Abstract
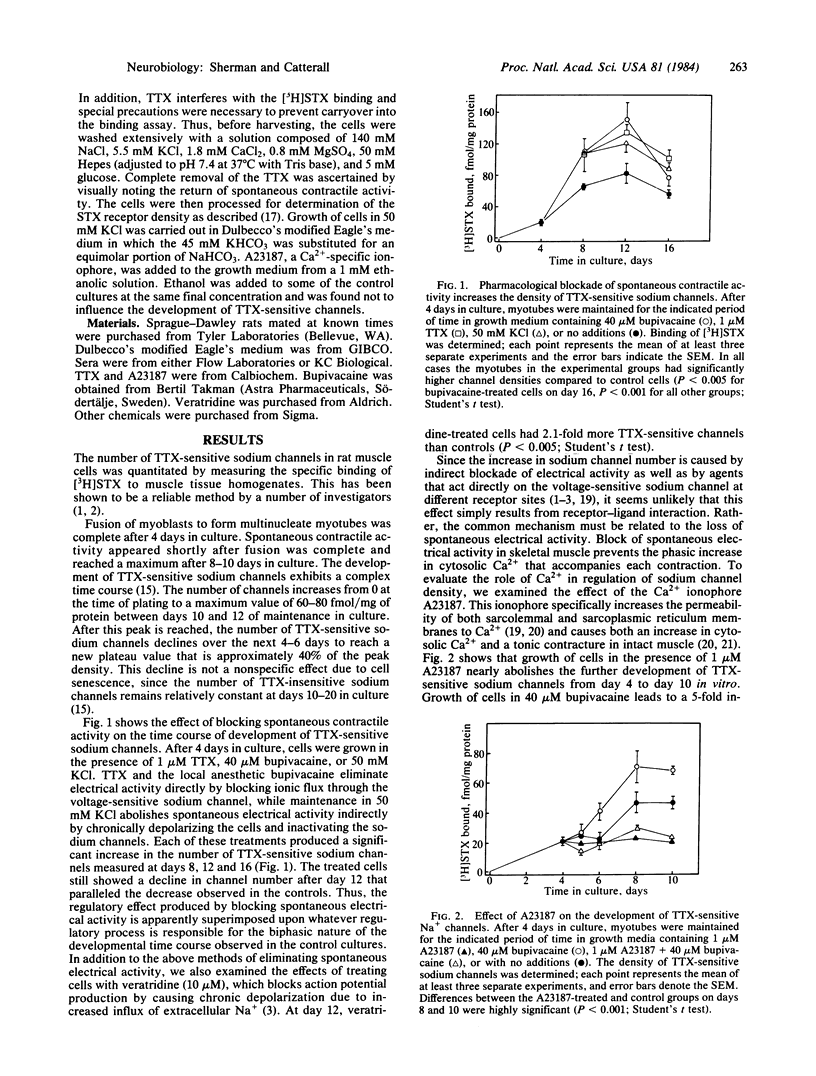
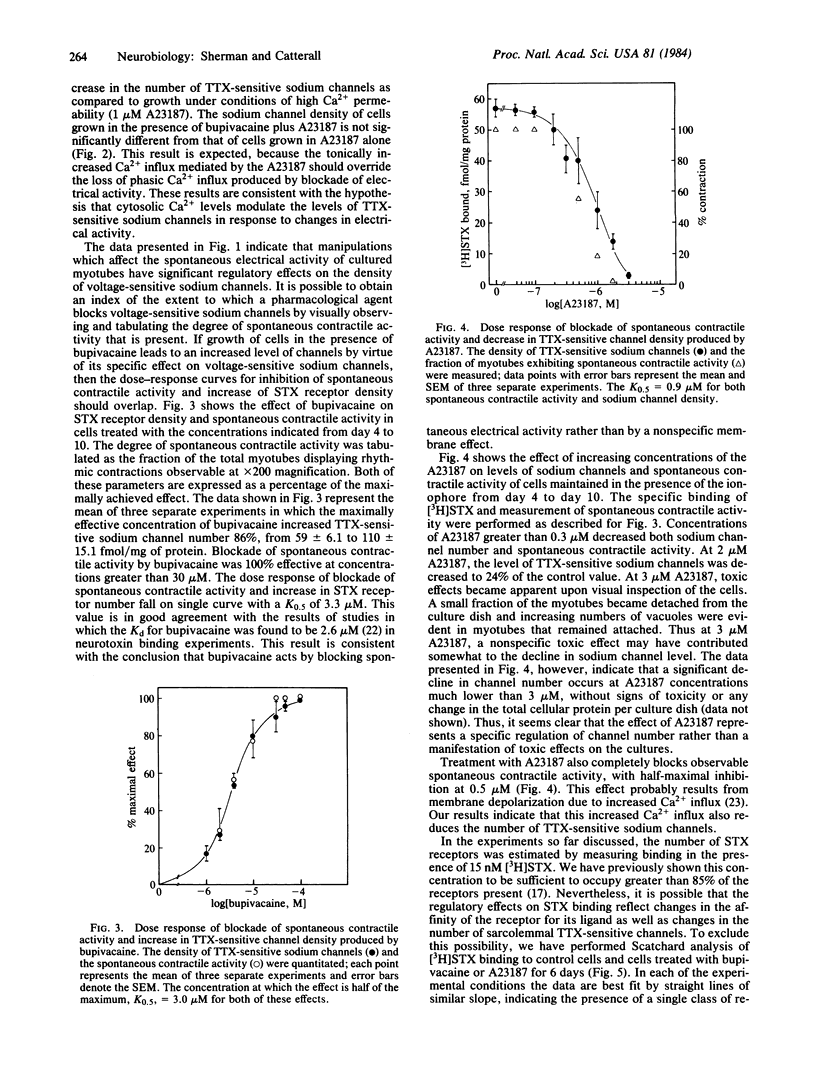
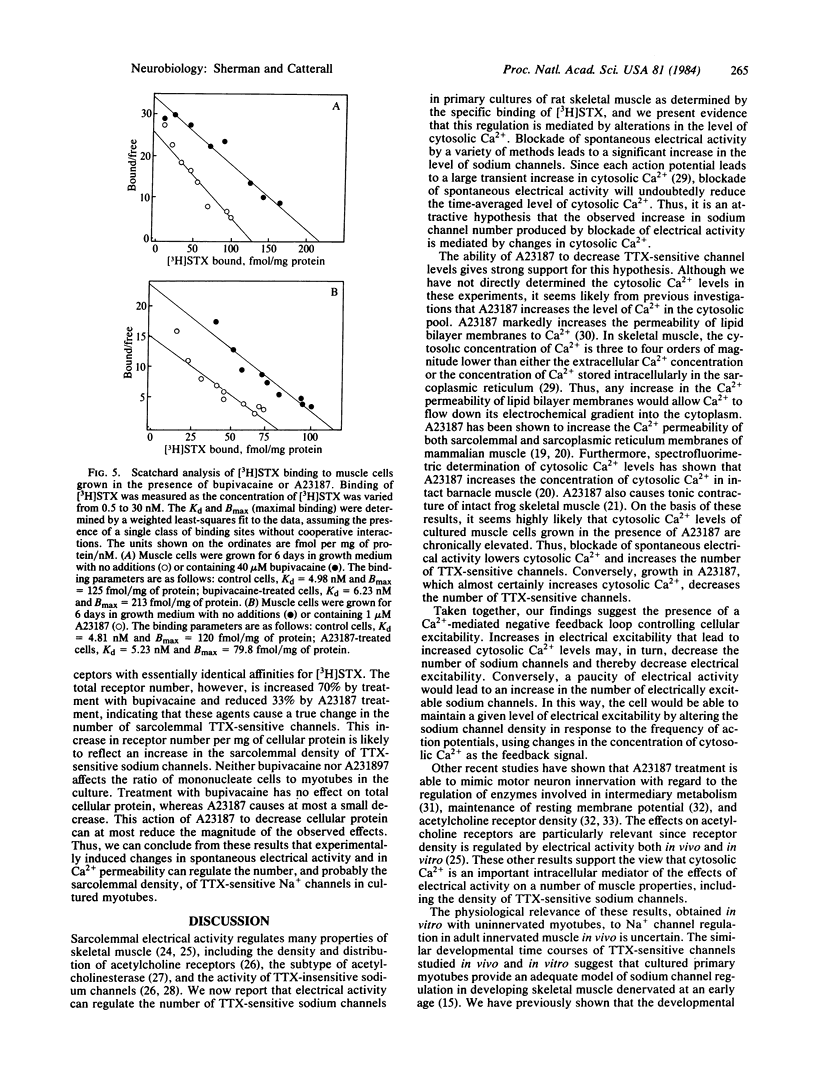
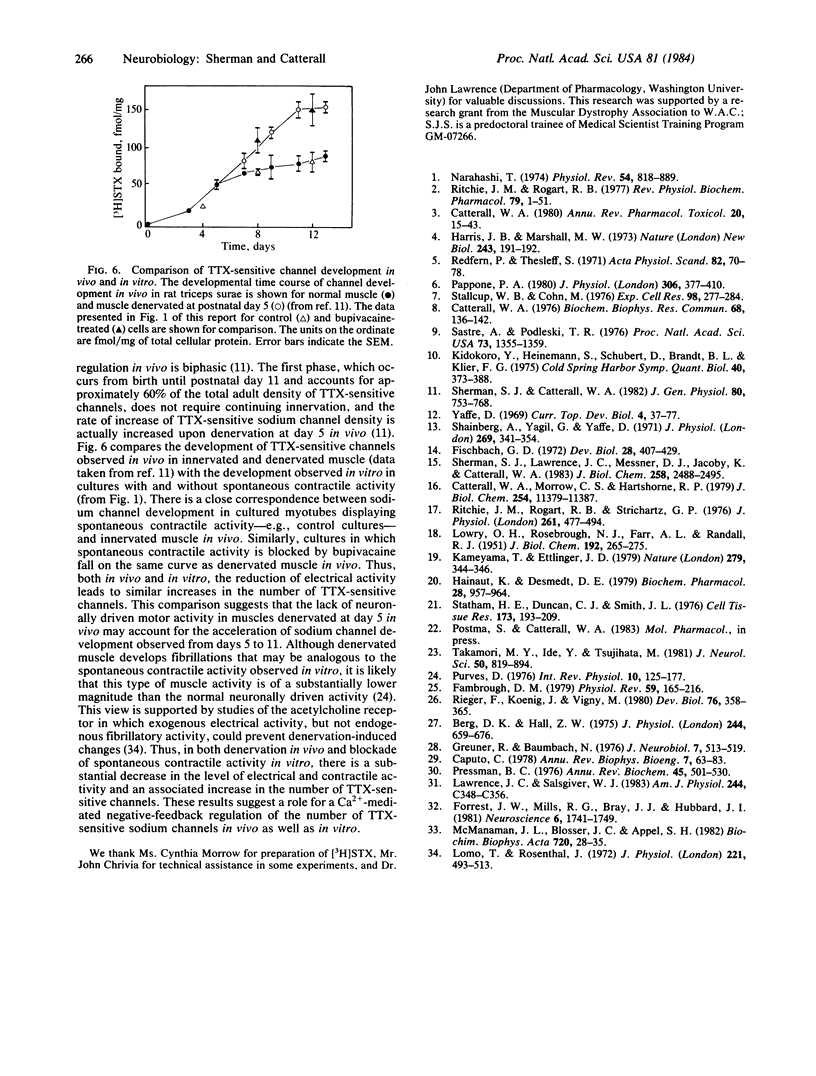
Pharmacological blockade of the spontaneous electrical activity present in primary cultures of rat myotubes by growth in bupivacaine, tetrodotoxin, or KCl was found to increase the number of voltage-sensitive Na+ channels 38-83% as measured by the specific binding of [3H]saxitoxin. The inhibition of spontaneous electrical activity and increase in channel density by bupivacaine displayed an identical dose response, with a half-maximal effect at 3.0 microM. Growth of myotubes in the presence of 1 microM A23187, a Ca2+-specific ionophore, resulted in a 30-60% decrease in the number of tetrodotoxin-sensitive channels with no change in affinity for [3H]saxitoxin. A23187 was able to overcome the increase in channel density produced by bupivacaine. These results suggest the presence of a Ca2+-mediated negative feedback system in which electrical excitability may be regulated by altering the number of tetrodotoxin-sensitive Na+ channels.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. K., Hall Z. W. Increased extrajunctional acetylcholine sensitivity produced by chronic acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol. 1975 Jan;244(3):659–676. doi: 10.1113/jphysiol.1975.sp010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. Excitation and contraction processes in muscle. Annu Rev Biophys Bioeng. 1978;7:63–83. doi: 10.1146/annurev.bb.07.060178.000431. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Activation and inhibition of the action potential Na+ ionophore of cultured rat muscle cells by neurotoxins. Biochem Biophys Res Commun. 1976 Jan 12;68(1):136–142. doi: 10.1016/0006-291x(76)90020-6. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S., Hartshorne R. P. Neurotoxin binding to receptor sites associated with voltage-sensitive sodium channels in intact, lysed, and detergent-solubilized brain membranes. J Biol Chem. 1979 Nov 25;254(22):11379–11387. [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Desmedt J. E., Hainaut K. Dantrolene and A13187 ionophore: specific action on calcium channels revealed by the aequorin method. Biochem Pharmacol. 1979 Apr 1;28(7):957–964. doi: 10.1016/0006-2952(79)90286-7. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Gruener R., Baumbach N. Muscle insensitivity to tetrodotoxin: induction by alpha-bungarotoxin and removal by submechanical threshold stimulation. J Neurobiol. 1976 Nov;7(6):513–519. doi: 10.1002/neu.480070605. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Marshall M. W. Tetrodotoxin-resistant action potentials in newborn rat muscle. Nat New Biol. 1973 Jun 6;243(127):191–192. doi: 10.1038/newbio243191a0. [DOI] [PubMed] [Google Scholar]
- Kameyama T., Etlinger J. D. Calcium-dependent regulation of protein synthesis and degradation in muscle. Nature. 1979 May 24;279(5711):344–346. doi: 10.1038/279344a0. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y., Heinemann S., Schubert D., Brandt B. L., Klier F. G. Synapse formation and neurotrophic effects on muscle cell lines. Cold Spring Harb Symp Quant Biol. 1976;40:373–388. doi: 10.1101/sqb.1976.040.01.036. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Salsgiver W. J. Levels of enzymes of energy metabolism are controlled by activity of cultured rat myotubes. Am J Physiol. 1983 May;244(5):C348–C355. doi: 10.1152/ajpcell.1983.244.5.C348. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaman J. L., Blosser J. C., Appel S. H. Inhibitors of membrane depolarization regulate acetylcholine receptor synthesis by a calcium-dependent, cyclic nucleotide-independent mechanism. Biochim Biophys Acta. 1982 Feb 10;720(1):28–35. doi: 10.1016/0167-4889(82)90035-0. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Pappone P. A. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980 Sep;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Rieger F., Koenig J., Vigny M. Spontaneous contractile activity and the presence of the 16 S form of acetylcholinesterase in rat muscle cells in culture: reversible suppressive action of tetrodotoxin. Dev Biol. 1980 May;76(2):358–365. doi: 10.1016/0012-1606(80)90385-1. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B., Strichartz G. R. A new method for labelling saxitoxin and its binding to non-myelinated fibres of the rabbit vagus, lobster walking leg, and garfish olfactory nerves. J Physiol. 1976 Oct;261(2):477–494. doi: 10.1113/jphysiol.1976.sp011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Sastre A., Podleski T. R. Pharmacologic characterization of the Na+ ionophores in L6 myotubes. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1355–1359. doi: 10.1073/pnas.73.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. J., Catterall W. A. Biphasic regulation of development of the high-affinity saxitoxin receptor by innervation in rat skeletal muscle. J Gen Physiol. 1982 Nov;80(5):753–768. doi: 10.1085/jgp.80.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. J., Lawrence J. C., Messner D. J., Jacoby K., Catterall W. A. Tetrodotoxin-sensitive sodium channels in rat muscle cells developing in vitro. J Biol Chem. 1983 Feb 25;258(4):2488–2495. [PubMed] [Google Scholar]
- Stallcup W. B., Cohn M. Electrical properties of a clonal cell line as determined by measurement of ion fluxes. Exp Cell Res. 1976 Mar 15;98(2):277–284. doi: 10.1016/0014-4827(76)90439-0. [DOI] [PubMed] [Google Scholar]
- Statham H. E., Duncan C. J., Smith J. L. The effect of the ionophore A23187 on the ultrastructure and electrophysiological properties of frog skeletal muscle. Cell Tissue Res. 1976 Oct 6;173(2):193–209. doi: 10.1007/BF00221375. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Cellular aspects of muscle differentiation in vitro. Curr Top Dev Biol. 1969;4:37–77. doi: 10.1016/s0070-2153(08)60480-9. [DOI] [PubMed] [Google Scholar]



