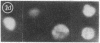
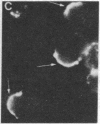
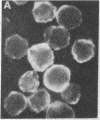
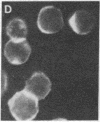
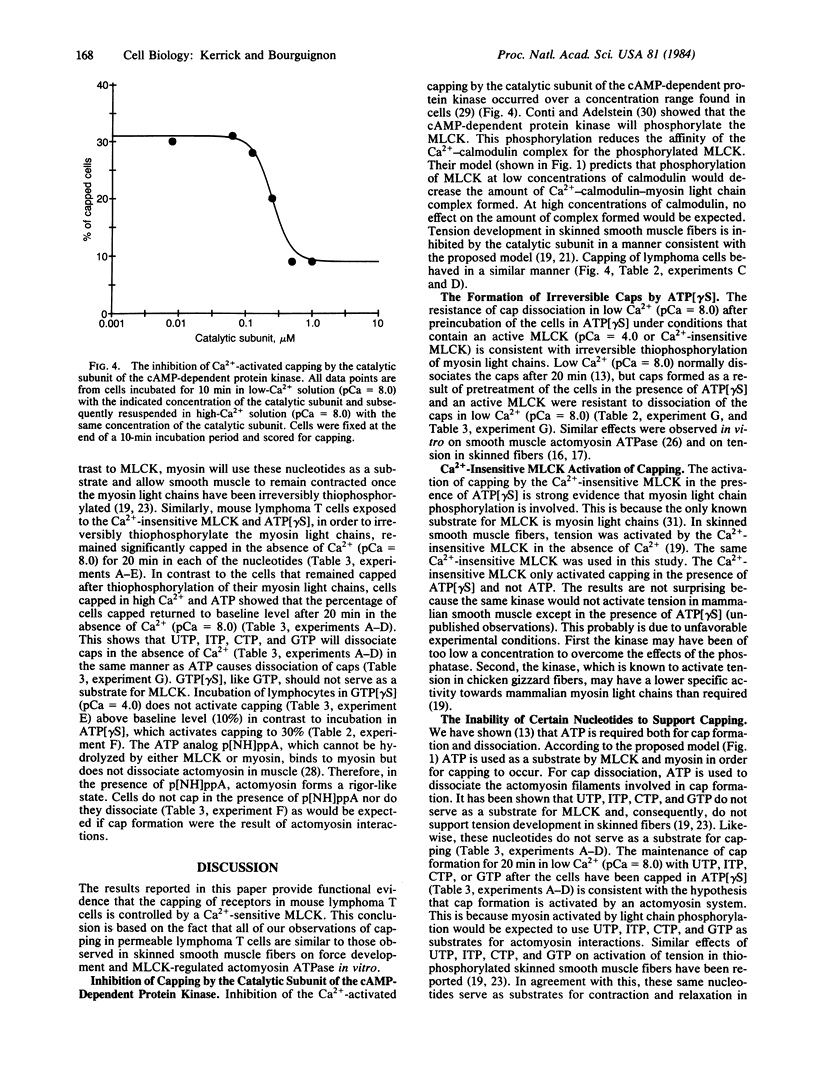
Abstract
Several characteristics of receptor capping in lymphocyte membranes suggest similarities with mechanisms underlying control of contraction in smooth muscle fibers. Both capping and contraction are Ca2+ dependent and require metabolic energy. Contractile proteins such as actin and myosin are associated with the cap, as is calmodulin, which mediates the Ca2+ dependence of smooth muscle contraction. Recent studies have shown that myosin light chain kinase (MLCK), which plays a central role in regulation of smooth muscle contraction, is also present in isolated lymphocyte membrane-cytoskeleton complexes. We have explored this analogy further, using mouse lymphoma T cells whose membranes were rendered permeable to small proteins by using a low-Ca2+ EGTA solution similar to that used to chemically skin smooth muscle cells. Permeabilized lymphocytes were then exposed to solutions containing various combinations of high or low Ca2+, ATP, or other nucleotides (5'-adenylyl imidodiphosphate, adenosine 5'-[gamma-thio]triphosphate, guanosine 5'-[gamma-thio]triphosphate, CTP, ITP, UTP, and GTP), calmodulin, Ca2+-insensitive MLCK (MLCK subunit that has been stripped of the Ca2+ binding site), and the catalytic subunit of cAMP-dependent protein kinase that phosphorylates (and thereby inactivates) MLCK. Capping of concanavalin A-labeled receptors in these various test solutions was scored. In all solutions the capping observed in permeable lymphoma cells correlated well with contraction previously observed in similarly treated skinned smooth muscle fibers, providing strong evidence for the involvement of myosin light chain phosphorylation in the regulation of receptor capping.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Bourguignon L. Y., Balazovich K. Effect of the antidepressant drug-Stelazine on lymphocyte capping. Cell Biol Int Rep. 1980 Oct;4(10):947–952. doi: 10.1016/0309-1651(80)90197-6. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Kerrick W. G. Receptor capping in mouse T-lymphoma cells: a Ca2+ and calmodulin-stimulated ATP-dependent process. J Membr Biol. 1983;75(1):65–72. doi: 10.1007/BF01870800. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Nagpal M. L., Balazovich K., Guerriero V., Means A. R. Association of myosin light chain kinase with lymphocyte membrane-cytoskeleton complex. J Cell Biol. 1982 Dec;95(3):793–797. doi: 10.1083/jcb.95.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Nagpal M. L., Hsing Y. C. Phosphorylation of myosin light chain during capping of mouse T-lymphoma cells. J Cell Biol. 1981 Dec;91(3 Pt 1):889–894. doi: 10.1083/jcb.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Rubin R. W., Krishan A. Cell cycle dependent receptor capping in mouse leukemic cells. Cell Biol Int Rep. 1983 Feb;7(2):109–119. doi: 10.1016/0309-1651(83)90023-1. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y. Simultaneous localization of intracellular myosin and surface concanavalin A receptor clusters using immuno-electron microscopy. Cell Biol Int Rep. 1980 Jun;4(6):541–547. doi: 10.1016/0309-1651(80)90019-3. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Singer S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5031–5035. doi: 10.1073/pnas.74.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Tokuyasu K. T., Singer S. J. The capping of lymphocytes and other cells, studied by an improved method for immunofluorescence staining of frozen sections. J Cell Physiol. 1978 Jun;95(3):239–257. doi: 10.1002/jcp.1040950302. [DOI] [PubMed] [Google Scholar]
- Butman B. T., Bourguignon G. J., Bourguignon L. Y. Lymphocyte capping induced by polycationized ferritin. J Cell Physiol. 1980 Oct;105(1):7–15. doi: 10.1002/jcp.1041050103. [DOI] [PubMed] [Google Scholar]
- Butman B. T., Jacobsen T., Cabatu O. G., Bourguignon L. Y. The involvement of cAMP in lymphocyte capping. Cell Immunol. 1981 Jul 1;61(2):397–403. doi: 10.1016/0008-8749(81)90387-7. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATP gamma S. J Biol Chem. 1979 Nov 10;254(21):11148–11153. [PubMed] [Google Scholar]
- Cassidy P., Kerrick W. G. Superprecipitation of gizzard actomyosin, and tension in gizzard muscle skinned fibers in the presence of nucleotides other than ATP. Biochim Biophys Acta. 1982 Jul 12;705(1):63–69. doi: 10.1016/0167-4838(82)90336-3. [DOI] [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. Phosphorylation by cyclic adenosine 3':5'-monophosphate-dependent protein kinase regulates myosin light chain kinase. Fed Proc. 1980 Apr;39(5):1569–1573. [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Geiger B., Singer S. J. The participation of alpha-actinin in the capping of cell membrane components. Cell. 1979 Jan;16(1):213–222. doi: 10.1016/0092-8674(79)90202-2. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Kerrick W. G., Cassidy P. S. Chicken gizzard: relation between calcium-activated phosphorylation and contraction. Science. 1979 May 4;204(4392):503–506. doi: 10.1126/science.432654. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Bolles L. L. Evidence that myosin light chain phosphorylation regulates contraction in the body wall muscles of the sea cucumber. J Cell Physiol. 1982 Sep;112(3):307–315. doi: 10.1002/jcp.1041120302. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Donaldson S. K. The effects of Mg 2+ on submaximum Ca 2+ -activated tension in skinned fibers of frog skeletal muscle. Biochim Biophys Acta. 1972 Jul 12;275(1):117–122. doi: 10.1016/0005-2728(72)90030-8. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Hoar P. E., Cassidy P. S., Bolles L., Malencik D. A. Calcium-regulatory mechanisms. Functional classification using skinned fibers. J Gen Physiol. 1981 Feb;77(2):177–190. doi: 10.1085/jgp.77.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Hoar P. E. Inhibition of smooth muscle tension by cyclic AMP-dependent protein kinase. Nature. 1981 Jul 16;292(5820):253–255. doi: 10.1038/292253a0. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Krasner B. Disruption of the sarcolemma of mammalian skeletal muscle fibers by homogenization. J Appl Physiol. 1975 Dec;39(6):1052–1055. doi: 10.1152/jappl.1975.39.6.1052. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Nelson G. A., Andrews M. L., Karnovsky M. J. Participation of calmodulin in immunoglobulin capping. J Cell Biol. 1982 Dec;95(3):771–780. doi: 10.1083/jcb.95.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Maihle N. J., Satir P. Calmodulin localization during capping and receptor-mediated endocytosis. Nature. 1981 Nov 12;294(5837):163–166. doi: 10.1038/294163a0. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M. P., Bridenbaugh R., Hartshorne D. J., Kerrick W. G. Phosphorylation-dependent activated tension in skinned gizzard muscle fibers in the absence of Ca2+. J Biol Chem. 1982 Jun 10;257(11):5987–5990. [PubMed] [Google Scholar]
- Walsh M. P., Dabrowska R., Hinkins S., Hartshorne D. J. Calcium-independent myosin light chain kinase of smooth muscle. Preparation by limited chymotryptic digestion of the calcium ion dependent enzyme, purification, and characterization. Biochemistry. 1982 Apr 13;21(8):1919–1925. doi: 10.1021/bi00537a034. [DOI] [PubMed] [Google Scholar]
- Yakara I., Kakimoto-Sameshima F. Microtubule organization of lymphocytes and its modulation by patch and cap formation. Cell. 1978 Sep;15(1):251–259. doi: 10.1016/0092-8674(78)90100-9. [DOI] [PubMed] [Google Scholar]