Abstract
Background
The purpose of this study was to determine whether adding the anti-epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor erlotinib to carboplatin/paclitaxel improved pathologic complete response (pCR) at reassessment surgery in epithelial ovarian, fallopian tube, or primary peritoneal cancers (OFPC).
Methods
Patients with stage III-IV OFPC initiated treatment within 12 weeks of initial cytoreductive surgery or, after histologic confirmation of diagnosis, neoadjuvantly. Treatment included paclitaxel (175 mg/m2) and carboplatin (AUC 6) every 3 weeks for up to 6 cycles, plus oral erlotinib 150 mg daily. The primary objective was to determine whether the pCR rate at reassessment surgery was at least 60% after optimal cytoreduction at initial surgery (< 1 cm residual disease), or at least 40% after suboptimal cytoreduction (at least 1 cm residual disease) using a two-stage design (alpha = 0.10, beta =0.10).
Results
The study population included 56 patients with stage III-IV OFPC. EGFR gene amplification was present in 15% of the 20 tumors evaluated. Twenty-eight patients had protocol therapy after optimal cytoreduction (stratum I), 23 had protocol therapy either after suboptimal cytoreduction (stratum II), and 5 received neoadjuvant therapy prior to cytoreduction (stratum III). Pathologic CR was confirmed in 8 patients (29%; 95% confidence intervals 13%, 49%) in stratum I and 3 patients (11%, 95% C.I. 2%, 28%) in stratum II, which did not meet the prespecified efficacy endpoint in either stratum.
Conclusions
Among unselected patients, erlotinib plus carboplatin-paclitaxel is not more effective than historical experience with carboplatin-paclitaxel alone in patients with stage III-IV OFPC.
Introduction
Epithelial ovarian cancer accounts for approximately 5% of all cancer deaths in women, and is the most common cause of death among the gynecologic malignancies in North America, Western Europe, and Scandinavia.1, 2 More than 75% patients with epithelial ovarian cancer present with advanced stage disease at presentation.3 Despite improvements in survival using a combination of platinum and paclitaxel-based chemotherapeutic regimens,4 only about 30% of patients with advanced stage ovarian carcinoma survive 5 years, and only about 10% are long term survivors.3 Recent evidence suggests new cytotoxic regimens are not more effective than the standard regimen of carboplatin and paclitaxel.5 New therapeutic strategies are needed.
The epidermal growth factor receptor (EGFR) pathway is a potential therapeutic target in epithelial ovarian cancer and other gynecological cancers arising from coelomic epithelium, including epithelial cancers of the fallopian tube and peritoneal cavity. EGFR protein expression is observed in about 30% of epithelial ovarian cancer, and EGFR gene amplification is present in about 20%.6 High tumor EGFR expression has been associated with poor disease free and overall survival after debulking surgery and platinum based chemotherapy.7 Therapeutic agents targeting EGFR include antibodies (cetuximab, panitumumab8,9) and small molecule tyrosine kinase inhibitors (gefitinib, erlotinib10, 11). Some evidence suggests that chemotherapy resistant disease may be more sensitive to EGFR-directed therapies, and that EGFR-directed therapies may exhibit complementary antitumor effects to standard cytotoxic therapy. For example, the paclitaxel resistant A2780 ovarian cancer cell line has been shown to exhibit enhanced sensitivity to the inhibitory effects of erlotinib.12 In addition, treatment with an EGFR inhibitor in the Caov3 ovarian cancer cell line enhanced paclitaxel-induced cell death in vitro.13
Based upon these considerations, we initiated a phase II trial of the EGFR tyrosine kinase inhibitor erlotinib in combination with standard carboplatin-paclitaxel chemotherapy in patients with advanced epithelial ovarian, fallopian tube, and peritoneal cancer (OFPC) after initial cytoreductive surgery. The primary endpoint was pathologic complete response observed at reassessment laparotomy in the study population compared with that expected for a similar population treated with standard chemotherapy alone. Pathologic CR at reassessment surgery after protocol therapy was selected as the primary study endpoint because it is a short term surrogate endpoint known to be associated with improved survival.14 A secondary objective was to determine the prevalence of EFGR gene amplification in this population as a biomarker that may predictive of benefit from this therapeutic approach.
Methods
Eligibility Criteria
Eligibility criteria including histologically-documented International Federation of Gynecologist and Oncologist (FIGO) stage III-IV epithelial ovarian, primary peritoneal or fallopian tube cancer with no prior chemotherapy for treatment of the disease. Patients were required to have had an appropriate debulking surgical procedure by a gynecologic oncologist within 12 weeks of registration. Patients who were deemed by a gynecologist oncologist to be not eligible for primary debulking surgery were also eligible if histologic confirmation of diagnosis was obtained. Other eligibility criteria included: (1) Gynecologic Oncology Group (GOG) performance status 0, 1, or 2, (2) adequate bone marrow (absolute neutrophil count ≥ 1500/uL, platelet count ≥ 100,000/uL), hepatic (transaminases ≤ 2.5-fold above the upper limit of normal, total bilirubin ≤ 1.5-fold above the upper limit of normal) and renal (serum creatinine ≤ 1.5-fold the upper limit of normal) function, (3) no neuropathy, (4) no medical contraindications to the planned regimen, including ongoing or active infection, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, or psychiatric illness/social situations that would limit compliance with study requirements, (5) no other malignancy within five years of study enrollment, (6) no treatment with other investigational agents, (7) no known brain metastases, (8) no history of allergic reaction attributed to agents of similar biologic or chemical composition as those used in the study, (9) not known to be pregnant or lactating, or (9) not known to be infected with the human immunodeficiency virus.
Informed consent and regulatory approval
The study was reviewed and approved by the Cancer Evaluation Therapy Program of the National Cancer Institute (P5886), and by the institutional review board (IRB) at each participating institution (Clinical Trials.gov identifier NCT00059787). All patients provided written informed consent reviewed and approved by the local IRB at each participating site.
Treatment plan
All patients received paclitaxel and carboplatin administered intravenously on day 1 of each 21-day cycle for a total of six cycles. The initial paclitaxel dose was 175 mg/m2 (maximum paclitaxel dose per cycle was 350 mg). Carboplatin was dosed according to the Calvert formula, at an area under the curve (AUC) of 6, with the calculated creatinine clearance (utilizing the Jellife method) estimating glomerular filtration rate (GFR).15 Carboplatin/paclitaxel cycles were repeated every 21 days if the absolute neutrophil count was 1500/uL, platelets at least 100,000/uL, and there was satisfactory recovery from toxicity associated with previous treatment cycles. The treatment plan also included erlotinib, 150 mg PO daily on a continuous basis beginning with day 1 of the first carboplatin-paclitaxel cycle. Erlotinib compliance was monitored by pill counts at each visit for chemotherapy. Chemotherapy treatment was discontinued prior to six cycles if there was disease progression, intercurrent illness which prevented further treatment, unacceptable adverse events which precluded continued treatment, patient refusal to continue, or a change in the patient’s condition which rendered continued treatment unacceptable to the patient or treating physician. Clinicians were permitted to obtain CA-125 levels, but treatment was given according the guidelines outlined above.
Erlotinib was held in patients who developed grade 3 or greater rash or diarrhea (or grade 2 rash or diarrhea poorly tolerated by the patient). Erlotinib was resumed after the toxicity improved to grade 0-1, with the erlotinib dose decreased to 100 mg daily for a first dose reduction, and 50 mg daily for a second dose reduction. Patients with unresolved toxicity after 2 weeks or requiring a delay in erlotinib dosing for 3 weeks or more were removed from study. After completion of carboplatin-paclitaxel plus erlotinib therapy, patients were eligible to receive erlotinib maintenance therapy (150 mg PO daily for up to 12 months). Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events v 3.0 (CTCAE). Erlotinib (Tarceva®, OSI Pharmaceuticals, Melville, NY) was supplied by the Division of Cancer Treatment and Diagnosis (DCTD) of the National Cancer Institute (NCI) under a Clinical Trials Agreement between OSI Pharmaceuticals Inc. and the DCTD, NCI.
Evaluation of EGFR Gene Amplicificaiton
DNA was purified from 15-33 mg of frozen tumor tissue using steps detailed in and materials provided in a Qiagen puregene tissue kit. For each sample, a total of 2.2 μg of ssDNA, labeled and fragmented with the NuGEN kit, was hybridized to Affymetrix arrays (Human U133 plus 2.0), following the manufacturer’s instructions as described previously.16 After washing and staining with biotinylated antibody and streptavidin phycoerythrin, the arrays were scanned with the Affymetrix GeneChip Scanner 3000 for data acquisition. The degree of expression of EGFR was determined by a comparison with a pool of DNA samples processed in the same manner as above.
Response assessment
Surgical reassessment was performed by either laparoscopy or laparotomy to determine whether pathologic complete response (pCR) was achieved within eight weeks of completion of chemotherapy. pCR was defined as having no pathologic or cytologic evidence of disease following surgical reassessment. 14
Maintenance therapy after surgery
Following surgical reassessment, patients with pCR resumed erlotinib (150 mg daily) for up to 12 months. Additionally, patients from any stratum exhibiting complete clinical response but declining surgical reassessment, or patients from stratum 2 or 3 exhibiting response, even if less than pCR, had the option of continuing erlotinib monotherapy. Erlotinib was discontinued prior to 12 months if there was disease progression, unacceptable toxicity, and the patient declined further therapy.
Statistical considerations
The primary study endpoint was pCR determined at the time of reassessment surgery after completion of 6 cycles of carboplatin-paclitaxel plus erlotinib. pCR was evaluated separately in patients with optimally debulked disease (stratum I) and non-optimally debulked disease (stratum II) at initial surgery. Given the additional toxicity expected for the addition of erlotinib, we believed that we need to define a benchmark that would clearly be indicative of significant activity for the combination. In previous studies, the pCR rate at second look laparotomy after platinum-paclitaxel therapy was approximately 20% in patients with suboptimally debulked disease, and would be expected to be higher in patients who had prior optimal debulking. 17, 18 For stratum I, the trial was designed to distinguish between a pCR rate of 40% versus 60%.. For stratum II, the trial was designed to distinguish between a pCR rate of 20% versus 40%. .. Both strata employed Simon’s minimax two-stage design (alpha = 0.10, beta =0.10). In stratum I, accrual would proceed to the second stage if at least 12 of 28 evaluable subjects had a pCR; the regimen would be considered promising if at least 21 of 41 eligible/evaluable subjects had a pCR. In stratum II, accrual would proceed to the second stage if at least 4 of 19 evaluable subjects had a pCR; the regimen would be considered promising if at least 11 of 36 evaluable subjects had a pCR. The analysis plan for stratum III was to provide descriptive results regarding the efficacy and safety of the regimen, and no formal statistical objectives were established.
Results
Patient characteristics
Fifty six patients were enrolled between June 2003 and December 2006. The characteristics of 56 enrolled patients are shown in Table 1, including 28 patients enrolled after optimal debulking surgery (stratum I), 23 patients who enrolled after suboptimal debulking surgery (stratum II), and 5 patients with stage IV disease and who were deemed not to be appropriate candidates for cytoreductive surgery (stratum III).
Table 1.
Patient characteristics
| All patients | Stratum I Optimally Debulked |
Stratum II Suboptimally Debulked |
Stratum III Neoadjuvant |
|
|---|---|---|---|---|
| Total | 56 | 28 | 23 | 5 |
| Age (years) | ||||
| Median | 55.5 | 57.5 | 55 | 53 |
| Range | 22-79 | 23-78 | 30-75 | 45-78 |
| Premenopausal | 17 (30%) | 8 (29%) | 7 (30%) | 2 (40%) |
| Ethnicity | ||||
| Hispanic | 5 (9%) | 2 (7%) | 2 (9%) | 1 (20%) |
| Non-Hispanic | 51 (91%) | 26 (93%) | 21 (91%) | 4 (80%) |
| Race | ||||
| Black | 2 (4%) | 0 (0%) | 1 (4%) | 1 (20%) |
| Asian | 5 (9%) | 2 (7%) | 3 (13%) | 0 (0%) |
| White | 42 (75%) | 22 (79%) | 17 (74%) | 3 (60%) |
| Ashkenazi Jewish | 13 (23%) | 8 (29%) | 3 (13%) | 2 (40%) |
| Smoking history | 18 (32%) | 11 (39%) | 6 (26%) | 1 (20%) |
| Primary Tumor | ||||
| Ovary | 45 (80%) | 22 (79%) | 18 (78%) | 3 (60%) |
| Fallopian Tube | 3 (5%) | 2 (7%) | 1 (4%) | 0 (0%) |
| Primary Peritoneal | 8 (14%) | 4 (14%) | 2 (9%) | 2 (40%) |
| Histology | ||||
| Papillary serous | 37 (66%) | 22 (79%) | 13 (57%) | 2 (40%) |
| Papillary serous + other |
2 (4%) | 2 (7%) | 0 (0%) | 0 (0%) |
| Other | 13 (23%) | 3 (11%) | 7 (30%) | 3 (60%) |
| Stage | ||||
| III | 37 (66%) | 28 (100%) | 9 (39%) | 0 (0%) |
| IV | 14 (25%) | 0 (0%) | 9 (39%) | 5 (100%) |
Administration of erlotinib plus carboplatin/paclitaxel
Of the 56 patients enrolled, 36 (64%) completed six cycles of the erlotinib-chemotherapy combination, and 20 (36%) did not. Reasons for discontinuing protocol therapy prior to completing six cycles included adverse events in 13 patients (23%), disease progression in 2 patients (4%), protocol ineligibility in 1 patient (2%), and other medical problems not associated with treatment in 4 patients (7%), including postoperative infection (N=2), liver disease (N=1), and cardiac disease (N=1).
Administration of maintenance erlotinib alone
Sixteen patients (29%) received maintenance erlotinib therapy for a maximum of one year, including 11 patients with surgically confirmed pCR, 3 patients with less than a pCR at surgery, and 2 patients with clinical complete response who did not undergo reassessment surgery. Twelve of 16 patients (75%) completed one year of maintenance therapy without recurrence, and 4 patients (25%) recurred during maintenance therapy. No patients discontinued maintenance therapy due to adverse events.
Adverse events
Adverse events are summarized in Table 2. The most common grade 3-4 adverse events occurring in at least 5% of patients included neutropenia (18%), skin rash (17%), thrombocytopenia (7%), infection (7%), and fatigue (5%). Erlotinib-associated toxicities including skin rash occurred in 46% of patients (including 21% grade 2, 13% grade 3, 4% grade 4), and diarrhea occurred in 25% (including 7% grade 2 and 4% grade 3).
Table 2.
Adverse events
| Grades 1-4 | Grade 2 | Grade 3 | Grade 4 |
|
||||
|---|---|---|---|---|---|---|---|---|
| Toxicity |
No. of
Patients |
% |
No. of
Patients |
% |
No. of
Patients |
% |
No. of
Patients |
% |
| Hematologic & Infectious | ||||||||
| Hemoglobin (anemia) | 11 | 20 | 6 | 11 | 2 | 4 | 0 | 0 |
| Neutropenia | 14 | 25 | 3 | 5 | 8 | 14 | 2 | 4 |
| Thrombocytopenia | 13 | 23 | 4 | 7 | 3 | 5 | 1 | 2 |
| Infection | 5 | 4 | 0 | 0 | 3 | 5 | 1 | 2 |
| Non-Hematologic | ||||||||
| Constitutional | ||||||||
| Anorexia | 6 | 11 | 2 | 4 | 0 | 0 | 0 | 0 |
| Fatigue | 16 | 29 | 5 | 9 | 3 | 5 | 0 | 0 |
| Hepatic | ||||||||
| Alkaline phosphatase | ||||||||
| Alanine transaminase (ALT/SGPT) | 2 | 4 | 0 | 0 | 0 | 0 | 1 | 2 |
| Aspartate transaminase (AST/SGOT) | 3 | 8 | 0 | 0 | 0 | 0 | 1 | 2 |
| Mucoenterocutaneous | ||||||||
| Constipation | 11 | 20 | 3 | 8 | 1 | 2 | 0 | 0 |
| Diarrhea | 14 | 25 | 4 | 7 | 2 | 4 | 0 | 0 |
| Mucositis: Oral | 6 | 11 | 3 | 8 | 0 | 0 | 0 | 0 |
| Nausea | 11 | 20 | 5 | 9 | 1 | 2 | 0 | 0 |
| Rash: desquamation | 26 | 46 | 12 | 21 | 7 | 13 | 2 | 4 |
| Vomiting | 7 | 13 | 5 | 9 | 0 | 0 | 1 | 2 |
| Neurologic | ||||||||
| Neuropathy: Sensory | 12 | 21 | 4 | 7 | 0 | 0 | 0 | 0 |
| Pain | ||||||||
| Pain: Abdomen | 6 | 11 | 2 | 4 | 1 | 2 | 0 | 0 |
| Renal/Metabolic | ||||||||
| Hypokalemia | 2 | 4 | 0 | 0 | 1 | 2 | 0 | 0 |
| Hyperglycemia | 2 | 4 | 1 | 2 | 1 | 2 | 0 | 0 |
Note: includes all adverse events occurring in at least 5% or where there is at least one occurrence of a grade 3 or 4 event
The combination of erlotinib plus carboplatin-paclitaxel was discontinued due to adverse events prior to completing 6 cycles of therapy in 13 patients (23%), including erlotinib-associated toxicities in 9 patients (16%). Erlotinib-associated toxicities which contributed to treatment discontinuation included skin rash in 7 patients (13%), nausea in 1 patient (2%), and hypersensitivity in 1 patient (2%). Other adverse events not attributed to erlotinib contributed to discontinuation of therapy in 4 patients (7%), including hypersensitivity to paclitaxel in 1 patient (2%).
Response evaluation after chemotherapy plus erlotinib
Response data are shown in Table 3. In stratum I, 18 of 28 patients (64%) underwent reassessment surgery. No patient had progressive disease during therapy. Eight of 28 patients achieved pCR (29%, 95% confidence intervals [C.I.] 13%, 49%), 10 patients (36%) had residual disease at reassessment surgery, and 10 patients (36%) did not undergo reassessment surgery for a variety of reasons. Two of 10 patients who did not have reassessment surgery had a complete clinical response but declined surgery and remain without evidence of disease two years after completing erlotinib maintenance therapy (three years following completion of paclitaxel, carboplatin and erlotinib).
Table 3.
Results of second look laparotomy
| Stratum I Optimally Debulked |
Stratum II Suboptimally Debulked |
|
|---|---|---|
| No. | 28 | 23 |
|
| ||
| No Residual Disease as Surgery | 8 (29%) (95% CI 13%, 48%) |
3 (13%) (95% CI 3%, 36%) |
|
| ||
| Residual Disease at Surgery | 10 (36%) | 18 (64%) |
|
| ||
| Surgery Not Performed | 10 (36%) | 2 (9%) |
| Clinical CR, decline surgery | 2 | 0 |
| Toxicity or other reasons | 8 | 2 |
In stratum II, 21 of 23 patients (75%) underwent reassessment surgery. Three of 23 achieved pCR patients (13%, 95% C.I. 3%, 36%), 18 patients (78%) had residual disease at surgery, and 2 patients (9%) did not undergo surgery.
Of the 5 who received neoadjuvant therapy in stratum III, no patient underwent initial cytoreductive surgery. Three patients achieved complete clinical response, one patient had stable disease, and one patient did not complete treatment due to medical comorbidities. At surgery following treatment, four neoadjuvant patients were able to be optimally cytoreduced.
EGFR gene amplification and response to therapy
Tumor specimens were evaluated for EGFR gene amplification by in 20 patients. No amplification was observed in 11 patients (55%), low level amplification in 6 patients (30%), and moderate-high amplification was observed in 3 patients (15%). There was no significant difference in the likelihood of having a pCR for patients with no amplification (2 of 11) compared with those who had some amplification (4 of 9) (p=0.33, Fisher’s exact test).
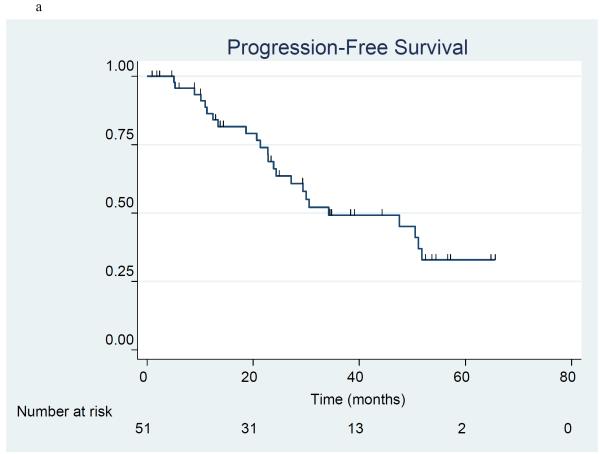
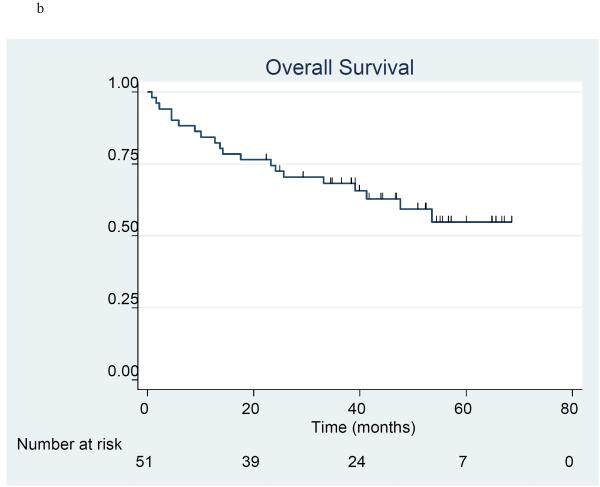
Progression-free survival and overall survival
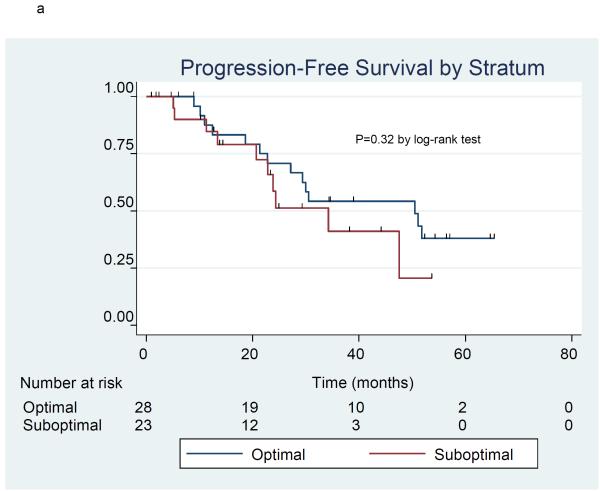
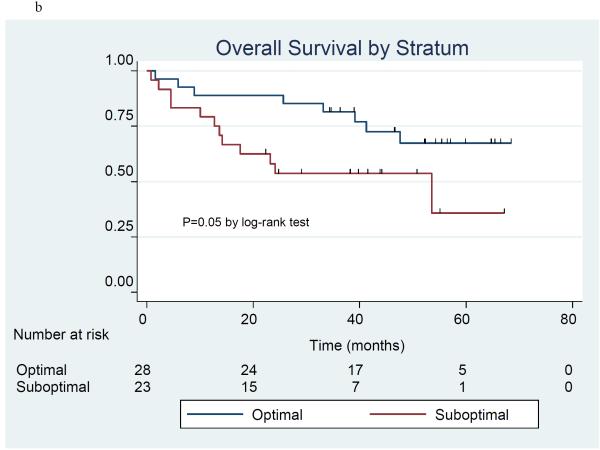
The product-limit method of Kaplan and Meier was used to estimate progression free survival (Figure 1a) and overall survival (Figure 1b) in the 51 patients included in stratum I and II. There were 24 patients who had disease progression and 20 patients who died at the time of the analysis. The 3-year progression-free survival (PFS) rate was 49.2% (95% C.I. 32.6%, 63.9%), and median PFS was 34.3 months (95% C.I. 23.9 - 51.8 months). The 3-year overall survival (OS) rate was 68.2% (95% C.I. 53.5%, 79.2%), and median OS l had not yet been reached. Information by stratum is provided for PFS (Figure 2a) and OS (Figure 2b). For PFS, the 3-year PFS rate was 54.2% (95% CI 32.7%, 71.4%) and median PFS was 50.5 months (95% CI 22.8 months, upper limit not estimated) in the optimal stratum; in the suboptimal stratum, the 3-year PFS rate was 41.0% (95% CI 15.5%, 65.3%) and median PFS was 34.3 months (95% CI 20.7 months, upper limit not estimated). For OS, the 3-year OS rate was 81.5% (95% CI 61.1%, 91.8%) and median OS has not been reached in the optimal stratum; in the suboptimal stratum, the 3-year OS rate was 53.6% (95% CI 32.0%, 71.1%), and the median OS was 53.5 months. (95% CI 13.6 months, upper limit not estimated).
Figure 1a-b.
Progression-free survival (1a) and overall survival (1b) in 51 patients treated with erlotinib plus carboplatin/paclitaxel prior to surgery.
Figure 2a-b.
Progression-free survival (2a) and overall survival (2b) in 51 patients treated with erlotinib plus carboplatin/paclitaxel prior to surgery by treatment stratum.
Discussion
We performed a phase II trial of the oral EGFR tyrosine kinase inhibitor erlotinib in combination with first-line carboplatin-paclitaxel chemotherapy in patients with stage III-IV epithelial ovarian, fallopian tube, and peritoneal cancer who had undergone prior cytoreductive surgery, of whom about 80% had a ovarian primary cancer. Our objective was to increase by two-fold the pCR rate at reassessment surgery in those who had gross residual disease at initial cytoreductive surgery (from 20% expected with historical data to 40%), and by 50% in those has had been optimally debulked at initial cytoreductive surgery (from 40% expected with historical data to 60%). We found no evidence that addition of erlotinib to carboplatin-paclitaxel improved the likelihood of achieving a pCR in either population, and conclude that this approach should not be further pursued among unselected patients.
A secondary objective of the study was to evaluate the incidence of EGFR gene amplification. We found moderate-high amplification in 15%, low level amplification in 30%, and no amplification in 55% of the 20 specimens evaluated. Our findings are consistent with another report which included 398 cases of serous ovarian carcinoma, in which EGFR gene amplification was detected in detected in 12% and low-level gain in 43%.19 Low level or greater EGFR amplification was not associated with a higher response rate in our trial, suggesting that enrichment for tumors exhibiting EGFR amplification would not likely identify subjects likely to benefit from erlotinib plus chemotherapy.
Some evidence suggests anti-EGFR directed therapies produce clinical benefit in selected patients with chemotherapy resistant ovarian cancer. For example, a phase II study of cetuximab (400 mg/m2 IV loading dose, then 250 mg/m2 weekly) in 25 patients with refractory ovarian cancer unselected for EGFR tumor overexpression revealed objective response in 1 patient (4%) and stable disease for at least 8 weeks in 9 patients (36%).20 A phase II trial of gefitinib (500 mg daily) in 27 evaluable patients with refractory ovarian cancer not selected for tumor EGFR overexpression revealed one patient who had an objective response (4%) and four patients who were progression free for at least 6 months (15%); the only responding patient had a mutation in the EGFR catalytic domain.21 Finally, a phase II study of erlotinib (150 mg daily) in 32 patients with refractory ovarian cancer selected for EGFR tumor overexpression revealed objective response in 2 patients (6%) and stable disease for at least 8 weeks in 15 patients (44%); the development of rash was associated with improved survival.22 Other studies have likewise reported an association between drug-induced rash and improved outcomes in other cancers treated with EGFR inhibitors, including cetuximab for colorectal cancer23 and erlotinib for non-small cell lung cancer.24, 25 Although these studies suggest that selected patients may benefit from erlotinib monotherapy, our study found no evidence for improved outcomes when erlotinib was combined with chemotherapy. These finding are consistent with the activity of erlotinib in non-small cell lung cancer, in which erlotinib has been shown to be associated with improved survival when used as monotherapy26, 27, but is not associated with clinical benefit when added to cytotoxic therapy.28,29
In conclusion, we found no evidence that erlotinib produces clinical benefit when added to standard chemotherapy after initial debulking surgery in patients with ovarian, fallopian tube, or peritoneal cancer. A phase III trial is being conducted by the European Organization of Research and Treatment (EORTC) to evaluate the role of maintenance erlotinib after first line chemotherapy in this population (NCT00263822). This trial will define if there is a role for erlotinib in the management of advanced ovarian cancer.
Acknowledgement
The authors acknowledge the late Dr. Scott Wadler, our colleague and friend, who conceived of and founded the New York Cancer Consortium and who played a key role in the development of this trial.
Supported by NIH M01-RR-00096, N01-CM-62204, SAIC-Frederick, Inc. Contract 23XS010A, and the Lynne Cohen Foundation for Ovarian Cancer Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Society of Gynecologic Oncologists 38th Annual Meeting in San Diego, CA, March 3-7, 2007, and at the American Society for Clinical Oncology Annual Meeting 2005 and 2006
Disclosures: The authors report no disclosures or conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Wethington SL, Herzog TJ, Seshan VE, et al. Improved survival for fallopian tube cancer: a comparison of clinical characteristics and outcome for primary fallopian tube and ovarian cancer. Cancer. 2008;113:3298–306. doi: 10.1002/cncr.23957. [DOI] [PubMed] [Google Scholar]
- 4.Huang L, Cronin KA, Johnson KA, Mariotto AB, Feuer EJ. Improved survival time: what can survival cure models tell us about population-based survival improvements in late-stage colorectal, ovarian, and testicular cancer? Cancer. 2008;112:2289–300. doi: 10.1002/cncr.23425. [DOI] [PubMed] [Google Scholar]
- 5.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–25. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stadlmann S, Gueth U, Reiser U, et al. Epithelial growth factor receptor status in primary and recurrent ovarian cancer. Mod Pathol. 2006;19:607–10. doi: 10.1038/modpathol.3800575. [DOI] [PubMed] [Google Scholar]
- 7.Psyrri A, Kassar M, Yu Z, et al. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin Cancer Res. 2005;11:8637–43. doi: 10.1158/1078-0432.CCR-05-1436. [DOI] [PubMed] [Google Scholar]
- 8.Blick SK, Scott LJ. Cetuximab: a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs. 2007;67:2585–607. doi: 10.2165/00003495-200767170-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hoy SM, Wagstaff AJ. Panitumumab: in the treatment of metastatic colorectal cancer. Drugs. 2006;66:2005–14. doi: 10.2165/00003495-200666150-00011. discussion 2015-6. [DOI] [PubMed] [Google Scholar]
- 10.Tang PA, Tsao MS, Moore MJ. A review of erlotinib and its clinical use. Expert Opin Pharmacother. 2006;7:177–93. doi: 10.1517/14656566.7.2.177. [DOI] [PubMed] [Google Scholar]
- 11.Frampton JE, Easthope SE. Gefitinib: a review of its use in the management of advanced non-small-cell lung cancer. Drugs. 2004;64:2475–92. doi: 10.2165/00003495-200464210-00008. [DOI] [PubMed] [Google Scholar]
- 12.Dai Q, Ling YH, Lia M, et al. Enhanced sensitivity to the HER1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib hydrochloride in chemotherapy-resistant tumor cell lines. Clin Cancer Res. 2005;11:1572–8. doi: 10.1158/1078-0432.CCR-04-0993. [DOI] [PubMed] [Google Scholar]
- 13.Qiu L, Di W, Jiang Q, et al. Targeted inhibition of transient activation of the EGFR-mediated cell survival pathway enhances paclitaxel-induced ovarian cancer cell death. Int J Oncol. 2005;27:1441–8. [PubMed] [Google Scholar]
- 14.Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007;25:2873–83. doi: 10.1200/JCO.2007.11.0932. [DOI] [PubMed] [Google Scholar]
- 15.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–22. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- 16.Caretti E, Devarajan K, Coudry R, et al. Comparison of RNA amplification methods and chip platforms for microarray analysis of samples processed by laser capture microdissection. J Cell Biochem. 2008;103:556–63. doi: 10.1002/jcb.21426. [DOI] [PubMed] [Google Scholar]
- 17.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 18.Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000;18:106–15. doi: 10.1200/JCO.2000.18.1.106. [DOI] [PubMed] [Google Scholar]
- 19.Lassus H, Sihto H, Leminen A, et al. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med. 2006;84:671–81. doi: 10.1007/s00109-006-0054-4. [DOI] [PubMed] [Google Scholar]
- 20.Schilder RJ, Pathak HB, Lokshin AE, et al. Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecol Oncol. 2009;113:21–7. doi: 10.1016/j.ygyno.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schilder RJ, Sill MW, Chen X, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin Cancer Res. 2005;11:5539–48. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 22.Gordon AN, Finkler N, Edwards RP, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Cancer. 2005;15:785–92. doi: 10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non--small-cell lung cancer. J Clin Oncol. 2004;22:3238–47. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23:5235–46. doi: 10.1200/JCO.2005.00.6916. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd FA, Pereira J Rodrigues, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 27.Spigel DR, Lin M, O’Neill V, Hainsworth JD. Final survival and safety results from a multicenter, open-label, phase 3b trial of erlotinib in patients with advanced nonsmall cell lung cancer. Cancer. 2008;112:2749–55. doi: 10.1002/cncr.23490. [DOI] [PubMed] [Google Scholar]
- 28.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 29.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–52. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]