Abstract
Background
3-Aminopyridine-2-carboxaldehydethiosemicarbazone (3-AP) is a novel small molecule ribonucleotide reductase (RR) inhibitor which is more potent than hydroxyurea, the prototype of RR inhibitors. 3-AP enhances the cellular uptake and DNA incorporation of gemcitabine in tumor cell lines. We evaluated the combination of 3-AP plus gemcitabine in advanced biliary tract adenocarcinoma.
Methods
Thirty-three patients with advanced adenocarcinoma of the gall bladder or biliary tract received gemcitabine (1,000 mg/m2 on days 1, 8, and 15 every 28 days) 1 h after completing a 4-h infusion of 3-AP given at a dose of 105 mg/m2 in patients with normal liver function (stratum A) or 80 mg/m2 if abnormal liver function (stratum B). The trial was designed to determine whether the response rate was at least 30% in stratum A and 20% in stratum B.
Results
Objective response occurred in 3 of 23 patients (13%, 95% confidence intervals [CI] 3, 34%) with normal liver function, and in 0 of 10 patients with abnormal liver function. The most common grade 3–4 adverse events in all patients included neutropenia (42%), infection (33%), thrombocytopenia (27%), anemia (18%), and fatigue (15%). Fine needle aspiration of tumor samples obtained before and 24 h after 3-AP therapy showed increased R2 mRNA expression by in situ RT–PCR, suggesting RR inhibition.
Conclusions
Despite evidence for RR inhibition in vivo, the 3-AP plus gemcitabine combination is not likely to be associated with a response rate exceeding 30% in patients with adenocarcinoma of the biliary tract.
Keywords: Biliary tract cancer, Gemcitabine, 3-aminopyridine-2-Carboxaldehydethiosemicarbazone, Ribonucleotide reductase
Introduction
Adenocarcinoma of the gallbladder and biliary tract is uncommon, but not rare; it was diagnosed in approximately 9,800 individuals in the United States, and it caused about 3,400 deaths in 2009 [1]. This cancer is most commonly observed in northwest Thailand, where biliary flukes are common [2]. Chronic viral hepatitis B and C have also been implicated [3]. Although tumors arising distally in the Ampulla of Vater are generally discovered at an early stage and are associated with a relatively good prognosis, tumors arising in the gallbladder or other parts of the biliary tract are associated with a poor prognosis [4]. Gemcitabine has been reported to have some clinical activity in biliary tumors, with variable response rates of approximately 30% or less [5–9], with a response of 15.5% in the largest trial which included 202 patients who received single agent gemcitabine [10]. The latter trial also demonstrated a significant survival benefit for the cisplatin–gemcitabine combination compared with gemcitabine alone (median survival 11.7 vs. 8.2 months. P = 0.002) [10]. However, survival remains generally poor, and new therapeutic targets and options are needed.
Ribonucleotide reductase (RR) is a potential therapeutic target for cancer therapy. It catalyzes the reduction step converting nucleotide diphosphates to their 2′-deoxy forms, the rate-limiting step in the synthesis of the 2′-deoxy nucleoside triphosphates from purine- and cytidine-based DNA precursors [11]. RR is a heterodimeric compound consisting of two subunits, R1 and R2. R2 acts in concert with various activated oncogenes [12], and R2 overexpression is associated with increased Raf-1 membrane– associated protein and mitogen-activated kinase (MAPK) activity [13] and resistance to cytotoxic therapy [14]. Regulation of RR activity is complex; in addition to cytokinetically regulated synthesis, activity is also regulated by allosteric control mechanisms with both positive and negative effectors [15]. Hydroxyurea (HU) inactivates the tyrosyl free radical on the R2 subunit, but its efficacy is limited by its short half-life and the need for parenteral administration to maintain serum levels required to inhibit R2 (>1 μM).
Mammalian RR has a dimeric structure, with each subunit (M1 and M2) consisting of a nucleotide-binding site (M1) and a metal-binding site (M2). The M1-affecting RR inhibitors are nucleoside analogs (i.e., gemcitabine), whereas the M2 subunit contains non-heme iron and a tyrosine free radical, which are required for the enzymatic reduction of ribonucleotides. Inhibitors of the M2 subunit act by destroying the free radical. In the case of hydroxyurea, which is the only clinically approved RR inhibitor acting at the M2 subunit, inhibition is reversible due to the ease in regenerating the tyrosine free radical by mammalian cells [16]. 3-Aminopyridine-2-carboxaldehydethiosemicarbazone (3-AP; Triapine®, formerly Vion Pharmaceuticals, Inc, New Haven, CT) is a heterocyclic carboxaldehyde thiosemicarbazone RR inhibitor that also acts on the M2 subunit of RR but is up to 5,000 times more potent than hydroxyurea [17]. 3-AP exhibits broad antitumor activity in the National Cancer Institute (NCI) 60 cell line screen in the micromolar range [17, 18]. 3-AP also enhances cellular uptake and DNA incorporation of gemcitabine into tumor cell lines and exhibits marked synergistic cytotoxicity in vitro [19]. A phase I study established the recommended phase II dose and safety of 3-AP given as a 4-h infusion followed by a 30-min gemcitabine infusion weekly for 3 of 4 weeks [20]. Based upon these considerations, we initiated a phase II trial of 3-AP in patients with advanced adenocarcinoma of the gallbladder and biliary tract and sought to determine the effects of 3-AP on RR activity in tumor and in peripheral blood mononuclear cells.
Methods
Patient selection
Eligible criteria included histologically or cytologically confirmed, unresectable or metastatic adenocarcinoma of the gallbladder or biliary tree with measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST, Version 1.0). Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status score of 0, 1, or 2, be 18 years of age or older, have no prior chemotherapy for the cancer, and be able to provide written informed consent. Other requirements included adequate bone marrow function (leukocyte count at least 3,500/μL, neutrophil count at least 1,500/μL, platelets at least 150,000/μL) and kidney function (normal serum creatinine). Patients were permitted to have mildly abnormal liver function, defined as a total bilirubin above the upper limits of normal (ULN) and less than or equal to threefold above the institutional upper limits of normal (includes CTCAE version 3 grades 1–2 hyperbilirubinemia). Patients were excluded if they were pregnant or breastfeeding women, had glucose-6-phosphate dehydrogenase deficiency (due to the risk of methemoglobinemia associated with 3-AP) [21], brain metastases, another malignancy (except early stage squamous cell carcinoma of skin or cervix), or an uncontrolled intercurrent illness (e.g., infection, congestive heart failure, unstable angina, cardiac arrhythmia, congenital or acquired immune deficiency, or psychiatric illness that could potentially impact compliance).
Treatment regimen and strata
All patients received gemcitabine 1,000 mg/m2 via a 30 min IV infusion beginning 1 h after completing the 3-AP infusion on days 1, 8, and 15 every 28 days. Patients with normal liver function (total bilirubin within normal limits) were assigned to stratum A and received 3-AP 105 mg/m2 as a 4-h IV infusion. Patients with mildly abnormal liver function were assigned to stratum B (up to threefold above the upper limits of normal for total bilirubin) and received 3-AP 80 mg/m2 as a 4-h IV infusion. Treatment was continued until progression of disease, unacceptable toxicity, intercurrent condition, declining performance status preventing further treatment, or patient withdrawal. Patients developing emesis with the initial or a subsequent treatment received prophylactic antiemetic treatment prior to every subsequent dose. All patients were observed clinically for 3–4 h after the 3-AP infusion during the first week of treatment for the development of dyspnea, hypoxemia, and possible methemoglobinemia. All patients received dexamethasone, 4 mg intravenously prior to each 3-AP infusion. Acute reactions to 3-AP, occurring either during the infusion or soon after the infusion is completed, have been observed, primarily at doses ≥140 mg/m2 infused IV over 2–4 h [20]. The reactions include hypoxia (with or without dyspnea and with or without associated cough) and hypotension. The cause of hypoxia and dyspnea is thought to be an increase in methemoglobin levels that usually resolve quickly (within hours) after the completion of the infusion.
For hematologic toxicity, gemcitabine was held if the neutrophil count was less than 1,500/μL and platelets less than 100,000/μL. The dose of gemcitabine in the new cycle was permanently reduced by 20% for the following: (1) toxicity that required holding a dose in the previous cycle, (2) grade 4 neutropenia occurring prior to the administration of the third dose, or lasting more than 3 days, or associated with febrile neutropenia, (3) platelet count less than or equal to 20,000/μL prior to the third dose of the previous cycle, or lasting more than 5 days, or associated with bleeding requiring a transfusion, (4) grade 3 non-hematologic toxicity with the exception of nausea/vomiting controlled with supportive care. Treatment was discontinued permanently for grade 4 non-hematologic toxicity or toxicity requiring delay of a new cycle for more than 2 weeks.
Response and toxicity evaluation
Computed tomography or magnetic resonance imaging scans of measurable lesions were obtained at baseline and every 8 weeks. Responses were classified according to RECIST criteria (version 1.0) [22]. National Cancer Institute Common Adverse Events Criteria version 3.0 was used to grade toxicity.
Statistical considerations
Simon’s two-stage optimal design was used to determine the number of patients required. For patients with normal liver function, the trial was designed to distinguish between a response rate of 15% or less versus 30% or higher (alpha 0.05, beta 0.80). If 4 or more responses were observed among the first 21 patients, the trial would continue to up to 49 evaluable subjects; if 12 or more responses were seen, the regimen would be declared promising. For patients with abnormal liver dysfunction, the trial was designed to distinguish between a response rate of 5% or less versus 20% or higher (alpha 0.05, beta 0.80). If 1 or more responses were observed among the first 10 patients, accrual would continue to up to 29 evaluable patients; if 3 or more responses, the regimen would be declared promising.
Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method, and 95% confidence intervals were constructed for median PFS and OS. PFS was defined as the time between registration and either progression of disease or death from any cause. All analyses were performed in SAS version 9.1 (SAS Institute, Inc., Cary, NC) and Stata version 10.0 (Stata Corporation, College Station, TX). All patients had progressive disease or discontinued therapy at the time of the analysis.
Correlative studies
Tumor samples
In situ RT–PCR for the small β2 homodimer of ribonucleotide reductase (M2) mRNA expression was evaluated in tumor samples before and 24 h after 3-AP/gemcitabine using previously described “Methods” [23]. Tumors were aspirated under computerized tomography guidance and smeared on the glass slides. Polymerase chain reaction (PCR) was performed in 50 μl of standard PCR mixture with 1.6 μM of RRM2 upstream primer, 5′TGAGAGA AAACCCCCGCCGCTTT-3′ and RRM2 downstream primer, 5′-GTGAGGCCAGGCATAGTCCTCGT-3′ (Invitrogen, Carlsbad, CA). Hot-start PCR was carried out on an In Situ PCR System 1000 with initial denaturation at 94°C for 1 min and annealing/extension, at 62°C for 2 min. The PCR products were detected by hybridizing with a RRM2 region-specific probe labeled with digoxigenin: 5′-TTTGTCCCCAATCCAAGGCAAG-3′ (Invitrogen) at 42°C overnight, in hybridization solution (10% deionized formamide, Sigma–Aldrich, St. Louis, MO), 10% (wt/vol) dextran sulfate (Sigma–Aldrich), 2× SSC and 5 pmol/100 μl probe. After hybridization, slides were washed in 1× SSC and 0.1% BSA for 10 min at 54°C, followed by washes in buffer 1(0.1 M Tris–HCL, pH 7.5, 0.15 M NaCl) and then incubated at room temperature for 30 min in 1:50 diluted anti-digoxigenin-AP Fab fragments in Buffer 1. Following a rinse in buffer 2 (0.1 M Tris-HCI, pH 9.5, 0.15 M NaCl, 50 mM MgCI2) at room temperature for 5 min, the slides were incubated in nitro blue tetrazolium(NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP) (Boehringer Mannheim)(Roche Applied Science, Indianapolis, IN) at a ratio of 1:400:1 for NBT:Buffer:BCIP.
Peripheral blood mononuclear cells
QRTPCR analysis was performed using ten milliliters of peripheral blood that was ficolled for isolation of mononuclear cells (PBMC) and evaluated for mRNA expression. PBMCs were isolated, homogenized, and frozen in RLT (Qiagen, Valencia, CA) buffer. The Qiagen RNeasy or PAXgene kits (Valencia, CA) were used to extract RNA from blood samples, and RNA was quantified using the Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). The integrity of the RNA samples was determined by using the Agilent Bioanlayzer (Agilent, Santa Clara, CA). Total RNA (0.6–1.0 μg) was converted to cDNA using the Quanta Bioscience (Gaithersburg, MD) qScript cDNA Supermix or the SuperScriptTM III Reverse Transcriptase Kit (Invitrogen, Calsbad, CA) for a total volume of 20 μl. The reactions were prepared according to manufacturer’s instructions. The qPCR reactions were carried out in triplicate using cDNA and the following assays from Applied Biosciences (Foster City, CA): Hs01072069_g1 (ribonucleotide reductase M1 [RRM1]), Hs01040698_m1 (ribonucleotide reductase M2 [RRM2]), and Hs00968432_m1 (ribonucleotide reductase M2B [p53R2]). Human TATA-box-binding protein assays, also from Applied Biosciences, were used as endogenous controls. All assays were performed in triplicate, and the relative gene expression data compared with pre-treatment samples were quantified using the comparative Ct (Δ-Δ Ct) method or the 2−ΔCT and converted to fold change relative to pre-treatment sample.
dCTP pool measurement was performed in PBMC via an enzymatic assay using Sequenase enzyme (USB Corporation, Cleveland, OH). The reaction mixture contained 0.25 μM annealed template-primer specific for dCTP (Invitrogen), 50 mMTris-HCl pH 7.5, 10 mM MgCl2, 5 mM DTT, 2.5 mM3H-dATP, and 0.078 U Sequenase 2.0. Ten microliters of extracts prepared from cells was added to 40 μl of reaction mixture. The reaction was carried out in triplicate at room temperature for 20 min, and 40 μl was blotted and dried onto disks of Whatman DE81 paper. The filters were washed 3 times for 15 min each with 5% Na2HPO4, washed once with ddH2O, and rinsed with 95% ethanol, dried, and counted using a Beckman Coulter LS6500 scintillation counter (Brea, CA). A one-tailed t-test was performed on the dCTP data comparing pre-treatment and post-treatment groups.
Informed consent and regulatory approval
The study was reviewed and approved by the Cancer Evaluation Therapy Program of the National Cancer Institute (P6254) and by the institutional review board at each participating institution in the New York Cancer Consortium (Montefiore Medical Center, New York Presbyterian Hospital, and Mt. Sinai) (Clinical Trials.gov identifier NCT00075504). All patients provided written informed consent which was reviewed and approved by the local institutional review board.
Results
Patient characteristics
Thirty-three patients were enrolled and treated between December 2003 and July 2007 at four institutions. The characteristics of the patients are shown in Table 1, including 23 patients in stratum A with normal liver function and 10 patients in stratum B with abnormal liver function.
Table 1.
Patient characteristics
| Characteristics | Stratum A: normal liver function |
Stratum B: abnormal liver function |
|---|---|---|
| No. | 23 | 10 |
| Age (years) | ||
| Median | 57 | 67.5 |
| Gender | ||
| Male | 11 | 6 |
| Female | 12 | 4 |
| Race | ||
| White | 20 | 10 |
| Asian | 2 | 0 |
| Black | 1 | 0 |
| Ethnicity | ||
| Non-Hispanic | 22 | 8 |
| Hispanic | 1 | 2 |
| Unknown | 0 | 0 |
| ECOG PS | ||
| 0 | 10 | 1 |
| 1 | 12 | 6 |
| 2 | 1 | 2 |
| Unknown | 0 | 1 |
| Primary site | ||
| Gallbladder | 12 | 6 |
| Other | 11 | 4 |
Treatment administered
For patients in stratum A, the median number of treatment cycles given was 4 (range 1–19 cycles), and reasons for discontinuation of therapy included disease progression in 12 patients (52%) after a median of 4 cycles (range 2–18), toxicity or patient withdrawal in 9 patients (39%) after a median of 2 cycles (range 1–8), and other reasons in 2 patients (9%) after a median of 12 cycles (range 5–19). Ten patients (43%) required a dose reduction/modification.
For patients in stratum B, the median number of treatment cycles given was 2.5 (range 1–16 cycles), and reasons for discontinuation of therapy included disease progression in 2 patients (20%) after a median of 8.5 cycles (range 1–16), toxicity or patient withdrawal in 7 patients (70%) after a median of 2 cycles (range 1–6), and other reasons in 1 patient (10%) after a median of 12 cycles. Four patients (40%) required a dose reduction/modification.
Adverse events
The incidence of grade 3–4 adverse events occurring in at least 5% for patients (or any grade 4 event occurring in at least one patient) in both treatment strata is shown in Table 2. The most common grade 3–4 adverse events occurring in at least 10% of patients with normal liver function (stratum A) included neutropenia (43%), infection (30%), anemia (22%), thrombocytopenia (22%), and elevated liver transaminase levels (13%). The toxicity profile was similar in patients with abnormal liver function (stratum B).
Table 2.
Grade 3–4 adverse events
| Toxicity | Stratum A: normal liver function |
Stratum B: abnormal liver function |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All grades |
Grade 3 |
Grade 4 |
All grades |
Grade 3 |
Grade 4 |
|||||||
| No. of patients |
% | No. of patients |
% | No. of patients |
% | No. of patients |
% | No. of patients |
% | No. of patients |
% | |
| Hematologic and infectious | ||||||||||||
| Hemoglobin (anemia) | 5 | 22 | 5 | 22 | 0 | 0 | 1 | 10 | 1 | 10 | 0 | 0 |
| Neutropenia | 10 | 43 | 7 | 30 | 3 | 13 | 4 | 40 | 3 | 30 | 1 | 10 |
| Thrombocytopenia | 5 | 22 | 3 | 13 | 2 | 9 | 4 | 40 | 2 | 20 | 2 | 20 |
| Infection | 7 | 30 | 7 | 30 | 0 | 0 | 4 | 40 | 4 | 40 | 0 | 0 |
| Non-Hematologic | ||||||||||||
| Constitutional Fatigue |
1 | 4 | 1 | 4 | 0 | 0 | 2 | 20 | 2 | 20 | 0 | 0 |
| Hemorrhage/Thrombosis | ||||||||||||
| Venous thrombosis | 2 | 9 | 2 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatic | ||||||||||||
| Alkaline phosphatase | 1 | 4 | 1 | 4 | 0 | 0 | 3 | 30 | 2 | 20 | 1 | 10 |
| (ALT/SGPT) | 3 | 13 | 3 | 13 | 0 | 0 | 1 | 10 | 1 | 10 | 0 | 0 |
| Gastrointestinal | ||||||||||||
| Constipation | 1 | 4 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 1 | 4 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 1 | 4 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 1 | 4 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain | ||||||||||||
| Pain: Abdomen | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 10 | 1 | 10 | 0 | 0 |
| Pulmonary | ||||||||||||
| Cough | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis/fibrosis | 2 | 9 | 2 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Renal/Metabolic | ||||||||||||
| Hypokalemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 10 | 1 | 10 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 20 | 2 | 20 | 0 | 0 |
Response and progression-free survival
Response, progression-free survival (PFS), and overall survival (OS) data are summarized in Table 3. For the 23 patients with normal liver function in stratum A, objective response occurred in 3 of 23 patients (13%; 95% confidence intervals [CI] 3, 34%), median PFS was 3.7 months, and median OS was 10.3 months. For the 10 patients with abnormal liver function in stratum B, objective response occurred in 0 of 10 patients, median PFS was 3.6 months, and median OS was 3.6 months.
Table 3.
Response and progression-free survival
| Stratum A: Normal liver function |
Stratum B: Abnormal liver function |
|
|---|---|---|
| No of patients | 23 | 10 |
| Partial response | ||
| No. | 3 | 0 |
| Percent | 13% | |
| (95% C.I.) | (3%, 34%) | |
| Progression-free survival | ||
| Median (months) | 3.7 | 3.6 |
| (95% Confidence intervals) |
(2.7–5.8 months) | (1.9–11.6 months) |
| Overall survival | ||
| Median (months) | 10. 3 | 3.6 |
| (95% Confidence intervals) |
(5.9 months—upper limit not reached) |
(2.1–26.2 months) |
Correlative studies in tumor samples and peripheral blood mononuclear cells
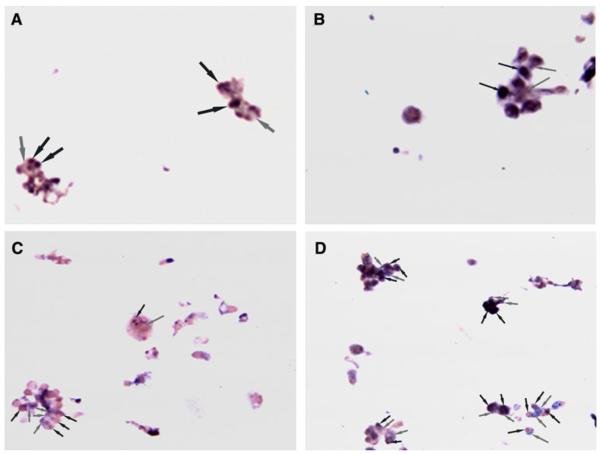
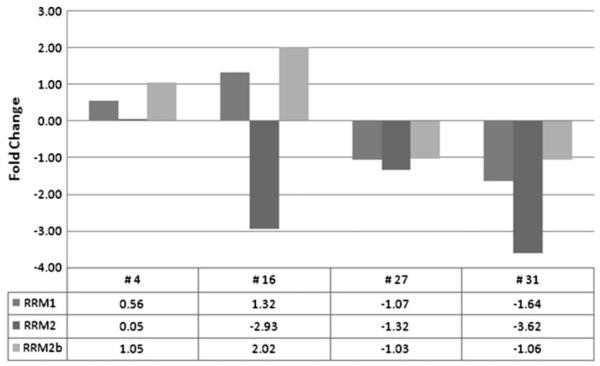
The clinical outcome for patients who underwent correlative studies and the results of these studies performed in tumor samples and PBMCs are summarized in Table 4. Fine needle aspiration samples were collected from two patients before and 24 h after 3-AP therapy for in situ RT PCR analysis of RRM2 mRNA expression (Fig. 1a–d). There was qualitatively increased RRM2 mRNA expression when compared with pre-treatment samples in both patients, suggesting in vivo inhibition of ribonucleotide reductase. Neither patient had an objective response; patient 4 discontinued therapy after 1.3 months and 1 cycle of therapy due to disease progression, and patient 6 discontinued therapy 5.2 months and 6 cycles of therapy due to disease progression. There was a quantitative decrease in PBMC RRM2 mRNA expression after 3-AP/gemcitabine therapy in 3 of 4 patients evaluated, and variable effects observed in RRM1 and RRM2b (Fig. 2), There was a significant decrease after 3-AP/gemcitabine therapy in PBMC dCTP pools in patient 30 (P = 0.012), whereas a significant increase was seen in patient 31 (P = 0.044) (Fig. 3).
Table 4.
Summary of correlative study results and relation to clinical outcomes
| Patient | Best response |
Time to progression (months) |
Change in tumor R2 β2 homodimer by in situ RT–PCR after 3-AP (Fig. 1) |
Change in PBMC RRM2 after 3-AP (Fig. 2) |
Change in PBMC dCTP pools after 3-AP (Fig. 3) |
|---|---|---|---|---|---|
| 4 | PD | 1.3 | Increased | Unchanged | Not done |
| 6 | SD | 5.2 | Increased | Not done | Not done |
| 16 | PD | 2.7 | Not done | Decreased | Not done |
| 27 | SD | 8.3 | Not done | Decreased | Not done |
| 30 | NE | NE | Not done | Not done | Significantly decreased |
| 31 | SD | 13.8 | Not done | Decreased | Significantly increased |
All patients except patient 4 had normal liver function; patient 30 was not evaluable for response because of treatment discontinuation after 1 dose due to toxicity
PD progressive disease, SD stable disease, NE not evaluable, PBMC peripheral blood mononuclear cells
Fig. 1.
a–d In situ RT–PCR analysis of RRM2 expression in fine needle tumor aspirate of tumor samples pre-treatment (a, c) and 24-h post-treatment (b, d) with gemcitabine and 3-AP in patients 4 (a, b) and 6 (c, d). Black arrows indicate nuclear staining, while gray arrows indicate cytoplasmic staining. There was qualitatively increased RRM2 mRNA expression when compared with pre-treatment samples in both patients, suggesting in vivo inhibition of ribonucleotide reductase
Fig. 2.
QRT-PCR analysis of RRM1, RRM2, and RRM2b represents fold change in RRM1, RRM2, and RRM2b mRNA expression in peripheral blood mononuclear cells 24-h post-treatment with 3-AP plus gemcitabine in patients 4, 16, 27 and 31. Fold change is relative to pre-treatment sample, and all samples were normalized to TATA-binding protein (TBP). There was a quantitative decrease in PBMC RRM2 mRNA expression after 3-AP/gemcitabine therapy in 3 of 4 patients evaluated, and variable effects observed in RRM1 and RRM2b
Fig. 3.
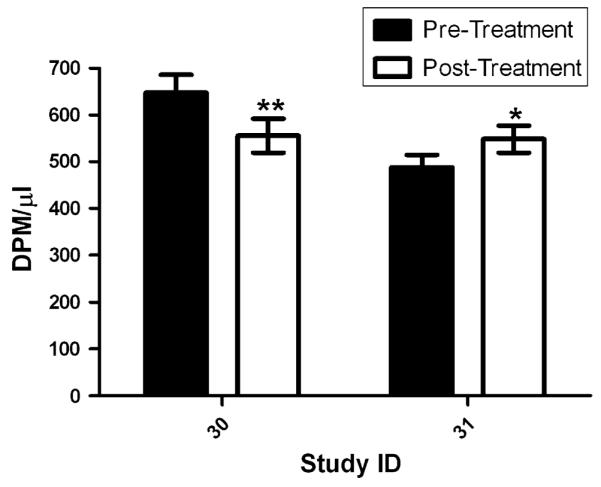
Deoxynucleotide cytidine triphosphate pools (dCTP) in peripheral blood mononuclear cells (PBMCs) before and 24-h post-treatment with 3-AP plus gemcitabine in patients 30 and 31. dCTP pool assays were performed in triplicate and repeated 3 times. There was a significant decrease after 3-AP/gemcitabine therapy in PBMC dCTP pools in patient 30 (P = 0.012), whereas a significant increase was seen in patient 31 (P = 0.044)
Discussion
We performed a phase II trial of the RR inhibitor 3-AP plus gemcitabine in patients with advanced adenocarcinoma of the gallbladder or biliary tract as first-line chemotherapy for treatment of advanced disease. The dose and schedule of 3-AP (105 mg/m2 given as a 4-h infusion prior to each gemcitabine dose) and gemcitabine (1,000 mg/m2 IV over 30 min on days 1, 8, and 15 every 28 days) used in patients with normal liver function was based upon a prior phase I trial of the combination [20]. Because patients with biliary tract cancer often have abnormal liver function, we also evaluated the same combination using a slightly attenuated 3-AP dose (80 mg/m2) in patients with mildly abnormal liver function, defined as a total bilirubin between one- and threefold above the upper limits of normal (consistent with grade 1–2 elevation). Based upon our findings, we conclude that the combination of 3-AP plus gemcitabine is not likely to be associated with a response rate of at least 30% in patients with normal liver function and at least 20% in those with abnormal liver function. The toxicity profile was similar to that observed with gemcitabine alone.
Several other trials have evaluated the antitumor effects of 3-AP used alone or in combination with other cytotoxic agents for a variety of cancer types. A phase II trial of 3-AP (96 mg/m2 2-h infusion daily for 4 days every 2 weeks) in 19 patients with metastatic renal cell cancer revealed objective response in one patient (5%); grade 3–4 neutropenia occurred in 79%, grade 1–2 fatigue, nausea and vomiting were common, and some patients exhibited acute reactions of hypoxia, hypotension, and methemoglobinemia [24]. Another trial evaluating a similar dose/schedule of 3-AP alone in 15 patients with pancreatic cancer failed to meet its efficacy endpoint [25]. A phase II trial of the 3-AP plus gemcitabine combination as first-line therapy in 25 patients with advanced pancreatic carcinoma (using a dose and schedule similar to our trial) revealed no objective responses, median time to progression of 4.1 months, 6-month progression-free survival rate of 29%, median overall survival of 9.0 months, and 1-year survival rate of 28%; this two-stage trial was stopped after the first stage due to insufficient antitumor activity [26]. A phase II trial of the 3-AP plus gemcitabine combination in non-small cell lung cancer likewise failed to meet its efficacy objective, with objective response occurring in 0 of 12 patients [27]. In contrast to trials in solid tumors, complete remission occurred in 4 of 31 patients with acute leukemia in a phase I trial of 3-AP plus cytarabine; the recommended phase II dose was 3-AP 105 mg/m2/day followed by cytarabine 600 mg/m2/day for 5 consecutive days every 3–6 weeks [28]. Additional clinical trials are ongoing evaluating the role of 3-AP in combination with fludarabine for patients with hematologic malignancies (NCT00381550), and in combination with radiation for pancreatic carcinoma (NCT00288093), or radiation and cisplatin for cervical and vaginal carcinoma (NCT0094 1070). Based upon the results of our trial and other reported trials, 3-AP is ineffective as a single agent for renal cell and pancreatic carcinoma and does not enhance the antineoplastic effects of gemcitabine in non-small cell lung, pancreatic, or biliary tract carcinoma when used as a weekly short infusion.
In order to determine whether 3-AP produced RR inhibition in vivo, we evaluated RRM2 mRNA expression by in situ RT–PCR in tumor fine needle aspiration samples performed in two patients before and 24 h after 3-AP administration. In human colon cancer cell lines treated with hydroxyurea in vitro, RRM2 mRNA expression increased within 24 h of treatment and was associated with decreased nucleotide triphosphates, indicating that increased RRM2 mRNA expression may serve as a surrogate for RR inhibition [29]. As shown in Fig. 1, there was a substantial increase in tumor RRM2 mRNA expression in both patients, providing evidence for RR inhibition in the tumor in vivo. In both samples, there was also evidence for nuclear translocation of RRM2, which is consistent with the potential role of RRM2 in DNA repair, suggesting a role for RRM2 inhibition in combination with gemcitabine and 3-AP [30]. In contrast, as shown in Fig. 2, the only patient who had both tumor and PBMC sampled (number 4) showed no significant change in PBMC RRM2, whereas the remaining three (patients 16, 27, and 31) demonstrated decreased RRM2 expression in PBMC. These differences may be due to differences in cell cycle distribution in tumor cells compared with normal PBMCs, where the proportion of cells in S phase may be high in the tumor compared with a normally low value in PBMCs. There were variable changes in RRM1 and RRM2B. RRM2B may be biomarker since this subunit is required for DNA synthesis and plays an important role in cell survival by repairing DNA damage [31]. Silencing or decreasing the expression of RRM2b (a p53 inducible subunit of RR) has been shown to enhance apoptosis when used with radiation or doxorubicin [32]. Given that only one patient had both tumor and PBMC sampled (number 4) and that there were no objective responses, however, we are not able to conclude whether evaluation of PBMC for RRM1, RRM2, or RRM2B would serve as useful surrogates for reflecting 3-AP-induced RR inhibition in human tumors in vivo, nor clinical benefit from 3-AP.
Inconsistent effects on intracellular PBMC dCTP pools were seen in the two patients evaluated, suggesting that the dose and schedule of 3-AP used in this study was insufficient to consistently produce clinically relevant depletion of nucleotides required to enhance the effectiveness of gemcitabine. A phase I trial that evaluated the combination of 3-AP given as a 24-h infusion (90 mg/m2) with fixed dose rate gemcitabine (1,000 mg/m2 over 100 min) every 2 weeks reported disease stabilization in 50% of patients enrolled, providing support for a need to further evaluate schedules used in this therapeutic regimen [33]. In addition, complete clinical response was observed in all 10 patients with stage IB2-IVB cervical carcinoma treated with thrice weekly infusions of 3-AP (25 mg/m2) in combination with cisplatin and pelvic irradiation [34]. Although our trial and others have shown no benefit for short weekly infusions combined with other cytotoxic agents, we provide in vivo evidence that additional studies evaluating more protracted infusion schedule or more frequent short infusions may be warranted.
Acknowledgments
The authors acknowledge the late Dr. Scott Wadler, our colleague and friend, who conceived of and designed this trial and founded the New York Cancer Consortium. He will be remembered for his skills as clinician, scholar, and mentor.
This study was performed by the New York Cancer Consortium (www.newyorkcancerconsortium.org), and was supported by a contract from the National Institute of Health, National Cancer Institute (N01-CM-62204 [PI: Joseph A. Sparano]) and NIH Subcontract 24XS07, P6254 (PI: Maureen E. Lane).
Footnotes
Conflict of interest None.
Presented in part at the American Society of Clinical Oncology Gastrointestinal Symposium, January 15–17, 2009, San Francisco, CA.
Contributor Information
Allyson J. Ocean, New York Presbyterian Hospital, Weill Cornell Medical College, New York, NY, USA
Paul Christos, New York Presbyterian Hospital, Weill Cornell Medical College, New York, NY, USA.
Joseph A. Sparano, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA
Dan Matulich, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA.
Andreas Kaubish, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA.
Abby Siegel, New York Presbyterian Hospital, Columbia University, New York, NY, USA.
Max Sung, Mt. Sinai School of Medicine, New York, NY, USA.
Maureen M. Ward, New York Presbyterian Hospital, Weill Cornell Medical College, New York, NY, USA
Nancy Hamel, New York Presbyterian Hospital, Weill Cornell Medical College, New York, NY, USA.
Igor Espinoza-Delgado, Cancer Therapy Evaluation Program, National Cancer Institute, Bethesda, MD, USA.
Yun Yen, City of Hope Medical Center, Duarte, CA, USA.
Maureen E. Lane, New York Presbyterian Hospital, Weill Cornell Medical College, New York, NY, USA
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Choi BI, Han JK, Hong ST, et al. Clonorchiasis and cholangiocarcinoma: etiologic relationship and imaging diagnosis. Clin Microbiol Rev. 2004;17:540–52. doi: 10.1128/CMR.17.3.540-552.2004. ( table of contents) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang KY, Chang JY, Yen Y. Increasing incidence of intrahepatic cholangiocarcinoma and its relationship to chronic viral hepatitis. J Natl Compr Canc Netw. 2009;7:423–427. doi: 10.6004/jnccn.2009.0030. [DOI] [PubMed] [Google Scholar]
- 4.de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Oh SY, Kim SH, et al. Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a phase II study. Jpn J Clin Oncol. 2005;35:68–73. doi: 10.1093/jjco/hyi021. [DOI] [PubMed] [Google Scholar]
- 6.Eng C, Ramanathan RK, Wong MK, et al. A phase II trial of fixed dose rate gemcitabine in patients with advanced biliary tree carcinoma. Am J Clin Oncol. 2004;27:565–569. doi: 10.1097/01.coc.0000135924.94955.16. [DOI] [PubMed] [Google Scholar]
- 7.Tsavaris N, Kosmas C, Gouveris P, et al. Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest New Drugs. 2004;22:193–198. doi: 10.1023/B:DRUG.0000011797.09549.53. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn R, Hribaschek A, Eichelmann K, et al. Outpatient therapy with gemcitabine and docetaxel for gallbladder, biliary, and cholangio-carcinomas. Invest New Drugs. 2002;20:351–356. doi: 10.1023/a:1016209901417. [DOI] [PubMed] [Google Scholar]
- 9.Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study—The UK ABC-01 Study. Br J Cancer. 2009;101:621–627. doi: 10.1038/sj.bjc.6605211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 11.Cory JG, Sato A. Regulation of ribonucleotide reductase activity in mammalian cells. Mol Cell Biochem. 1983;53–54:257–266. doi: 10.1007/BF00225258. [DOI] [PubMed] [Google Scholar]
- 12.Fan H, Villegas C, Huang A, et al. The mammalian ribonucleotide reductase R2 component cooperates with a variety of oncogenes in mechanisms of cellular transformation. Cancer Res. 1998;58:1650–1653. [PubMed] [Google Scholar]
- 13.Cory JG, Cory AH, Rappa G, et al. Inhibitors of ribonucleotide reductase. Comparative effects of amino- and hydroxy-substituted pyridine-2-carboxaldehyde thiosemicarbazones. Biochem Pharmacol. 1994;48:335–344. doi: 10.1016/0006-2952(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 14.Liu MC, Lin TS, Cory JG, et al. Synthesis and biological activity of 3- and 5-amino derivatives of pyridine-2-carboxaldehyde thiosemicarbazone. J Med Chem. 1996;39:2586–2593. doi: 10.1021/jm9600454. [DOI] [PubMed] [Google Scholar]
- 15.Elford HL, Freese M, Passamani E, et al. Ribonucleotide reductase and cell proliferation. I. Variations of ribonucleotide reductase activity with tumor growth rate in a series of rat hepatomas. J Biol Chem. 1970;245:5228–5233. [PubMed] [Google Scholar]
- 16.Zhou B, Mi S, Mo X, et al. Time and sequence dependence of hydroxyurea in combination with gemcitabine in human KB cells. Anticancer Res. 2002;22:1369–77. [PubMed] [Google Scholar]
- 17.Finch RA, Liu M, Grill SP, et al. Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone): a potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol. 2000;59:983–991. doi: 10.1016/s0006-2952(99)00419-0. [DOI] [PubMed] [Google Scholar]
- 18.Finch RA, Liu MC, Cory AH, et al. Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone; 3-AP): an inhibitor of ribonucleotide reductase with antineoplastic activity. Adv Enzyme Regul. 1999;39:3–12. doi: 10.1016/s0065-2571(98)00017-x. [DOI] [PubMed] [Google Scholar]
- 19.Sigmond J, Kamphuis JA, Laan AC, et al. The synergistic interaction of gemcitabine and cytosine arabinoside with the ribonucleotide reductase inhibitor triapine is schedule dependent. Biochem Pharmacol. 2007;73:1548–1557. doi: 10.1016/j.bcp.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Yen Y, Margolin K, Doroshow J, et al. A phase I trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone in combination with gemcitabine for patients with advanced cancer. Cancer Chemother Pharmacol. 2004;54:331–342. doi: 10.1007/s00280-004-0821-2. [DOI] [PubMed] [Google Scholar]
- 21.Foltz LM, Dalal BI, Wadsworth LD, et al. Recognition and management of methemoglobinemia and hemolysis in a G6PD-deficient patient on experimental anticancer drug Triapine. Am J Hematol. 2006;81:210–211. doi: 10.1002/ajh.20547. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Wadler S, Zhang H, Cammer M, et al. Quantification of ribonucleotide reductase expression in wild-type and hydroxy-urea-resistant cell lines employing in situ reverse transcriptase polymerase chain reaction and a computerized image analysis system. Anal Biochem. 1999;267:24–29. doi: 10.1006/abio.1998.2890. [DOI] [PubMed] [Google Scholar]
- 24.Knox JJ, Hotte SJ, Kollmannsberger C, et al. Phase II study of Triapine in patients with metastatic renal cell carcinoma: a trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC IND.161) Invest New Drugs. 2007;25:471–477. doi: 10.1007/s10637-007-9044-9. [DOI] [PubMed] [Google Scholar]
- 25.Attia S, Kolesar J, Mahoney MR, et al. A phase 2 consortium (P2C) trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) for advanced adenocarcinoma of the pancreas. Invest New Drugs. 2008;26:369–379. doi: 10.1007/s10637-008-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackenzie MJ, Saltman D, Hirte H, et al. A Phase II study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) and gemcitabine in advanced pancreatic carcinoma. A trial of the Princess Margaret hospital Phase II consortium. Invest New Drugs. 2007;25:553–558. doi: 10.1007/s10637-007-9066-3. [DOI] [PubMed] [Google Scholar]
- 27.Ma B, Goh BC, Tan EH, et al. A multicenter phase II trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Invest New Drugs. 2008;26:169–173. doi: 10.1007/s10637-007-9085-0. [DOI] [PubMed] [Google Scholar]
- 28.Yee KW, Cortes J, Ferrajoli A, et al. Triapine and cytarabine is an active combination in patients with acute leukemia or myelodysplastic syndrome. Leuk Res. 2006;30:813–822. doi: 10.1016/j.leukres.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Wadler S, Horowitz R, Zhang HY, et al. Effects of perturbations of pools of deoxyribonucleoside triphosphates on expression of ribonucleotide reductase, a G1/S transition state enzyme, in p53-mutated cells. Biochem Pharmacol. 1998;55:1353–1360. doi: 10.1016/s0006-2952(97)00641-2. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YW, Jones TL, Martin SE, et al. Implication of checkpoint kinase-dependent up-regulation of ribonucleotide reductase R2 in DNA damage response. J Biol Chem. 2009;284:18085–18095. doi: 10.1074/jbc.M109.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi T, Matsuda K, Sagiya Y, et al. p53R2-dependent pathway for DNA synthesis in a p53-regulated cell cycle checkpoint. Cancer Res. 2001;61:8256–8262. [PubMed] [Google Scholar]
- 32.Devlin HL, Mack PC, Burich RA, et al. Impairment of the DNA repair and growth arrest pathways by p53R2 silencing enhances DNA damage-induced apoptosis in a p53-dependent manner in prostate cancer cells. Mol Cancer Res. 2008;6:808–818. doi: 10.1158/1541-7786.MCR-07-2027. [DOI] [PubMed] [Google Scholar]
- 33.Mortazavi A, Dearn D, Ling Y, Harper EJ, Phelps MA, Espinoza-Delgado I, Monk JP, Otterson GA, Grever MR, Belkaii-Saab T. A phase I study of prolonged infusion of triapine incombination with fixed-dose rate of gemcitabine in patients with advanced solid tumors. Proceedings of the American Society of Clinical Oncology. 2010 Abstract 53259. [Google Scholar]
- 34.Kunos CA, Waggoner S, von Gruenigen V, et al. Phase I trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical cancer. Clin Cancer Res. 2010;16:1298–1306. doi: 10.1158/1078-0432.CCR-09-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]