Abstract
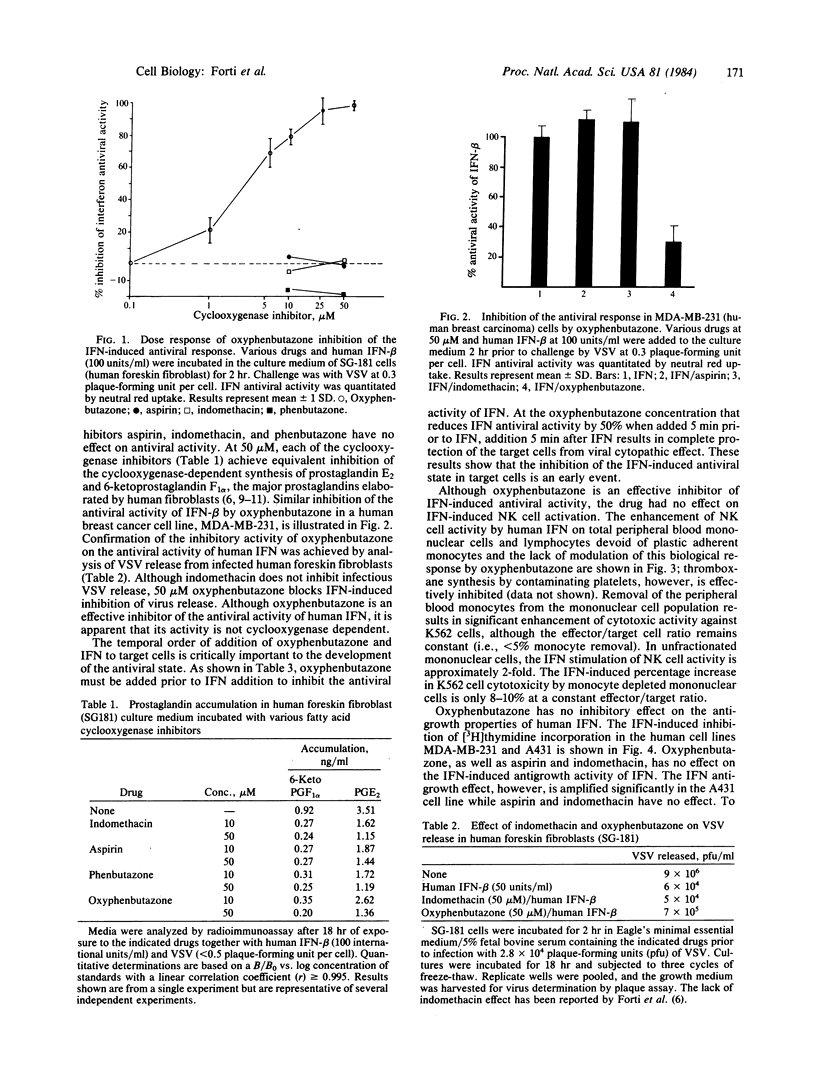
Among the pleiotropic effects of human interferon are the inhibition of viral replication, the activation of natural killer cells, and the inhibition of cellular growth. Oxyphenbutazone, a nonsteroidal antiinflammatory agent, is a potent inhibitor of the antiviral activity of human alpha and beta interferons as determined by cytopathic effect and vesicular stomatitis virus synthesis and release in human foreskin fibroblasts. The inhibition of interferon activity is dose dependent with maximal inhibition at 25-50 microM and minimal inhibition at 1 microM. In contrast, oxyphenbutazone at concentrations as high as 100 microM has no effect on the activation of natural killer cells by human interferon. Similarly, oxyphenbutazone has no inhibitory effect on interferon-induced antigrowth activity in the human breast carcinoma cell line MDA-MB-231. This cell line is sensitive to oxyphenbutazone inhibition of interferon-induced antiviral activity in vitro. In another human cell line, the vulvar carcinoma A431, oxyphenbutazone apparently augments the antigrowth activity of interferon. Although oxyphenbutazone inhibits the fatty acid cyclooxygenase enzyme in these systems, other inhibitors of cyclooxygenase fail to inactivate the antiviral activity of human interferon. Thus, oxyphenbutazone appears to inhibit the interferon antiviral cascade at a site distinct from prostaglandin biosynthesis. Moreover, the failure to inhibit natural killer cell activation or cellular antigrowth effects of human interferon suggests a pathway different from that associated with the antiviral effect of human interferon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M. High-affinity binding of 125I-labelled mouse interferon to a specific cell surface receptor. Nature. 1980 Apr 3;284(5755):459–461. doi: 10.1038/284459a0. [DOI] [PubMed] [Google Scholar]
- Attallah A. M., Needy C. F., Noguchi P. D., Elisberg B. L. Enhancement of carcinoembryonic antigen expression by interferon. Int J Cancer. 1979 Jul 15;24(1):49–52. doi: 10.1002/ijc.2910240109. [DOI] [PubMed] [Google Scholar]
- Branca A. A., Baglioni C. Evidence that types I and II interferons have different receptors. Nature. 1981 Dec 24;294(5843):768–770. doi: 10.1038/294768a0. [DOI] [PubMed] [Google Scholar]
- Chandrabose K. A., Cuatrecasas P., Pottathil R., Lang D. J. Interferon-resistant cell line lacks fatty acid cyclooxygenase activity. Science. 1981 Apr 17;212(4492):329–331. doi: 10.1126/science.6163214. [DOI] [PubMed] [Google Scholar]
- Evinger M., Rubinstein M., Pestka S. Antiproliferative and antiviral activities of human leukocyte interferons. Arch Biochem Biophys. 1981 Aug;210(1):319–329. doi: 10.1016/0003-9861(81)90195-8. [DOI] [PubMed] [Google Scholar]
- Familletti P. C., Costello L., Rose C. A., Pestka S. Induction, production, and concentration of interferon produced by a myeloblast culture. Methods Enzymol. 1981;78(Pt A):83–86. doi: 10.1016/0076-6879(81)78101-1. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Stringfellow D. A. Virus and interferon effects on cellular prostaglandin biosynthesis. J Immunol. 1980 Jul;125(1):431–437. [PubMed] [Google Scholar]
- Forti R. L., Mitchell W. M., Workman R. J., Forbes J. T., Hubbard W. C. A functional cyclooxygenase enzyme is not required for mediation of the pleiotropic effects of human alpha or beta interferon. Prostaglandins. 1983 Sep;26(3):409–420. doi: 10.1016/0090-6980(83)90176-4. [DOI] [PubMed] [Google Scholar]
- Fuse A., Mahmud I., Kuwata T. Mechanism of stimulation by human interferon of prostaglandin synthesis in human cell lines. Cancer Res. 1982 Aug;42(8):3209–3214. [PubMed] [Google Scholar]
- Gupta S. L., Rubin B. Y., Holmes S. L. Interferon action: induction of specific proteins in mouse and human cells by homologous interferons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4817–4821. doi: 10.1073/pnas.76.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAACS A., LINDENMANN J., VALENTINE R. C. Virus interference. II. Some properties of interferon. Proc R Soc Lond B Biol Sci. 1957 Sep 12;147(927):268–273. doi: 10.1098/rspb.1957.0049. [DOI] [PubMed] [Google Scholar]
- ISAACS A., LINDENMANN J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957 Sep 12;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Koblet H., Wyler R., Kohler U. Altered or increased transfer-RNA methylation in the course of Interferon action on cells in culture? Experientia. 1979 May 15;35(5):576–578. doi: 10.1007/BF01960327. [DOI] [PubMed] [Google Scholar]
- Leong S. S., Horoszewicz J. S. Production and preparation of human fibroblast interferon for clinical trials. Methods Enzymol. 1981;78(Pt A):87–101. doi: 10.1016/0076-6879(81)78102-3. [DOI] [PubMed] [Google Scholar]
- Mitchell W. M., Forti R. L. Progress in the monitoring of human interferon in body fluids and the phenotypic expression of human interferon activity. Prog Clin Pathol. 1984;9:101–119. [PubMed] [Google Scholar]
- NAGANO Y., KOJIMA Y. Inhibition de l'infection vaccinale par un facteur liquide dans le tissu infecté par le virus homologue. C R Seances Soc Biol Fil. 1958;152(11):1627–1629. [PubMed] [Google Scholar]
- Pestka S. The human interferons--from protein purification and sequence to cloning and expression in bacteria: before, between, and beyond. Arch Biochem Biophys. 1983 Feb 15;221(1):1–37. doi: 10.1016/0003-9861(83)90118-2. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Wang E., Tamm I. Interferon effects on microfilament organization, cellular fibronectin distribution, and cell motility in human fibroblasts. J Cell Biol. 1980 Apr;85(1):9–17. doi: 10.1083/jcb.85.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer L. M., Wang E., Tamm I. Interferon inhibits the redistribution of cell surface components. J Exp Med. 1980 Aug 1;152(2):469–474. doi: 10.1084/jem.152.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottathil R., Chandrabose K. A., Cuatrecasas P., Lang D. J. Establishment of the interferon-mediated antiviral state: role of fatty acid cyclooxygenase. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5437–5440. doi: 10.1073/pnas.77.9.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg E., Kelder B., Hoal E. G., Pestka S. Specific molecular activities of recombinant and hybrid leukocyte interferons. J Biol Chem. 1982 Oct 10;257(19):11497–11502. [PubMed] [Google Scholar]
- Rubin B. Y., Gupta S. L. Differential efficacies of human type I and type II interferons as antiviral and antiproliferative agents. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5928–5932. doi: 10.1073/pnas.77.10.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Zilberstein A., Shulman L., Federman P., Berissi H., Revel M. Interferon action: isolation of nuclease F, a translation inhibitor activated by interferon-induced (2'-5') oligo-isoadenylate. FEBS Lett. 1978 Nov 15;95(2):257–264. doi: 10.1016/0014-5793(78)81006-0. [DOI] [PubMed] [Google Scholar]
- Yaron M., Yaron I., Gurari-Rotman D., Revel M., Lindner H. R., Zor U. Stimulation of prostaglandin E production in cultured human fibroblasts by poly(I)-poly(C) and human interferon. Nature. 1977 Jun 2;267(5610):457–459. doi: 10.1038/267457a0. [DOI] [PubMed] [Google Scholar]
- Yoshida R., Imanishi J., Oku T., Kishida T., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci U S A. 1981 Jan;78(1):129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Azhary R., Mannering G. J. Effects of interferon inducing agents (polyriboinosinic acid. Polyribocytidylic acid, tilorone) on hepatic hemoproteins (cytochrome P-450, catalase, tryptophan 2,3-dioxygenase, mitochondrial cytochromes), heme metabolism and cytochrome P-450-linked monooxygenase systems. Mol Pharmacol. 1979 May;15(3):698–707. [PubMed] [Google Scholar]



