Abstract
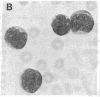
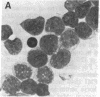
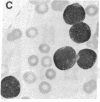
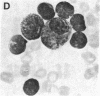
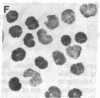
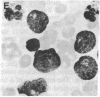
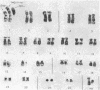
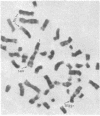
Selective failure of lymphoid development occurs in genetic deficiency of adenosine deaminase (ADA). We examined the in vivo effects of a potent inhibitor of ADA, 2'-deoxycoformycin, which was used to treat a patient with refractory acute leukemia. Unexpectedly, within 7 days of starting treatment, the leukemic phenotype underwent complete conversion from T lymphoblastic to promyelocytic, with kinetics that suggested a precursor-product relationship between the two cell populations. Pretreatment T lymphoblasts and posttreatment promyelocytes had the same abnormal karyotype. Upon culture in vitro, the former transformed spontaneously over several weeks into mature myeloid cells. We conclude that the leukemia arose from a multipotent stem cell capable of both lymphoid and myeloid differentiation. Effects of ADA inhibition on leukemia cells during treatment included expansion of the deoxyadenosine nucleotide pool and accumulation of S-adenosylhomocysteine, a potent inhibitor of S-adenosylmethionine-dependent methylation. The influence of these changes on the leukemic phenotype is discussed in terms of (i) selective cytotoxicity to T lymphoblasts, which accumulated deoxyadenosine nucleotides more efficiently than did the patient's promyelocytes during in vitro incubation with deoxycoformycin plus deoxyadenosine, and (ii) induction of an altered program of differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D. R. Editorial: Hematopoietic stem cell theory in relation to possible lymphoblastic conversion of chronic myeloid leukemia. Blood. 1974 Sep;44(3):449–453. [PubMed] [Google Scholar]
- Bolen P. L., Grant D. M., Swinton D., Boynton J. E., Gillham N. W. Extensive methylation of chloroplast DNA by a nuclear gene mutation does not affect chloroplast gene transmission in chlamydomonas. Cell. 1982 Feb;28(2):335–343. doi: 10.1016/0092-8674(82)90351-8. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Differential sensitivity of human leukemic T cell lines and B cell lines to growth inhibition by deoxyadenosine. J Immunol. 1978 Nov;121(5):1726–1731. [PubMed] [Google Scholar]
- Coleman C. N., Johns D. G., Chabner B. A. Studies on mechanisms of resistance to cytosine arabinoside: problems in the determination of related enzyme activities in leukemic cells. Ann N Y Acad Sci. 1975 Aug 8;255:247–251. doi: 10.1111/j.1749-6632.1975.tb29231.x. [DOI] [PubMed] [Google Scholar]
- Creusot F., Acs G., Christman J. K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2'-deoxycytidine. J Biol Chem. 1982 Feb 25;257(4):2041–2048. [PubMed] [Google Scholar]
- DE LA HABA G., CANTONI G. L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959 Mar;234(3):603–608. [PubMed] [Google Scholar]
- Eisenbarth G. S., Haynes B. F., Schroer J. A., Fauci A. S. Production of monoclonal antibodies reacting with peripheral blood mononuclear cell surface differentiation antigens. J Immunol. 1980 Mar;124(3):1237–1244. [PubMed] [Google Scholar]
- Fleischman R. A., Custer R. P., Mintz B. Totipotent hematopoietic stem cells: normal self-renewal and differentiation after transplantation between mouse fetuses. Cell. 1982 Sep;30(2):351–359. doi: 10.1016/0092-8674(82)90233-1. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Hensley L. L., Jegasothy B. V. Differentiation of human T lymphocytes: II. Phenotypic difference in skin and blood malignant T-cells in cutaneous T-cell lymphoma. J Invest Dermatol. 1982 Apr;78(4):323–326. doi: 10.1111/1523-1747.ep12507406. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. Human T lymphocyte antigens as defined by monoclonal antibodies. Immunol Rev. 1981;57:127–161. doi: 10.1111/j.1600-065x.1981.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Shimizu K., Eisenbarth G. S. Identification of human and rodent thymic epithelium using tetanus toxin and monoclonal antibody A2B5. J Clin Invest. 1983 Jan;71(1):9–14. doi: 10.1172/JCI110755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield M. S. Apparent suicide inactivation of human lymphoblast S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine and adenine arabinoside. A basis for direct toxic effects of analogs of adenosine. J Biol Chem. 1979 Jan 10;254(1):22–25. [PubMed] [Google Scholar]
- Hershfield M. S., Fetter J. E., Small W. C., Bagnara A. S., Williams S. R., Ullman B., Martin D. W., Jr, Wasson D. B., Carson D. A. Effects of mutational loss of adenosine kinase and deoxycytidine kinase on deoxyATP accumulation and deoxyadenosine toxicity in cultured CEM human T-lymphoblastoid cells. J Biol Chem. 1982 Jun 10;257(11):6380–6386. [PubMed] [Google Scholar]
- Hershfield M. S., Kredich N. M., Koller C. A., Mitchell B. S., Kurtzberg J., Kinney T. R., Falletta J. M. S-adenosylhomocysteine catabolism and basis for acquired resistance during treatment of T-cell acute lymphoblastic leukemia with 2'-deoxycoformycin alone and in combination with 9-beta-D-arabinofuranosyladenine. Cancer Res. 1983 Jul;43(7):3451–3458. [PubMed] [Google Scholar]
- Hershfield M. S., Kredich N. M., Ownby D. R., Ownby H., Buckley R. In vivo inactivation of erythrocyte S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine in adenosine deaminase-deficient patients. J Clin Invest. 1979 Apr;63(4):807–811. doi: 10.1172/JCI109367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield M. S., Krodich N. M. S-adenosylhomocysteine hydrolase is an adenosine-binding protein: a target for adenosine toxicity. Science. 1978 Nov 17;202(4369):757–760. doi: 10.1126/science.715439. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Snyder F. F., Seegmiller J. E. Adenine and adenosine are toxic to human lymphoblast mutants defective in purine salvage enzymes. Science. 1977 Sep 23;197(4310):1284–1287. doi: 10.1126/science.197600. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Jehn U., Thiel E. Auer bodies in acute lymphocytic leukaemia and B-cell lymphoma: evidence for a common progenitor of myeloid and lymphoid cells? Blut. 1981 Jul;43(1):7–14. doi: 10.1007/BF00319926. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Hershfield M. S. S-adenosylhomocysteine toxicity in normal and adenosine kinase-deficient lymphoblasts of human origin. Proc Natl Acad Sci U S A. 1979 May;76(5):2450–2454. doi: 10.1073/pnas.76.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Martin D. V., Jr Role of S-adenosylhomocysteine in adenosinemediated toxicity in cultured mouse T lymphoma cells. Cell. 1977 Dec;12(4):931–938. doi: 10.1016/0092-8674(77)90157-x. [DOI] [PubMed] [Google Scholar]
- McGraw T. P., Folds J. D., Bollum F. J., Stass S. A. Terminal deoxynucleotidyl transferase-positive acute myeloblastic leukemia. Am J Hematol. 1981;10(3):251–258. doi: 10.1002/ajh.2830100304. [DOI] [PubMed] [Google Scholar]
- Mitchell B. S., Mejias E., Daddona P. E., Kelley W. N. Purinogenic immunodeficiency diseases: selective toxicity of deoxyribonucleosides for T cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. B., Stass S., Kalwinsky D., Rivera G. Phenotypic conversion of acute leukaemia from T-lymphoblastic to myeloblastic induced by therapy with 2'-deoxycoformycin. Br J Haematol. 1983 Oct;55(2):285–293. doi: 10.1111/j.1365-2141.1983.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Perentesis J., Ramsay N. K., Brunning R., Kersey J. H., Filipovich A. H. Biphenotypic leukemia: immunologic and morphologic evidence for a common lymphoid-myeloid progenitor in humans. J Pediatr. 1983 Jan;102(1):63–67. doi: 10.1016/s0022-3476(83)80288-1. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- SANDBERG A. A., CARTWRIGHT G. E., WINTROBE M. M. Studies on leukemia. I. Uric acid excretion. Blood. 1956 Feb;11(2):154–166. [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Smyth J. F., Poplack D. G., Holiman B. J., Leventhal B. G., Yarbro G. Correlation of adenosine deaminase activity with cell surface markers in acute lymphoblastic leukemia. J Clin Invest. 1978 Sep;62(3):710–712. doi: 10.1172/JCI109179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Whang-Peng J., Canellos G. P., Carbone P. P., Tjio J. H. Clinical implications of cytogenetic variants in chronic myelocytic leukemia (CML). Blood. 1968 Nov;32(5):755–766. [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J., Oken M. M., Kaplan M. E., Ensrud K. M., Howe R. R., Theologides A. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin's lymphoma. N Engl J Med. 1982 Nov 11;307(20):1231–1236. doi: 10.1056/NEJM198211113072002. [DOI] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Déchamps M., Buttin G. The modulation of the thymidine triphosphate pool of Chinese hamster cells by dCMP deaminase and UDP reductase. Thymidine auxotrophy induced by CTP in dCMP deaminase-deficient lines. J Biol Chem. 1980 Jan 10;255(1):162–167. [PubMed] [Google Scholar]