40 min in ice. After washing with three times, the fluorescence was evaluated using an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software (Ashland, OR). Results are presented as percentage tetramer-positive cells of the CD8+/MHC-II negative population. To determine whether CD8 T cells were able to recognize tumor cell lines (TC-1, C3.43) expressing the naturally processed peptide, IFNγ, enzyme-linked immunosorbent spot (EliSpot) assays were performed as described [26]. Briefly, CD8 T cells from spleens of vaccinated mice were purified by positive selection using antibody-coated magnetic beads (Miltenyi Biotec, Auburn, CA). Responder (CD8-purified) cells were incubated at 3 × 105, 1 × 105, and 3 × 104 per well, together with 1 × 105 stimulator cells (EL4, plus/minus peptide, TC-1, and C3.43 cells pretreated or not for 24 h with 100 ng/ml IFNγ). Cultures were incubated at 37 °C for 20 h, and spots (IFNγ-producing cells) were developed as described by the EliSpot kit manufacturer (Mabtech, Inc., Mariemont, OH). Spot counting was done with an AID EliSpot Reader System (Autoimmun Diagnostika GmbH, Strassberg, Germany).
Evaluation of therapeutic anti-tumor effects
Mice received 3 × 105/mouse tumor cells (TC-1 or C3.43) s.c. in a shaved rear flank 6 or 11 days (as noted) before their first immunization. In some instances, survivor mice were re-challenged with the same number of tumor cells (in opposite flanks). To determine the contribution of different subsets of lymphocytes, the anti-tumor effect of the vaccine, NK, CD4, and CD8 cell antibody-depleted mice and KO mice was compared with B6 wild-type (WT) mice. For cell depletions, each mouse received 300 μg anti-NK1.1, 300 μg anti-CD4, or 500 μg anti-CD8 twice on days −2 and 0 before immunization. Depletions were confirmed by analysis of blood samples using flow cytometry (data not presented). Tumor growth was monitored every 2–4 days in individual tagged mice by measuring 2 opposing diameters with a set of calipers. Mice were killed when the tumor area reached 400 mm2. Results are presented as the mean tumor size (area in mm2) ± SD for every treatment group at various time points until the termination of the experiment.
Statistical analyses
Statistical significance of the numbers of antigen specific CD8 T cells (EliSpot), cytokine levels (ELISA) and absolute number of lung tumor nodules was assessed using unpaired Student’s t tests. Tumor sizes between 2 populations throughout time were analyzed for significance using 2-way ANOVA tests. All analysis and graphics were done using GraphPad Prism 5.01 (GraphPad Software, San Diego, CA).
Results
Evaluation of TriVax immunization using a peptide epitope from HPV16-E7
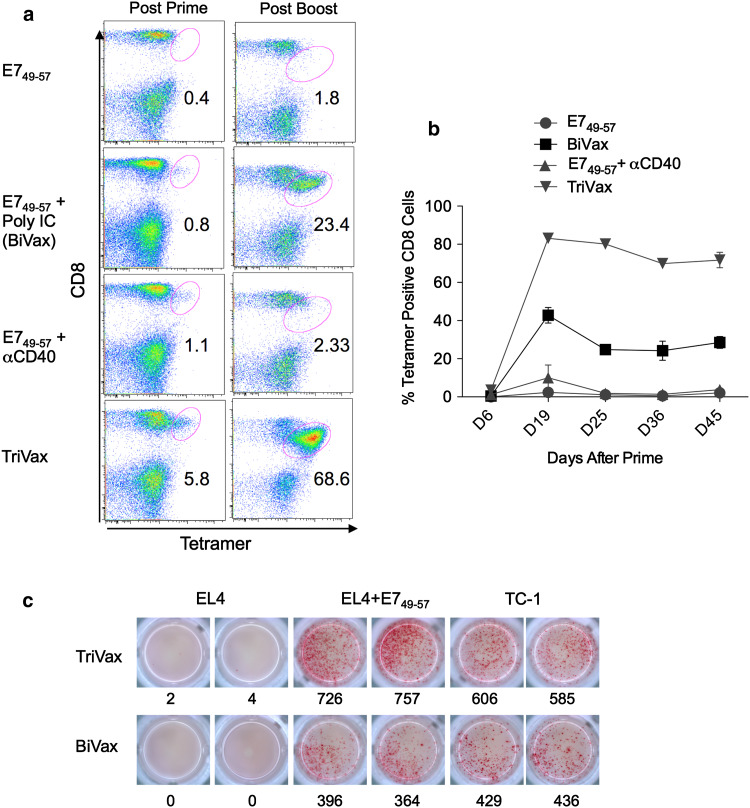
Residues 49-57 of the HPV16-E7 protein (RAHYNIVTF) correspond an immunodominant CD8 T-cell epitope restricted by the H-2Db MHC-I molecule [19, 25]. We first determined the ability of synthetic peptide E749-57 representing this sequence to elicit an immune response when administered to mice in combination with poly-IC and αCD40 mAb, a vaccine formulation known as TriVax. In addition, we compared the immunogenicity of vaccines containing peptide alone, peptide plus poly-IC (BiVax), or peptide plus αCD40 mAb. Antigen-specific immune responses were measured using tetramer analysis six days after the prime and booster immunizations and at various time points thereafter. As shown in Fig. 1a, TriVax and BiVax induced very high number of antigen-specific (tetramer positive) CD8 T cells after the booster immunization, which persisted at high levels for several weeks (Fig. 1b). On the other hand, administration of the E749-57 peptide alone or in combination with αCD40 mAb failed to generate a significant immune response. More importantly, spleen CD8 T cells from TriVax- and BiVax-immunized mice were shown to be very effective in recognizing E749-57 peptide-pulsed target cells (EL4) and TC-1 tumor cells, which express the HPV16-E7 protein and naturally process the antigen (Fig. 1c).
Fig. 1.
Synergy for the potentiation of the immunogenicity of E749-57 peptide by poly-IC and αCD40 mAb. a Frequencies of antigen-specific CD8 T cells in blood measured by tetramer analysis 6 days after prime and boost induced by immunization using various vaccine formulations. Numbers below the oval gates represent the % of tetramer-positive cells of the CD8-positive population. Dot plots showing the percentage of tetramer-positive cells in blood of a mouse from each group (3 mice/group). b Average percentage of tetramer-positive cells in the blood of mice in each group measured at different time points (same experiment as in a). c Separate experiment where CD8 T cells were purified from pooled splenocytes 7 days after the boost and antigen-induced responses to various target cells was evaluated using IFNγ EliSpot assays. Stimulator cells: EL4 cells pulsed or not with E749-57 peptide and TC-1 cells (expressing HPV16-E7) were used to evaluate CD8 T-cell responses from TriVax- (top row) and BiVax (bottom row)-vaccinated mice. In this experiment, each well contained 1 × 104 CD8 T cells and 1 × 105 stimulator cells. Numbers represent the total spots present in each well. Representative results of data obtained from two different experiments
Anti-tumor effectiveness of TriVax
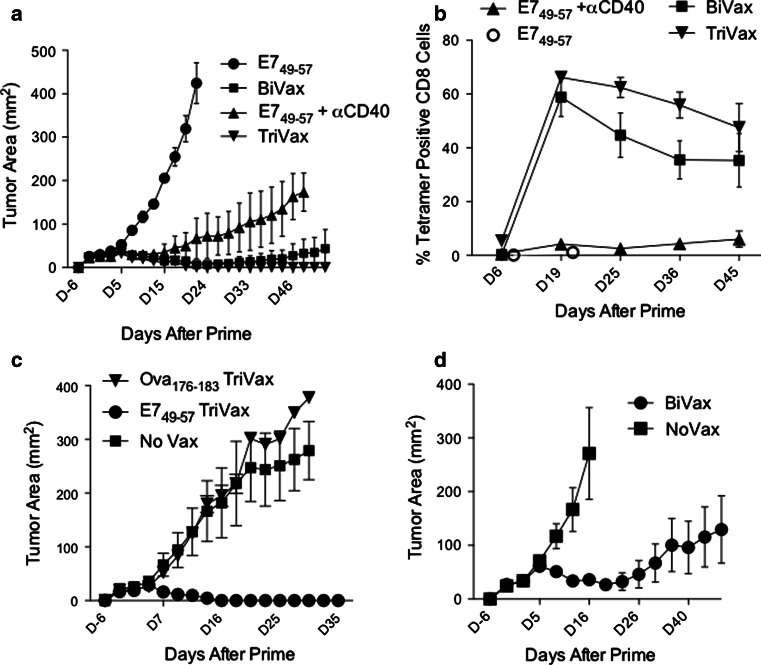
The therapeutic anti-tumor effects of the 4 modes of peptide vaccination were assessed. Mice bearing 6-day established TC-1 tumors received the peptide vaccines (prime/boost), and tumor sizes and immune responses were measured at various time points. As shown on Fig. 2a, tumor growth was effectively controlled in those mice that received TriVax, BiVax, and, to some extent, peptide E749-57 with αCD40 mAb, as compared to mice vaccinated with E749-57 alone (where tumors grew at an accelerated rate and mice had to be euthanatized by day 22). Notably, 100 % of the mice that received TriVax and 33 % of those receiving BiVax completely rejected their tumors, while in the case of the mice immunized with E749-57 with αCD40 mAb, although the tumors grew at a slow rate, none were rejected. Measurements of the immune responses elicited by these vaccines in the tumor-bearing mice (Fig. 2b) indicated that both TriVax and BiVax induced high numbers of persisting E749-57-specific CD8 T cells. Interestingly, the differences between TriVax and BiVax were less apparent as compared to those observed in tumor-free mice (Fig. 1b). E749-57 with αCD40 mAb induced a small CD8 T-cell response (between 3 and 5 % tetramer-positive cells), but apparently strong enough to reduce the tumor growth. No appreciable immune response was observed in mice that received E749-57 alone. The therapeutic effect of TriVax with E749-57 was antigen specific since TriVax prepared with an irrelevant peptide (Ova176-183) was not effective in reducing the rate of tumor growth (Fig. 2c). The therapeutic effects observed with E749-57 BiVax (Fig. 2a) were confirmed in an additional experiment using a larger number of mice (6), where in this case, this therapeutic vaccination strategy resulted in 50 % complete tumor rejections (Fig. 2d). Moreover, when BiVax was administered three times (prime plus 2 boosts, 7 days apart), rejections were observed in 100 % of the mice (data not presented).
Fig. 2.
Therapeutic effects induced by TriVax and BiVax immunization against established TC-1 tumors. a Mice (n = 3 per group) were inoculated (s.c.) with 3 × 105 TC-1 cells and vaccinated (i.v.) 6 days later with E749-57 peptide alone, BiVax, peptide plus αCD40 mAb, or TriVax. Identical booster immunizations were given on day 13. Tumor growth was measured (two opposing diameters) and recorded twice at week. None of mice from the E749-57 and E749-57 + αCD40 mAb groups rejected their tumors. One mouse from the BiVax group and all 3 mice from the TriVax group rejected their tumors. b The percentage of antigen-specific (tetramer positive) CD8 T cells in blood of the mice from the experiment shown on a measured at different time points. Only 2 measurements were done in the mice immunized with peptide alone because these mice did not survive beyond day 24. c Mice (n = 4 per group) were inoculated (s.c.) with 3 × 105 TC-1 cells and vaccinated (i.v.) 6 days later with TriVax prepared with either E749-57 or Ova176-183 as indicated. Identical booster immunizations were given on day 13. A non-vaccinated group (No Vax) was included as control. d The therapeutic effects of E749-57 BiVax were confirmed using a larger number of mice (6/group). In this experiment, half (3/6) of the mice in the BiVax-vaccinated group rejected their tumor, while all mice in the control group did not
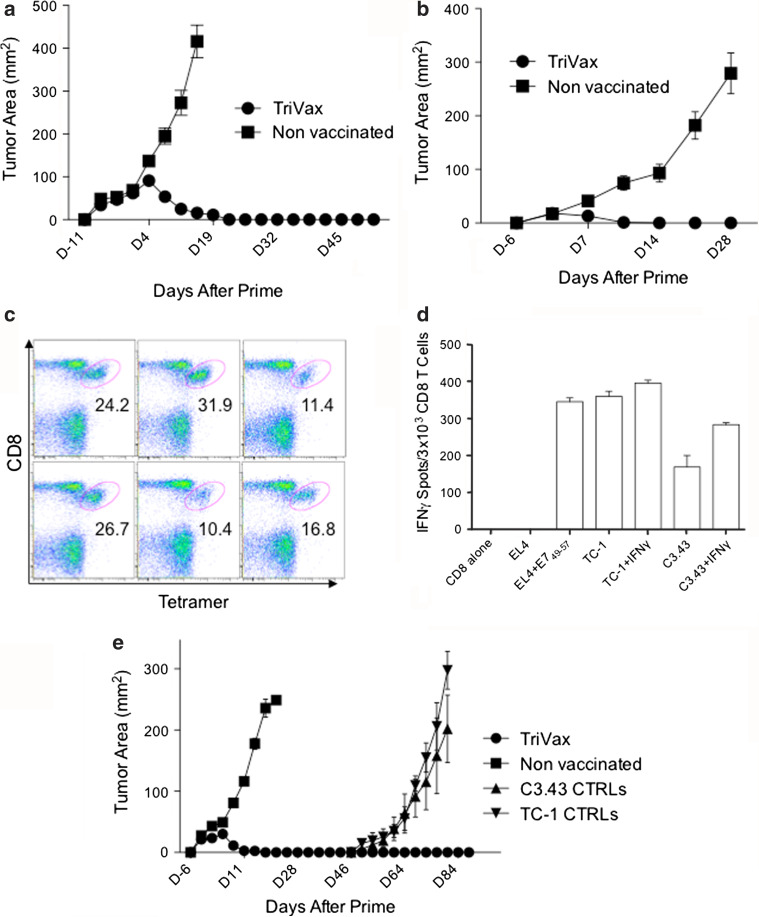
The impressive therapeutic anti-tumor effects observed with E749-57 TriVax was evaluated against larger and more established TC-1 tumors. The results shown in Fig. 3a demonstrate that therapeutic immunization with TriVax 11 days post-tumor inoculation resulted in 100 % complete rejections and increased survival. The therapeutic effectiveness of E749-57 TriVax was also examined using a different tumor cell line called C3.43, which also expresses the HPV16-E7 antigen. As presented in Fig. 3b, TriVax using the E749-57 peptide resulted in complete rejection of 6-day established C3.43 tumors. Furthermore, at the conclusion of this experiment (day 30), considerable numbers of E749-57-specific CD8 T cells remained present on the spleens of the TriVax-immunized mice (Fig. 3c). Moreover, the spleen CD8 T cells from the TriVax-immunized mice that rejected the C3.43 tumors were effective in recognizing C3.43 and TC-1 tumors, and such recognition was increased to some extent by interferon-gamma (IFNγ) pretreatment of the tumor cells (Fig. 3d), which enhances the expression levels of MHC-I on both tumors (data not shown).
Fig. 3.
Therapeutic effects induced by TriVax against HPV16-E7 expressing tumors. a Anti-tumor effects against large TC-1 tumors. Mice (6/group) were inoculated (s.c.) with 3 × 105 TC-1 cells and 11 days later were immunized with TriVax, and a booster was given 13 days after prime. All of the mice immunized with TriVax rejected their tumors. b Anti-tumor effects against C3.43 tumors. Mice (6/group) were inoculated with 3 × 105 C3.43 cells (s.c.) and vaccinated 6 and 13 days later with TriVax. All TriVax-immunized mice rejected their tumors. c Frequency of antigen-specific CD8 T cells in the spleens of mice from the TriVax-immunized group shown in b was determined 30 days after the prime. Numbers below each oval gate represent % tetramer-positive cells of the CD8 population. d CD8 T cells were purified from pooled splenocytes of TriVax-vaccinated mice shown in c, and tumor cell recognition was evaluated using IFNγ ELiSpot assays. Stimulator cells were as follows: EL4 cells loaded or not with E749-57 peptide, TC-1, and C3.43 tumor cells previously treated or not with IFNγ (100 U/ml, 24 h, to increase MHC-I expression). Results represent the average number of spots from triplicate wells with SD (error bars) of the means. e TriVax immunization confers long-term protection against tumors. Mice (6/group) were inoculated (s.c.) with 3 × 105 TC-1 cells and 6 and 13 days later received TriVax. By day 25, all mice in the TriVax group rejected their tumors. On day 47, the mice were re-challenged with 3 × 105 TC-1 on their right flanks and 3 × 105 C3.43 on their left flanks. None of the tumors grew. As controls (CTRLs), 3 naïve mice were inoculated with the same number of tumor cells
TriVax generates immunological memory that prevents tumor recurrences
An effective therapeutic vaccination strategy should generate immune responses capable not only of eliminating tumor masses but also should be successful in preventing tumor recurrences by generating long-term immune memory. Thus, we evaluated whether E749-57 TriVax-treated mice that had successfully rejected TC-1 tumors would be able to respond to subsequent tumor re-challenges. The experiment shown in Fig. 3e shows that ~1 month after rejecting their original TC-1 tumors, TriVax-immunized mice successfully resisted second tumor challenges using TC-1 and C3.43 cells, which were given separately to each mouse in opposite posterior flanks. Unvaccinated control mice developed both tumor challenges, which grew at an accelerated rate.
Effect of route of administration in TriVax
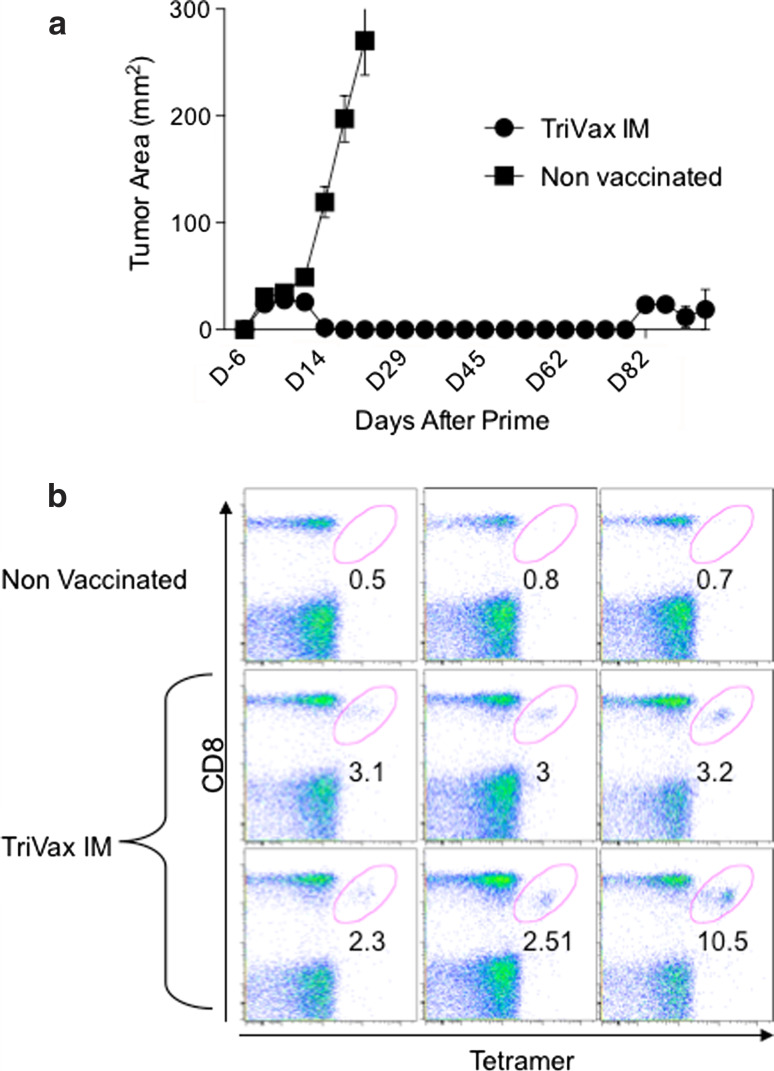
The results presented so far were obtained using immunizations that were administered intravenously (i.v.) since the goal was to generate a systemic immune response. However, since vaccines are generally not administered via the i.v. route, we examined whether TriVax would be effective against 6-day established TC-1 tumors if it were administered intramuscularly (i.m.). As shown in Fig. 4a, i.m. E749-57 TriVax induced tumor clearance of 100 % of treated mice, while tumors in the non-vaccinated mice grew at a fast rate. Notably, the frequency of antigen-specific (tetramer positive) CD8 T cells in blood of these mice, measured 11 days after the booster immunization, was rather low (range 2.3–10.5 %, Fig. 4b), compared to the values we routinely observed in mice immunized i.v. (>50 %). In addition, one of the six i.m. vaccinated mice in this experiment (the one with the lowest numbers of antigen-specific CD8 T cells) developed a tumor recurrence at the original injection site on day 82 (post prime), suggesting that the tumor had not been totally eradicated.
Fig. 4.
Anti-tumor efficacy of intramuscularly administered TriVax. a Mice were inoculated s.c. with 3 × 105 TC-1 cells. Six days later were vaccinated i.m. with TriVax (TriVax IM) and an identical booster was given on day 13. Tumor growth was measured as described previously. By day 25, all mice (6/6) vaccinated with TriVax rejected tumor, but by day 66, one of the mice developed a tumor mass at the original site (not shown). All of the (3/3) non-vaccinated mice developed large tumors and did not survive. b Frequency of antigen-specific CD8 T cells (tetramer positive) in the blood of the mice shown in a measured 11 days after boost. Numbers below the oval gates represent the % tetramer-positive cells of the CD8 T-cell population
Mechanism of anti-tumor effects of TriVax
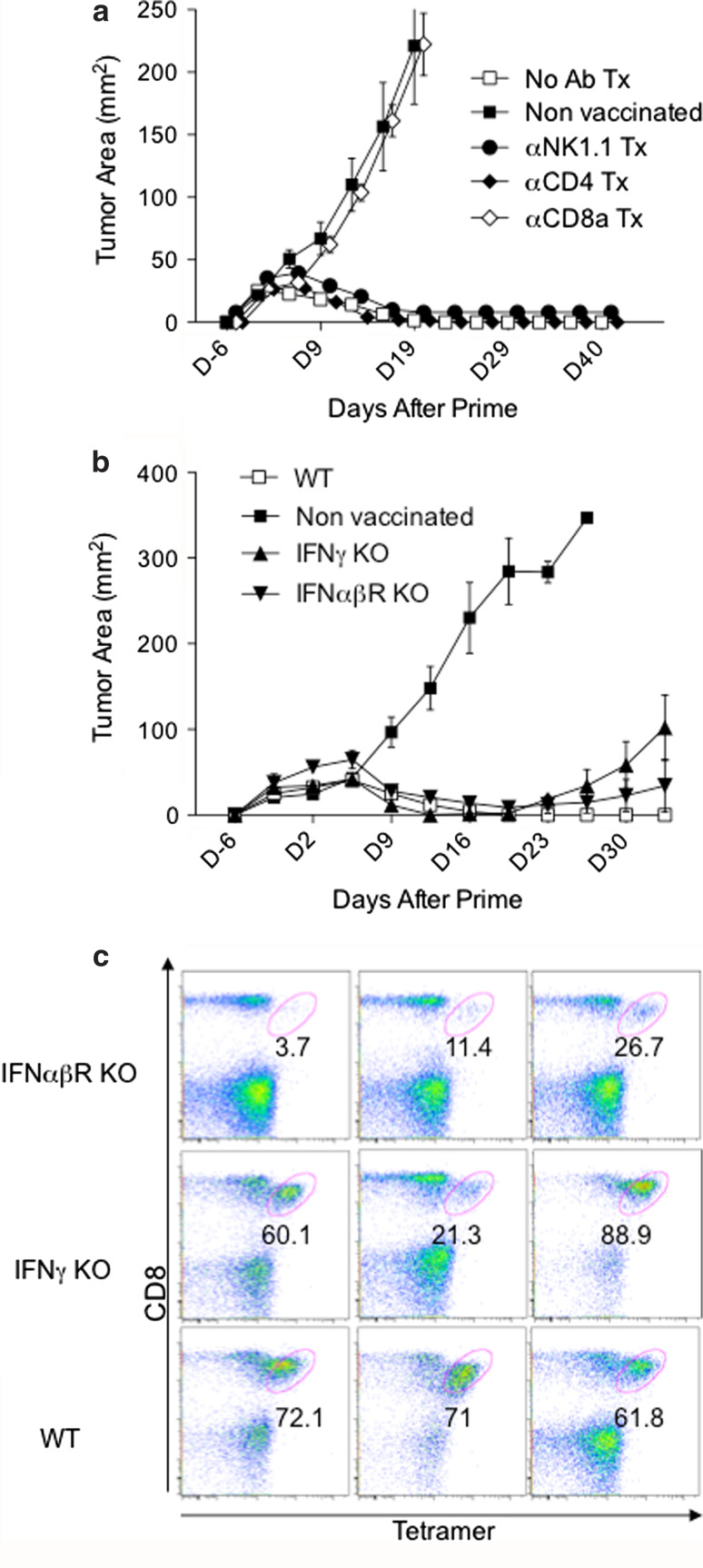
Lastly, to assess the roles of various lymphocyte subsets and the requirements of effector cytokines, the anti-tumor efficacy of E749-57 TriVax was examined in CD8-, CD4-, and NK-depleted mice and in IFNγKO and IFNαβR KO mice. The results in Fig. 5a show, as expected, that mice depleted of CD8 T cells were unable to control tumor growth and closely resembled the non-vaccinated mice. On the other hand, 100 % of the CD4- and NK cell-depleted mice rejected their tumors. Surprisingly, the TriVax-immunized IFNγ KO and IFNαβR KO mice were able to control tumor growth to a great degree, and in some instances, complete rejections were observed (Fig. 5b). When the intensity of the CD8 T-cell responses in immunized IFNγ ΚΟ and IFNαβR KO tumor-free mice was evaluated after TriVax immunization (prime/boost), it became evident that absence of IFNγ had little effect, while the role of type-I IFN in generating high CD8 T-cell numbers was clearly more important (Fig. 5c).
Fig. 5.
Mechanism of therapeutic anti-tumor effects of TriVax. a Tumor growth in mice depleted of NK cells, CD4 T cells, and CD8 T cells vaccinated (i.v.) with TriVax. Mice (6/group) were inoculated with 3 × 105 TC-1 cells and 6 and 13 days later received TriVax. 1 and 3 days before the TriVax prime mice received depleting antibodies via an i.p. injection as described in “Materials and methods”. b Tumor growth in IFNγ KO and IFNαβR KO mice vaccinated with TriVax in the same manner as described in a. Non-vaccinated mice and TriVax-vaccinated wild-type (WT) mice were used as controls in each experiment. All of the TriVax WT, CD4-, and NK cell-depleted mice rejected their tumors. One out of 6 mice in the IFNγ KO group and 2/4 mice from the IFNαβR KO group had complete tumor rejections. c Immune responses elicited by TriVax in naïve IFNγ KO and IFNαβR KO mice. In a separate experiment, non-tumor-bearing wild-type (WT), IFNγ KO, and IFNαβR KO mice were vaccinated with TriVax (prime/boost, 13 days apart), and the percentage of tetramer-positive cells in blood was determined 7 days after boost
Discussion
The goal of the present studies was to assess the effectiveness of a new and potent peptide vaccination strategy for its therapeutic effectiveness against tumors expressing the HPV16-E7 oncogene product. This viral product is an ideal TAA for developing T cell–based immunotherapy against HPV-transformed cells because it is recognized as a foreign antigen. Thus, the lack of immunological tolerance allows the generation of high-avidity T-cell responses, which contrasts with lower-avidity T cells generated against other types of TAAs that are expressed by normal tissues (e.g., melanosomal products, p53, HER2/neu). We have reported that TriVax using epitopes from melanosomal TAA such as Trp1 and Trp2 that are expressed on normal cells, although generate large numbers of antigen-specific T cells that diminish tumor growth, are incapable of rejecting established B16 melanomas in WT mice [23, 27].
Because the HPV16-E7 protein plays a critical role in maintaining the transformed phenotype of the tumor cells [28, 29], the possible appearance of antigen-loss mutants, which is often seen in immunotherapy [10], should be diminished. The results presented here using two HPV16 mouse tumor models (TC-1 and C3.43) demonstrate that immunization with a synthetic peptide representing an exact CD8 T-cell epitope in combination with poly-IC and αCD40 mAb (TriVax) resulted in extensive antigen-specific T-cell responses that were durable and capable of eradicating established tumors. Others have reported that vaccines prepared with the minimal CD8 T-cell epitopes, especially when administered systemically, are ineffective because the peptides can be presented by non-professional antigen-presenting cells (APCs) resulting in T-cell tolerance [30–32]. For this reason, some investigators advocate the use of long synthetic peptides that require antigen processing by professional APCs [20]. The present results and previous work by our group [22, 23] demonstrate that vaccines containing minimal CD8 T-cell epitopes can be highly immunogenic when provided together with poly-IC and αCD40 mAb and in some cases with poly-IC alone (Fig. 1). In fact, to the best of our knowledge, the magnitude of the responses we have observed with several minimal CD8 T-cell epitopes using TriVax (HPV16-E749-57, Trp1455-463/9M, Trp2180-188, Ova257-264, rNEU66-74) is far superior to what has been reported using other peptide vaccines (with either short or long synthetic peptides), when administered with conventional adjuvants (e.g., IFA), pulsed onto DCs or when using recombinant DNA vaccines. Although the magnitude of the T-cell responses achieved with TriVax in mice is impressive, we do not know whether similar effects can be accomplished in humans following the same strategy. Moreover, it will be important to assess and closely monitor those potential flu-like toxic effects that could accompany the generation of large numbers of activated T cells.
Although the combination of all three components of TriVax was clearly the most effective therapeutic strategy, possibly because it generated the strongest immune responses (Fig. 1a, b), immunization with peptide and poly-IC (BiVax) or peptide plus αCD40 mAb was also quite effective in eliciting CD8 T-cell responses that resulted in significant therapeutic benefit (Figs. 1, 2). These results indicate that the αCD40 mAb potentiates the effectiveness of TriVax, but is not essential and that poly-IC plays the major role of adjuvant in this vaccination strategy. It is possible that with additional immunization boosters, the efficacy of BiVax could improve to the level of TriVax to attain 100 % tumor rejections by increasing T-cell numbers. On the other hand, we cannot rule out that the αCD40 mAb may facilitate the generation of CD8 T cells with increased function and that simply inducing high numbers of T cells will be insufficient to achieve maximal therapeutic responses. Notwithstanding, our results indicate that even low numbers of antigen-specific CD8 T cells, which were induced by peptide plus αCD40 mAb, and TriVax administered i.m. were sufficient to effectively control the rate of tumor growth. However, complete and durable rejections in all mice were only achieved when high numbers of antigen-specific CD8 T cells were produced.
The rationale for the use of αCD40 mAb in the generation of CD8 T-cell responses is to provide the strong costimulatory signal to antigen-presenting dendritic cells (DCs), which is usually supplied by CD40 ligand (CD40L) expressing CD4 T helper cells. The CD40/CD40L costimulatory interaction has been proposed to be critical for the generation of memory CD8 T cells capable of persisting for long time periods [33]. Nevertheless, BiVax showed to be effective in generating high numbers of long-lasting CD8 T-cell responses, presumably in the absence of CD40 ligation. Furthermore, depletion of CD4 T cells does not decrease (but slightly increases) the generation of antigen-specific CD8 T cells by BiVax (data not presented), ruling out that this vaccine somehow also stimulates CD4 T cells. The ability of BiVax to trigger strong T-cell responses to the E749-57 peptide appears to be somewhat unique, since other potent CD8 T-cell epitopes such as Ova257-264 (SIINFEKL) and melanoma Trp1455-463/9M (TAPDNLGYM) are ineffective and require αCD40 mAb in addition to poly-IC (E. Celis, unpublished). Specific characteristics such as solubility that could influence the formation of micro-aggregates and serum protease resistance affecting the peptide’s pharmacokinetics are likely to determine in great part whether a peptide is able to trigger T-cell responses when administered in the BiVax format. Poly-IC is a double-stranded synthetic RNA that stimulates TLR3 and cytoplasmic RNA helicases (RIG-I and MDA5) resulting in the activation of DCs and the generation of high amounts of type-I IFN [34, 35], which is considered important for the induction of effective T-cell responses [36]. Our results indicate that in the absence of type-I IFN signaling (in IFNαβR KO mice), TriVax was less effective in generating sufficient numbers of CD8 T cells that could be necessary for total disease eradication (Fig. 5b, c). Nevertheless, it was interesting to note a substantial anti-tumor effect of TriVax in the absence of type-I IFN signals, which could be due to the participation of other T-cell stimulatory cytokines such as IL-12 generated by the combination of poly-IC and αCD40 mAb. It was somewhat unexpected to observe a significant anti-tumor effect of TriVax in the absence of IFNγ (Fig. 5b), since this cytokine is considered to be critical for the anti-tumor effects of CD8 T cells [37–39]. Nevertheless, our results indicate that IFNγ may play some role in the overall effectiveness of TriVax to completely reject the tumors. These results contrast with our recent findings in the B16 melanoma system in which IFNγ was shown to play a negative role in the anti-tumor effects of TriVax using Trp1455-463/9M and Trp2280-288, where complete rejections of established tumors were observed in IFNγ KO mice and not in WT mice [27]. Thus, it is possible that IFNγ plays an effector role with the TC-1 tumors, limiting cell proliferation or that the increase MHC-I expression induced by IFNγ in these cells enhances T-cell recognition.
In summary, the results presented herein in a mouse model of HPV-induced cancer demonstrate the feasibility of a novel and potent peptide vaccination strategy that could be adopted for CC or other HPV-induced malignancies. Both poly-IC and αCD40 mAb for human clinical use are in development and together with known human CD8 T-cell epitopes could be administered using the TriVax format with the goal of reducing tumor growth and, perhaps as shown here in mice, eradicate established disease.
Acknowledgments
We gratefully acknowledge Dr. T-C Wu and Dr. W. M. Kast for providing us with the tumor cell lines and are indebted to Dr. A. Salazar for providing large amounts of Poly-ICLC. We also thank Moffitt Cancer Center Flow Cytometry Core, especially J. Kroger for her help in flow cytometer training. This work was supported by NIH grants R01CA136828 and R01CA157303 to EC.
Conflict of interest
Esteban Celis has filed a patent application based on the use of synthetic peptides and poly-IC combinatorial complexes for vaccination. The rights of the patent application have been transferred to the Moffitt Cancer Center. Kelly Barrios declares no conflict of interest.
Abbreviations
- αCD40 mAb
Anti-CD40 monoclonal antibodies
- CC
Cervical carcinoma
- DC
Dendritic cell
- HPV
Human papillomavirus
- MHC-I
Major histocompatibility complex I
- IFNγ
Interferon-gamma
- IFNαβR
Interferon-alpha/beta receptor
- KO
Knockout
- TAA
Tumor-associated antigen
- TLR
Toll-like receptor
References
- 1.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 2.Fleurence RL, Dixon JM, Milanova TF, Beusterien KM. Review of the economic and quality-of-life burden of cervical human papillomavirus disease. Am J Obstet Gynecol. 2007;196:206–212. doi: 10.1016/j.ajog.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus–related disease. Am J Obstet Gynecol. 2004;191:114–120. doi: 10.1016/j.ajog.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 4.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, Jimenez SE, Lowy DR. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 5.FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann AM, Nieland JD, Jochmus I, Baur S, Friese K, Gabelsberger J, Gieseking F, Gissmann L, Glasschroder B, Grubert T, Hillemanns P, Hopfl R, Ikenberg H, Schwarz J, Karrasch M, Knoll A, Kuppers V, Lechmann M, Lelle RJ, Meissner H, Muller RT, Pawlita M, Petry KU, Pilch H, Walek E, Schneider A. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3) Int J Cancer. 2007;121:2794–2800. doi: 10.1002/ijc.23022. [DOI] [PubMed] [Google Scholar]
- 7.Bach PB. Gardasil: from bench, to bedside, to blunder. Lancet. 2010;375:963–964. doi: 10.1016/S0140-6736(09)62029-8. [DOI] [PubMed] [Google Scholar]
- 8.Cecchini S, Carozzi F, Confortini M, Zappa M, Ciatto S. Persistent human papilloma virus infection as an indicator of risk of recurrence of high-grade cervical intraepithelial neoplasia treated by the loop electrosurgical excision procedure. Tumori. 2004;90:225–228. doi: 10.1177/030089160409000211. [DOI] [PubMed] [Google Scholar]
- 9.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 10.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8 + T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung CF, Ma B, Monie A, Tsen SW, Wu TC. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther. 2008;8:421–439. doi: 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer. 2010;102:1129–1136. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, van der Burg SH, Melief CJ. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 15.Zeng Q, Peng S, Monie A, Yang M, Pang X, Hung CF, Wu TC. Control of cervicovaginal HPV-16 E7-expressing tumors by the combination of therapeutic HPV vaccination and vascular disrupting agents. Hum Gene Ther. 2011;22:809–819. doi: 10.1089/hum.2010.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CY, Monie A, Pang X, Hung CF, Wu TC. Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. J Biomed Sci. 2010;17:88. doi: 10.1186/1423-0127-17-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daftarian PM, Mansour M, Pohajdak B, Fuentes-Ortega A, Korets-Smith E, Macdonald L, Weir G, Brown RG, Kast WM. Rejection of large HPV-16 expressing tumors in aged mice by a single immunization of VacciMax encapsulated CTL/T helper peptides. J Transl Med. 2007;5:26. doi: 10.1186/1479-5876-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roman LD, Wilczynski S, Muderspach LI, Burnett AF, O’Meara A, Brinkman JA, Kast WM, Facio G, Felix JC, Aldana M, Weber JS. A phase II study of Hsp-7 (SGN-00101) in women with high-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2007;106:558–566. doi: 10.1016/j.ygyno.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 19.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, ter Schegget J, Melief CJ, Kast WM. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 20.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 21.van Driel WJ, Ressing ME, Kenter GG, Brandt RM, Krul EJ, van Rossum AB, Schuuring E, Offringa R, Bauknecht T, Tamm-Hermelink A, van Dam PA, Fleuren GJ, Kast WM, Melief CJ, Trimbos JB. Vaccination with HPV16 peptides of patients with advanced cervical carcinoma: clinical evaluation of a phase I-II trial. Eur J Cancer. 1999;35:946–952. doi: 10.1016/S0959-8049(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 22.Assudani D, Cho HI, DeVito N, Bradley N, Celis E. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Res. 2008;68:9892–9899. doi: 10.1158/0008-5472.CAN-08-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009;69:9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 25.Sadovnikova E, Zhu X, Collins SM, Zhou J, Vousden K, Crawford L, Beverley P, Stauss HJ. Limitations of predictive motifs revealed by cytotoxic T lymphocyte epitope mapping of the human papilloma virus E7 protein. Int Immunol. 1994;6:289–296. doi: 10.1093/intimm/6.2.289. [DOI] [PubMed] [Google Scholar]
- 26.Nava-Parada P, Forni G, Knutson KL, Pease LR, Celis E. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2007;67:1326–1334. doi: 10.1158/0008-5472.CAN-06-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho HI, Lee YR, Celis E. Interferon gamma limits the effectiveness of melanoma peptide vaccines. Blood. 2011;117:135–144. doi: 10.1182/blood-2010-08-298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Knebel DoeberitzM, Rittmuller C, zur Hausen H, Durst M. Inhibition of tumorigenicity of cervical cancer cells in nude mice by HPV E6-E7 anti-sense RNA. Int J Cancer. 1992;51:831–834. doi: 10.1002/ijc.2910510527. [DOI] [PubMed] [Google Scholar]
- 29.Hu G, Liu W, Hanania EG, Fu S, Wang T, Deisseroth AB. Suppression of tumorigenesis by transcription units expressing the antisense E6 and E7 messenger RNA (mRNA) for the transforming proteins of the human papilloma virus and the sense mRNA for the retinoblastoma gene in cervical carcinoma cells. Cancer Gene Ther. 1995;2:19–32. [PubMed] [Google Scholar]
- 30.Bennett SR, Carbone FR, Toy T, Miller JF, Heath WR. B cells directly tolerize CD8(+) T cells. J Exp Med. 1998;188:1977–1983. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suhrbier A, Burrows SR, Fernan A, Lavin MF, Baxter GD, Moss DJ. Peptide epitope induced apoptosis of human cytotoxic T lymphocytes. Implications for peripheral T cell deletion and peptide vaccination. J Immunol. 1993;150:2169–2178. [PubMed] [Google Scholar]
- 32.Toes RE, van der Voort EI, Schoenberger SP, Drijfhout JW, van Bloois L, Storm G, Kast WM, Offringa R, Melief CJ. Enhancement of tumor outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J Immunol. 1998;160:4449–4456. [PubMed] [Google Scholar]
- 33.Borrow P, Tough DF, Eto D, Tishon A, Grewal IS, Sprent J, Flavell RA, Oldstone MB. CD40 ligand-mediated interactions are involved in the generation of memory CD8(+) cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection. J Virol. 1998;72:7440–7449. doi: 10.1128/jvi.72.9.7440-7449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 36.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev. 2009;20:97–113. doi: 10.1016/j.cytogfr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, Schreiber RD. Interferon-gamma and cancer immunoediting. Immunol Res. 2005;32:231–245. doi: 10.1385/IR:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 39.Apte SH, Groves P, Olver S, Baz A, Doolan DL, Kelso A, Kienzle N. IFN-gamma inhibits IL-4-induced type 2 cytokine expression by CD8 T cells in vivo and modulates the anti-tumor response. J Immunol. 2010;185:998–1004. doi: 10.4049/jimmunol.0903372. [DOI] [PubMed] [Google Scholar]