Abstract
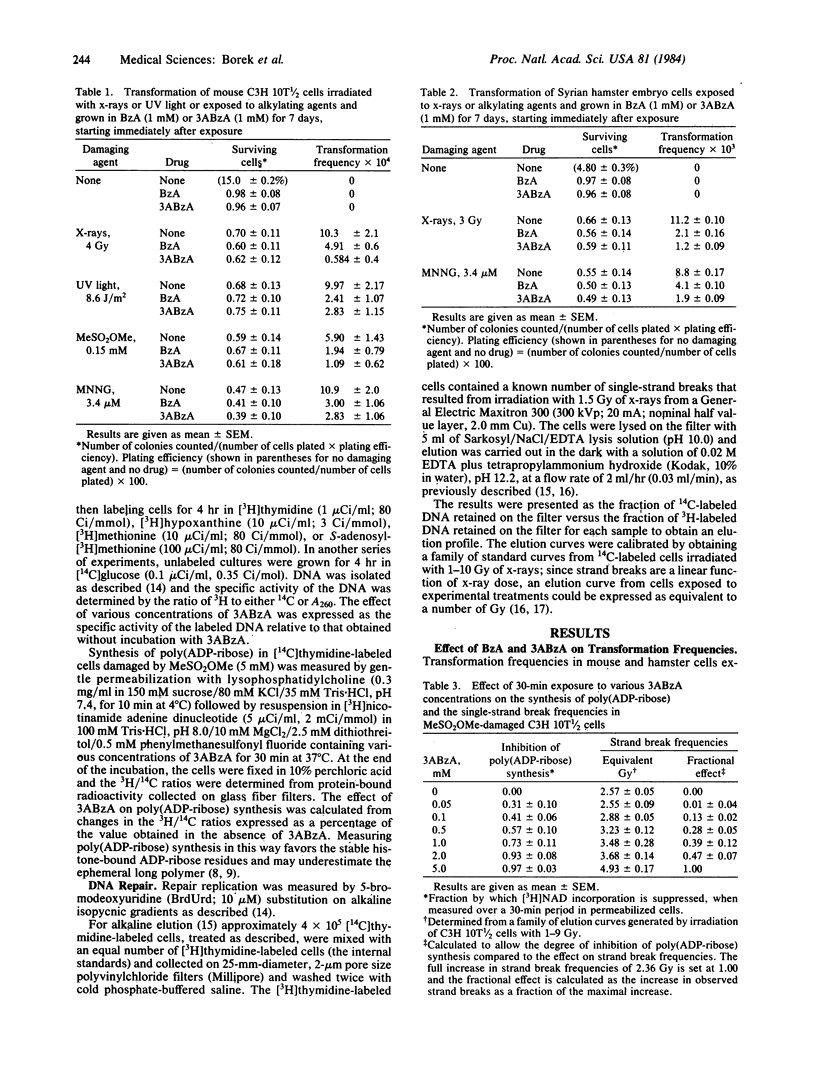
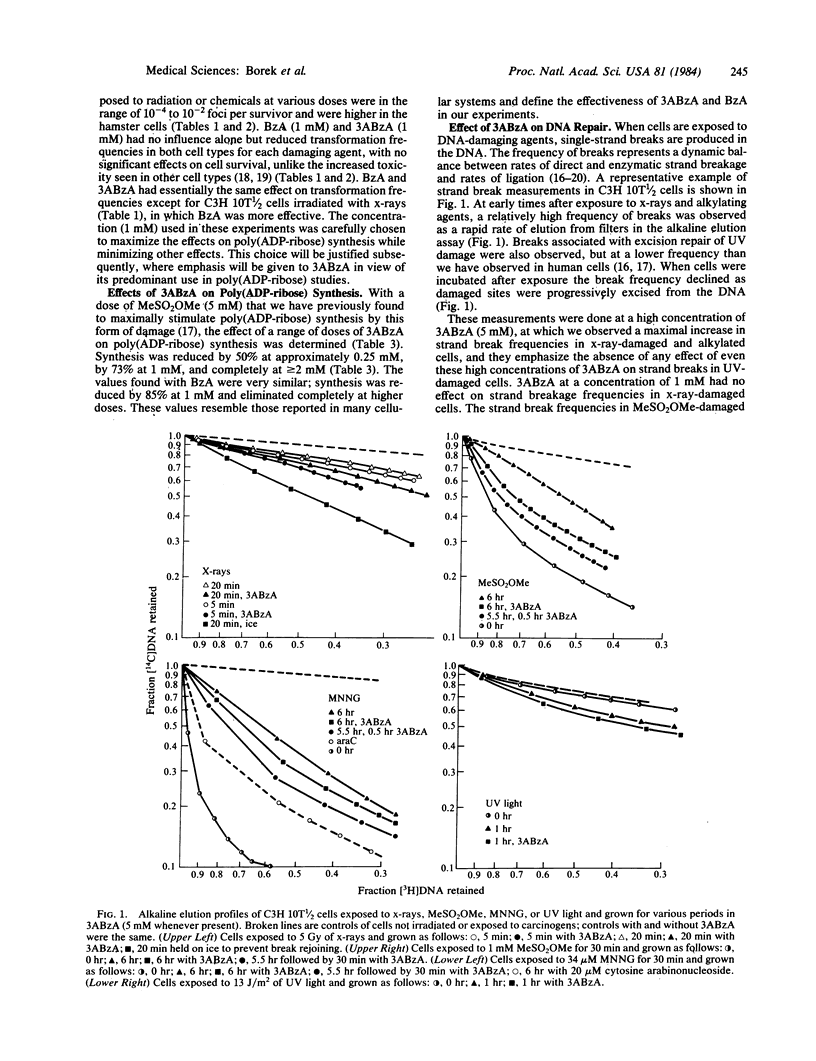
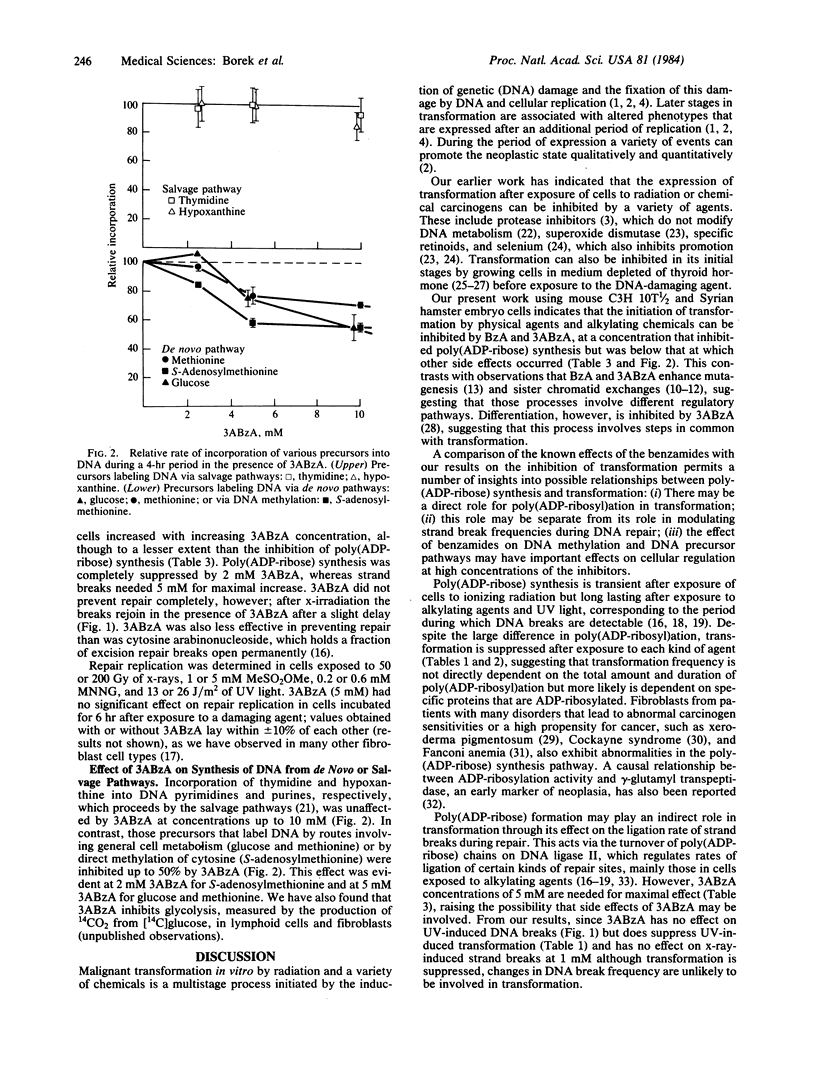
Malignant transformation in vitro of hamster embryo cells and mouse C3H 10T 1/2 cells by x-rays, ultraviolet light, and chemical carcinogens was inhibited by benzamide and by 3-aminobenzamide at concentrations that are specific for inhibition of poly(ADP-ribose) formation. These compounds slow the ligation stage of repair of x-ray and alkylation damage but not of ultraviolet light damage. At high concentrations they also inhibited de novo synthesis of DNA purines and DNA methylation by S-adenosylmethionine. The suppression of transformation by the benzamides is in striking contrast to their reported effectiveness in enhancing sister chromatid exchange, mutagenesis, and killing in cells exposed to alkylating agents. Our results suggest that mechanisms regulating malignant transformation are different from those regulating DNA repair, sister chromatid exchange, and mutagenesis and may be associated with changes in gene regulation and expression caused by alterations in poly(ADP-ribosyl)ation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Lawrence S. D., He Y. Z., Sattler G. L., Tsukada Y., Pitot H. C. Effects of altered [ADP-ribose]n metabolism on expression of fetal functions by adult hepatocytes. Nature. 1982 Nov 25;300(5890):366–368. doi: 10.1038/300366a0. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Berger S. J., Catino D. M. Abnormal NAD+ levels in cells from patients with Fanconi's anaemia. Nature. 1982 Sep 16;299(5880):271–273. doi: 10.1038/299271a0. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Sikorski G. W., Petzold S. J., Kurohara K. K. Defective poly(adenosine diphosphoribose) synthesis in xeroderma pigmentosum. Biochemistry. 1980 Jan 22;19(2):289–293. doi: 10.1021/bi00543a006. [DOI] [PubMed] [Google Scholar]
- Borek C., Cleaver J. E. Protease inhibitors neither damage DNA nor interfere with DNA repair or replication in human cells. Mutat Res. 1981 Jul;82(2):373–380. doi: 10.1016/0027-5107(81)90166-4. [DOI] [PubMed] [Google Scholar]
- Borek C., Guernsey D. L., Ong A., Edelman I. S. Critical role played by thyroid hormone in induction of neoplastic transformation by chemical carcinogens in tissue culture. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5749–5752. doi: 10.1073/pnas.80.18.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C., Hall E. J., Rossi H. H. Malignant transformation in cultured hamster embryo cells produced by X-rays, 460-keV monoenergetic neutrons, and heavy ions. Cancer Res. 1978 Sep;38(9):2997–3005. [PubMed] [Google Scholar]
- Borek C., Miller R., Pain C., Troll W. Conditions for inhibiting and enhancing effects of the protease inhibitor antipain on x-ray-induced neoplastic transformation in hamster and mouse cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1800–1803. doi: 10.1073/pnas.76.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C. Radiation oncogenesis in cell culture. Adv Cancer Res. 1982;37:159–232. doi: 10.1016/s0065-230x(08)60884-2. [DOI] [PubMed] [Google Scholar]
- Borek C., Sachs L. In vitro cell transformation by x-irradiation. Nature. 1966 Apr 16;210(5033):276–278. doi: 10.1038/210276a0. [DOI] [PubMed] [Google Scholar]
- Borek C., Troll W. Modifiers of free radicals inhibit in vitro the oncogenic actions of x-rays, bleomycin, and the tumor promoter 12-O-tetradecanoylphorbol 13-acetate. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1304–1307. doi: 10.1073/pnas.80.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Trosko J. E., Lett J. T. Excision repair (dimer excision, strand breakage and repair replication) in primary cultures of eukaryotic (bovine) cells. Exp Cell Res. 1972 Sep;74(1):67–80. doi: 10.1016/0014-4827(72)90482-x. [DOI] [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983 Jan 6;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun. 1983 Feb 28;111(1):47–54. doi: 10.1016/s0006-291x(83)80115-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Goto K., Kano Y. Ultraviolet hypersensitivity of Cockayne's syndrome fibroblasts. Effects of nicotinamide adenine dinucleotide and poly(ADP-ribose) synthesis. Exp Cell Res. 1982 May;139(1):207–215. doi: 10.1016/0014-4827(82)90334-2. [DOI] [PubMed] [Google Scholar]
- Guernsey D. L., Borek C., Edelman I. S. Crucial role of thyroid hormone in x-ray-induced neoplastic transformation in cell culture. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5708–5711. doi: 10.1073/pnas.78.9.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Hori T. High incidence of sister chromatid exchanges and chromatid interchanges in the conditions of lowered activity of poly(ADP-ribose)polymerase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):38–45. doi: 10.1016/0006-291x(81)91485-6. [DOI] [PubMed] [Google Scholar]
- Huberman E., Mager R., Sachs L. Mutagenesis and transformation of normal cells by chemical carcinogens. Nature. 1976 Nov 25;264(5584):360–361. doi: 10.1038/264360a0. [DOI] [PubMed] [Google Scholar]
- James M. R., Lehmann A. R. Role of poly(adenosine diphosphate ribose) in deoxyribonucleic acid repair in human fibroblasts. Biochemistry. 1982 Aug 17;21(17):4007–4013. doi: 10.1021/bi00260a016. [DOI] [PubMed] [Google Scholar]
- Meuth M. Role of deoxynucleoside triphosphate pools in the cytotoxic and mutagenic effects of DNA alkylating agents. Somatic Cell Genet. 1981 Jan;7(1):89–102. doi: 10.1007/BF01544750. [DOI] [PubMed] [Google Scholar]
- Morgan W. F., Cleaver J. E. 3-Aminobenzamide synergistically increases sister-chromatid exchanges in cells exposed to methyl methanesulfonate but not to ultraviolet light. Mutat Res. 1982 Jul;104(6):361–366. doi: 10.1016/0165-7992(82)90170-1. [DOI] [PubMed] [Google Scholar]
- Morgan W. F., Cleaver J. E. Effect of 3-aminobenzamide on the rate of ligation during repair of alkylated DNA in human fibroblasts. Cancer Res. 1983 Jul;43(7):3104–3107. [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Sugimura T., Miwa M., Saitô H., Kanai Y., Ikejima M., Terada M., Yamada M., Utakoji T. Studies of nuclear ADP-ribosylation. Adv Enzyme Regul. 1980;18:195–220. doi: 10.1016/0065-2571(80)90016-3. [DOI] [PubMed] [Google Scholar]



