Abstract
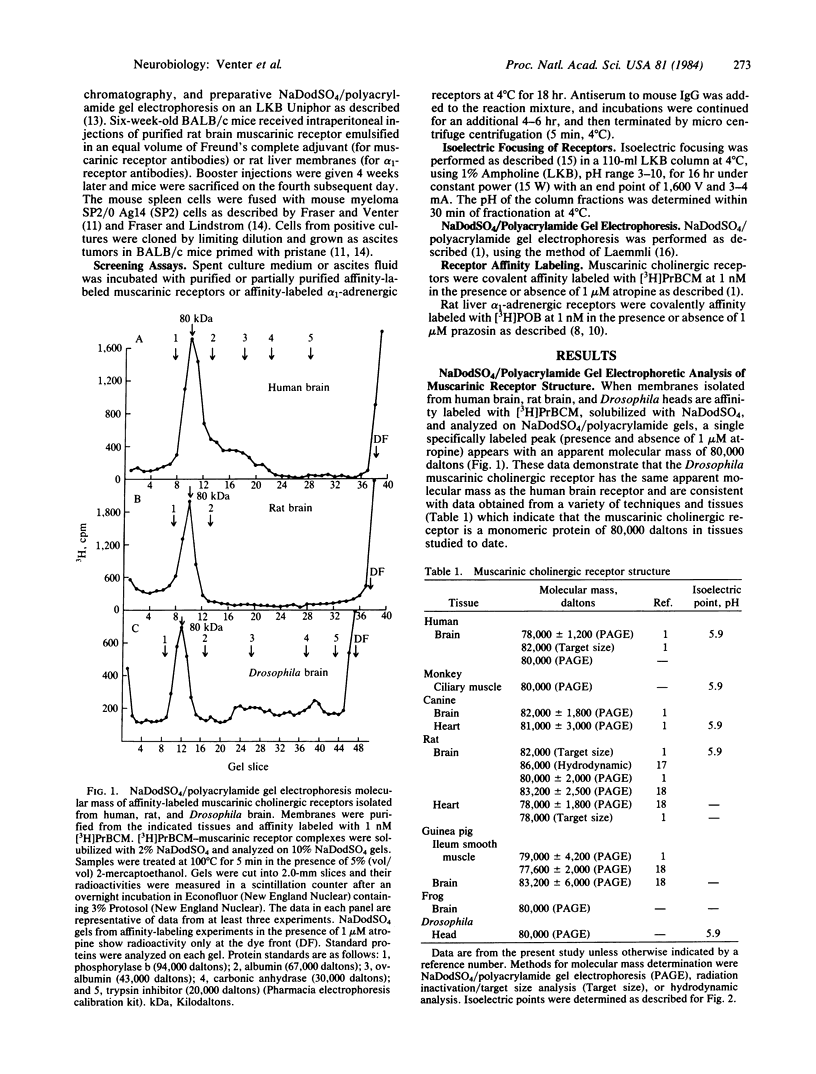
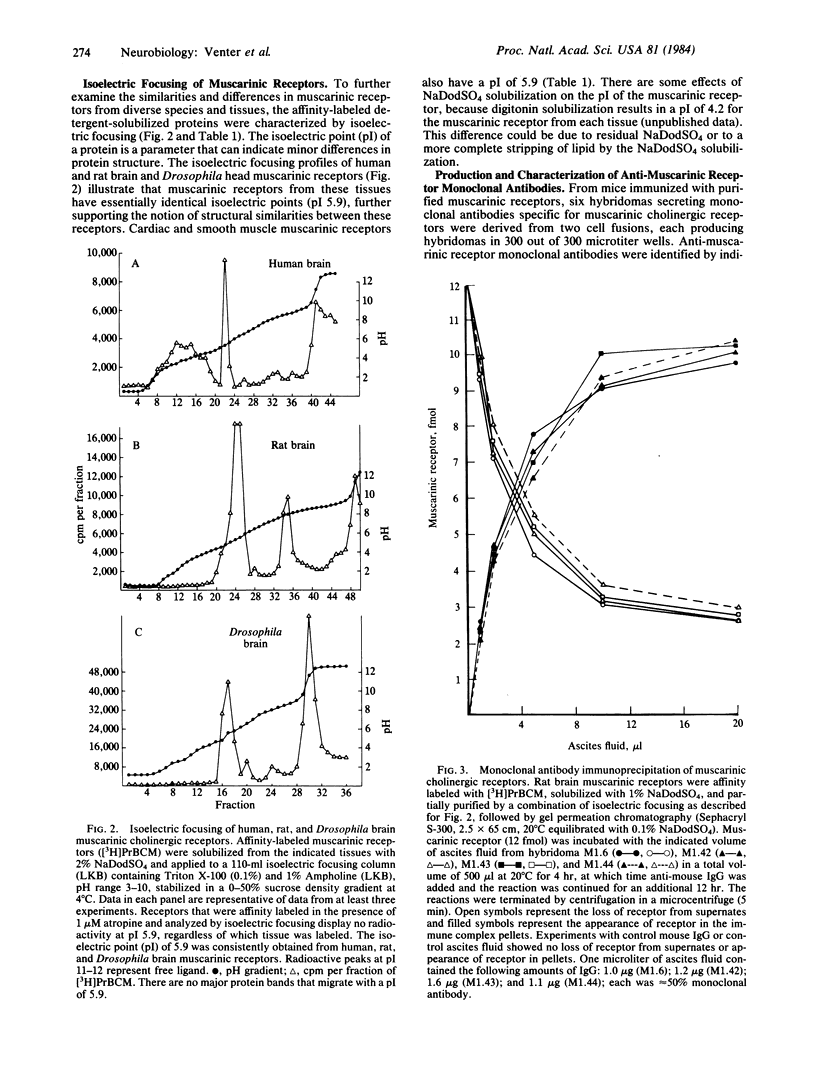
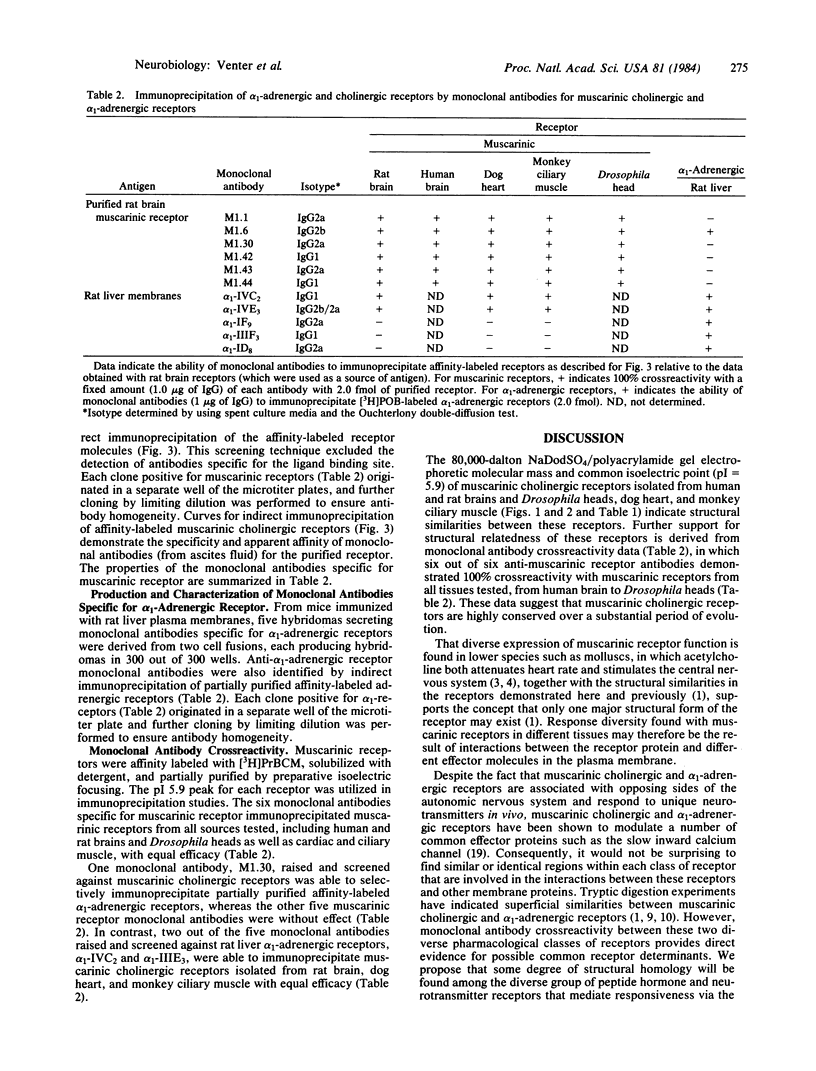
Muscarinic cholinergic receptors isolated from Drosophila heads, rat and human brain, dog heart, and monkey ciliary muscle were examined for structural similarities/differences by utilizing isoelectric focusing, sodium dodecyl sulfate/polyacrylamide gel electrophoresis, and monoclonal antibody crossreactivity. Muscarinic receptors were affinity labeled with [3H]propylbenzilylcholine mustard and subjected to isoelectric focusing. Muscarinic receptors from each species focused with an isoelectric point of 5.9. The same proteins all migrated with an apparent molecular mass of 80,000 daltons on sodium dodecyl sulfate gels. Six hybridomas secreting monoclonal antibodies specific for muscarinic receptors were developed by using purified rat brain muscarinic receptors as the antigen. The six different monoclonal antibodies immunoprecipitated muscarinic receptors from all tissues and species tested, including human and Drosophila brains, with equal efficacy. These data indicate that muscarinic receptors are highly conserved over a considerable evolutionary period. One of the six muscarinic receptor monoclonal antibodies also immunoprecipitated rat liver alpha 1-adrenergic receptors. Furthermore, two out of five monoclonal antibodies raised against alpha 1-receptors immunoprecipitated muscarinic receptors. These data suggest that some degree of structural homology exists between muscarinic cholinergic receptors and alpha 1-adrenergic receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birdsall N. J., Burgen A. S., Hulme E. C. A study of the muscarinic receptor by gel electrophoresis. Br J Pharmacol. 1979 Jun;66(2):337–342. doi: 10.1111/j.1476-5381.1979.tb13685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Ben-Barak J. Muscarinic receptor in Drosophila melanogaster demonstrated by binding of [3H]quinuclidinyl benzilate. FEBS Lett. 1977 Sep 1;81(1):134–136. doi: 10.1016/0014-5793(77)80945-9. [DOI] [PubMed] [Google Scholar]
- Fischer H. Animal evolution in the field of synaptic substances. Naturwissenschaften. 1972 Oct;59(10):425–435. doi: 10.1007/BF00592877. [DOI] [PubMed] [Google Scholar]
- Fraser C. M., Greguski R., Eddy B., Venter J. C. Autoantibodies and monoclonal antibodies in the purification and molecular characterization of neurotransmitter receptors. J Cell Biochem. 1983;21(3):219–231. doi: 10.1002/jcb.240210304. [DOI] [PubMed] [Google Scholar]
- Fraser C. M., Venter J. C. Monoclonal antibodies to beta-adrenergic receptors: use in purification and molecular characterization of beta receptors. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7034–7038. doi: 10.1073/pnas.77.12.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitschier J., Strichartz G. R., Hall L. M. Saxitoxin binding to sodium channels in head extracts from wild-type and tetrodotoxin-sensitive strains of Drosophila melanogaster. Biochim Biophys Acta. 1980 Jan 25;595(2):291–303. doi: 10.1016/0005-2736(80)90091-7. [DOI] [PubMed] [Google Scholar]
- Haga T. Molecular size of muscarinic acetylcholine receptors of rat brain. FEBS Lett. 1980 Apr 21;113(1):68–72. doi: 10.1016/0014-5793(80)80497-2. [DOI] [PubMed] [Google Scholar]
- Haim N., Nahum S., Dudai Y. Properties of a putative muscarinic cholinergic receptor from Drosophila melanogaster. J Neurochem. 1979 Feb;32(2):543–552. doi: 10.1111/j.1471-4159.1979.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Sumikawa K. Quinuclidinyl benzilate binding in house fly heads and rat brain. J Neurochem. 1981 Feb;36(2):454–459. doi: 10.1111/j.1471-4159.1981.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Kistler J., Stroud R. M., Klymkowsky M. W., Lalancette R. A., Fairclough R. H. Structure and function of an acetylcholine receptor. Biophys J. 1982 Jan;37(1):371–383. doi: 10.1016/S0006-3495(82)84685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunos G., Kan W. H., Greguski R., Venter J. C. Selective affinity labeling and molecular characterization of hepatic alpha 1-adrenergic receptors with [3H]phenoxybenzamine. J Biol Chem. 1983 Jan 10;258(1):326–332. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michelson M. J. Some aspects of evolutionary pharmacology. Biochem Pharmacol. 1974 Aug 15;23(16):2211–2224. doi: 10.1016/0006-2952(74)90551-6. [DOI] [PubMed] [Google Scholar]
- Venter J. C., Fraser C. M., Schaber J. S., Jung C. Y., Bolger G., Triggle D. J. Molecular properties of the slow inward calcium channel. Molecular weight determinations by radiation inactivation and covalent affinity labeling. J Biol Chem. 1983 Aug 10;258(15):9344–9348. [PubMed] [Google Scholar]
- Venter J. C. Muscarinic cholinergic receptor structure. Receptor size, membrane orientation, and absence of major phylogenetic structural diversity. J Biol Chem. 1983 Apr 25;258(8):4842–4848. [PubMed] [Google Scholar]



