Abstract
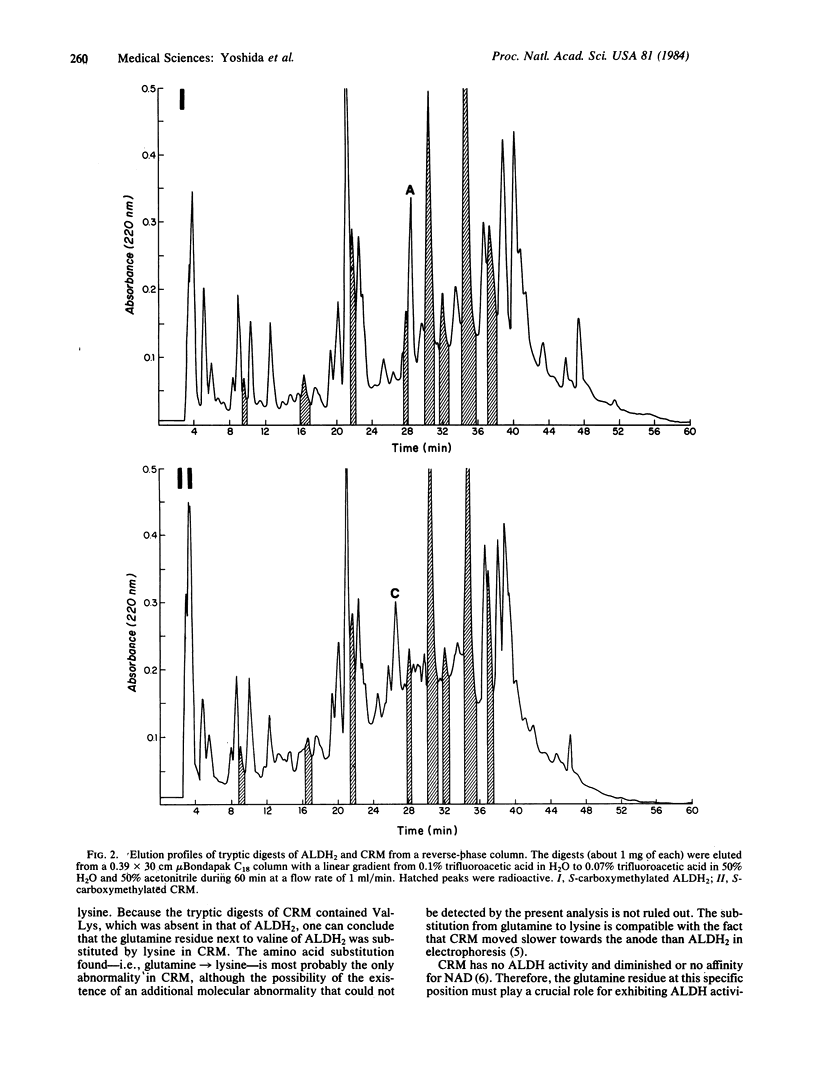
Usual human livers contain two major aldehyde dehydrogenase [(ALDH) aldehyde:NAD+ oxidoreductase] isozymes--i.e., a cytosolic ALDH1 component and a mitochondrial ALDH2 component--whereas approximately equal to 50% of Orientals are "atypical" and have only the ALDH1 isozyme and are missing the ALDH2 isozyme. We previously demonstrated that atypical livers contain an enzymatically inactive but immunologically crossreactive material (CRM) corresponding to the ALDH2 component. The enzymatically active ALDH2 obtained from a usual liver and the CRM obtained from an atypical liver were reduced, S-carboxymethylated, and digested by trypsin. Separation of their digests by high-performance reverse-phase chromatography and by two-dimensional paper chromatography and electrophoresis revealed that ALDH2 contained a peptide sequence of -Glu-Leu-Gly-Glu-Ala-Gly-Leu-Gln-Ala-Asn-Val-Gln-Val-Lys- and that the glutamine adjacent to lysine was substituted by lysine in CRM. All other tryptic peptides, including eight peptides containing S-carboxymethylcysteine, were common in ALDH2 and CRM. It is concluded that a point mutation in the human ALDH2 locus produced the glutamine leads to lysine substitution and enzyme inactivation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Crow K. E., Kitson T. M., MacGibbon A. K., Batt R. D. Intracellular localisation and properties of aldehyde dehydrogenases from sheep liver. Biochim Biophys Acta. 1974 May 20;350(1):121–128. doi: 10.1016/0005-2744(74)90209-5. [DOI] [PubMed] [Google Scholar]
- Eckfeldt J. H., Yonetani T. Subcellular localization of the F1 and F2 isozymes of horse liver aldehyde dehydrogenase. Arch Biochem Biophys. 1976 Aug;175(2):717–722. doi: 10.1016/0003-9861(76)90564-6. [DOI] [PubMed] [Google Scholar]
- Eckfeldt J., Mope L., Takio K., Yonetani T. Horse liver aldehyde dehydrogenase. Purification and characterization of two isozymes. J Biol Chem. 1976 Jan 10;251(1):236–240. [PubMed] [Google Scholar]
- Goedde H. W., Harada S., Agarwal D. P. Racial differences in alcohol sensitivity: a new hypothesis. Hum Genet. 1979 Oct 2;51(3):331–334. doi: 10.1007/BF00283404. [DOI] [PubMed] [Google Scholar]
- Greenfield N. J., Pietruszko R. Two aldehyde dehydrogenases from human liver. Isolation via affinity chromatography and characterization of the isozymes. Biochim Biophys Acta. 1977 Jul 8;483(1):35–45. doi: 10.1016/0005-2744(77)90005-5. [DOI] [PubMed] [Google Scholar]
- Huang I. Y., Rubinfien E., Yoshida A. Complete amino acid sequence of human phosphoglycerate kinase. Isolation and amino acid sequence of tryptic peptides. J Biol Chem. 1980 Jul 10;255(13):6408–6411. [PubMed] [Google Scholar]
- Ikawa M., Impraim C. C., Wang G., Yoshida A. Isolation and characterization of aldehyde dehydrogenase isozymes from usual and atypical human livers. J Biol Chem. 1983 May 25;258(10):6282–6287. [PubMed] [Google Scholar]
- Impraim C., Wang G., Yoshida A. Structural mutation in a major human aldehyde dehydrogenase gene results in loss of enzyme activity. Am J Hum Genet. 1982 Nov;34(6):837–841. [PMC free article] [PubMed] [Google Scholar]
- Kitabatake N., Sasaki R., Chiba H. Localization of bovine liver aldehyde dehydrogenase isozymes and their immunological properties. J Biochem. 1981 Apr;89(4):1223–1229. [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Chen S. H., Fukui M. Liver alcohol dehydrogenase in Japanese: high population frequency of atypical form and its possible role in alcohol sensitivity. Am J Hum Genet. 1975 Nov;27(6):789–796. [PMC free article] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Teng Y. S. Human liver aldehyde dehydrogenase in Chinese and Asiatic Indians: gene deletion and its possible implications in alcohol metabolism. Biochem Genet. 1981 Feb;19(1-2):107–114. doi: 10.1007/BF00486141. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Impraim C. C., Huang I. Y. Enzymatic and structural differences between usual and atypical human liver alcohol dehydrogenases. J Biol Chem. 1981 Dec 10;256(23):12430–12436. [PubMed] [Google Scholar]
- Yoshida A. The existence of atypical blood group galactosyltransferase which causes an expression of A2 character in A1B red blood cells. Am J Hum Genet. 1983 Nov;35(6):1117–1125. [PMC free article] [PubMed] [Google Scholar]
- von Bahr-Lindström H., Sohn S., Woenckhaus C., Jeck R., Jörnvall H. Characterization of a structure close to the coenzyme-binding site of liver aldehyde dehydrogenase. Eur J Biochem. 1981 Jul;117(3):521–526. doi: 10.1111/j.1432-1033.1981.tb06368.x. [DOI] [PubMed] [Google Scholar]




