Abstract
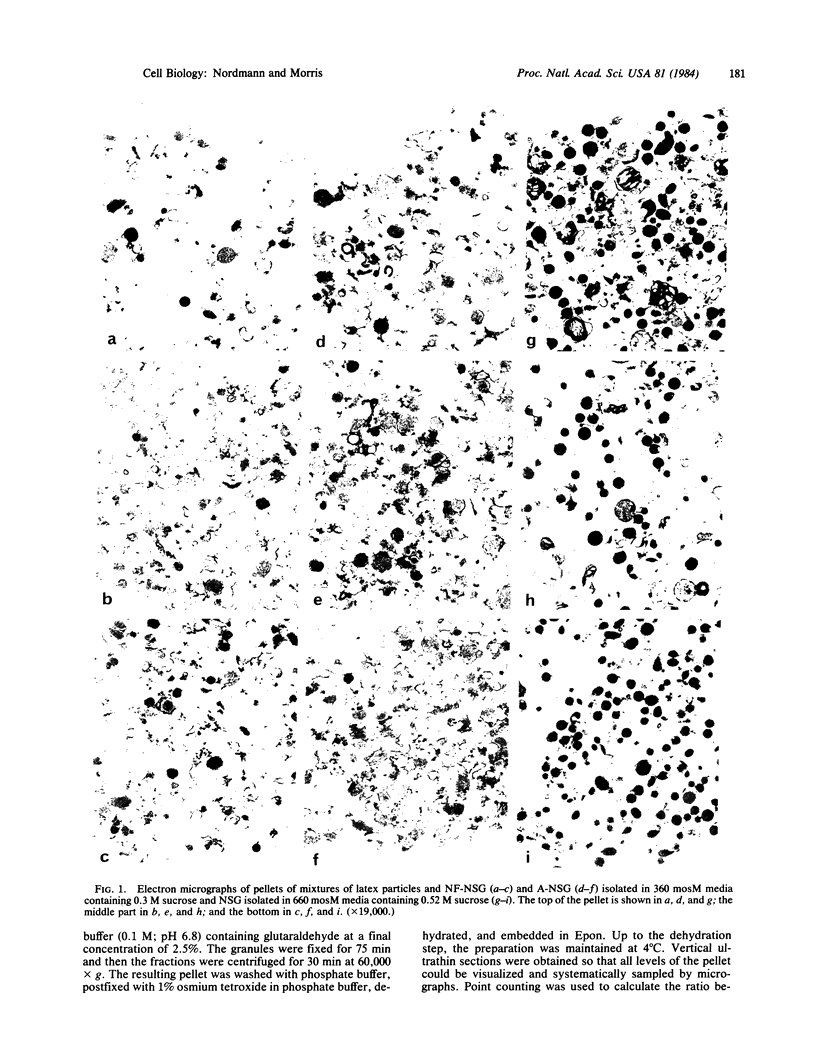
A method is described for the quantitative determination of the content of subcellular organelles such as secretory granules. Purified subcellular fractions of the organelle are prepared and aliquots are assayed for hormones, for example. To determine the number of organelles per fraction, known numbers of latex particles of a size similar to the organelle are added to other aliquots of the subcellular fractions. Latex particles and organelles are then pelleted together by centrifugation. The ratio between latex particles and organelles can be determined by morphometric analysis of ultrathin sections taken through the full thickness of the pellet. The number of organelles and hence their content of the substance assayed can then be calculated. We have applied this technique to posterior pituitary neurosecretory granules, the content of which has already been estimated by a different method. Newly formed neurosecretory granules from oxen and rats were found to have a content of approximately equal to 85,000 molecules of hormone and neurophysin. Aged neurosecretory granules from the same neural lobes appeared to contain less hormone and neurophysin, but this was shown to be the result of loss of material from the granules during isolation in media of 360 mosM. Such loss could be prevented by isolation in hypertonic (660 mosM) media.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen P., Nicolas P., Camier M. Biochemical aspects of neurosecretion: neurophysin--neurohypophyseal hormone complexes. Curr Top Cell Regul. 1979;15:263–318. doi: 10.1016/b978-0-12-152815-7.50011-9. [DOI] [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- Lescure H., Nordmann J. J. Neurosecretory granule release and endocytosis during prolonged stimulation of the rat neurohypophysis in vitro. Neuroscience. 1980;5(3):651–659. doi: 10.1016/0306-4522(80)90062-7. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Morris J. F. Hormone storage in individual neurosecretory granules of the pituitary gland: A quantitative ultrastructural approach to hormone storage in the neural lobe. J Endocrinol. 1976 Feb;68(02):209–224. doi: 10.1677/joe.0.0680209. [DOI] [PubMed] [Google Scholar]
- Morris J. F., Nordmann J. J., Dyball R. E. Structure-function correlation in mammalian neurosecretion. Int Rev Exp Pathol. 1978;18:1–95. [PubMed] [Google Scholar]
- Morris J. F., Nordmann J. J. Membrane recapture after hormone release from nerve endings in the neural lobe of the rat pituitary gland. Neuroscience. 1980;5(3):639–659. doi: 10.1016/0306-4522(80)90061-5. [DOI] [PubMed] [Google Scholar]
- Morris J. F. The Brattleboro magnocellular neurosecretory system: a model for the study of peptidergic neurons. Ann N Y Acad Sci. 1982;394:54–71. doi: 10.1111/j.1749-6632.1982.tb37412.x. [DOI] [PubMed] [Google Scholar]
- Nicolas P., Batelier G., Rholam M., Cohen P. Bovine neurophysin dimerization and neurohypophyseal hormone binding. Biochemistry. 1980 Jul 22;19(15):3565–3573. doi: 10.1021/bi00556a023. [DOI] [PubMed] [Google Scholar]
- Nicolas P., Camier M., Lauber M., Masse M. J., Möhring J., Cohen P. Immunological identification of high molecular weight forms common to bovine neurophysin and vasopressin. Proc Natl Acad Sci U S A. 1980 May;77(5):2587–2591. doi: 10.1073/pnas.77.5.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann J. J., Aunis D. Distribution of secretory granules from adrenal medulla and neurohypophysis on continuous isoosmotic density gradients formed with a new ioxaglic derivative AG-6227. Anal Biochem. 1980 Nov 15;109(1):94–101. doi: 10.1016/0003-2697(80)90015-9. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Labouesse J. Neurosecretory granules: evidence from an aging process within the neurohypophysis. Science. 1981 Feb 6;211(4482):595–597. doi: 10.1126/science.7455700. [DOI] [PubMed] [Google Scholar]
- Nordmann J. J., Louis F., Morris S. J. Purification of two structurally and morphologically distinct populations of rat neurohypophysial secretory granules. Neuroscience. 1979;4(9):1367–1379. doi: 10.1016/0306-4522(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Hong J. S. Storage and release of vasopressin from neurosecretory granules and the neurohypophysis. Adv Cytopharmacol. 1974;2:303–310. [PubMed] [Google Scholar]
- Russell J. T., Brownstein M. J., Gainer H. Biosynthesis of vasopressin, oxytocin, and neurophysins: isolation and characterization of two common precursors (propressophysin and prooxyphysin). Endocrinology. 1980 Dec;107(6):1880–1891. doi: 10.1210/endo-107-6-1880. [DOI] [PubMed] [Google Scholar]
- Russell J. T., Holz R. W. Measurement of delta pH and membrane potential in isolated neurosecretory vesicles from bovine neurohypophyses. J Biol Chem. 1981 Jun 25;256(12):5950–5953. [PubMed] [Google Scholar]
- Scherman D., Nordmann J. J. Internal pH of isolated newly formed and aged neurohypophysial granules. Proc Natl Acad Sci U S A. 1982 Jan;79(2):476–479. doi: 10.1073/pnas.79.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]




