Abstract
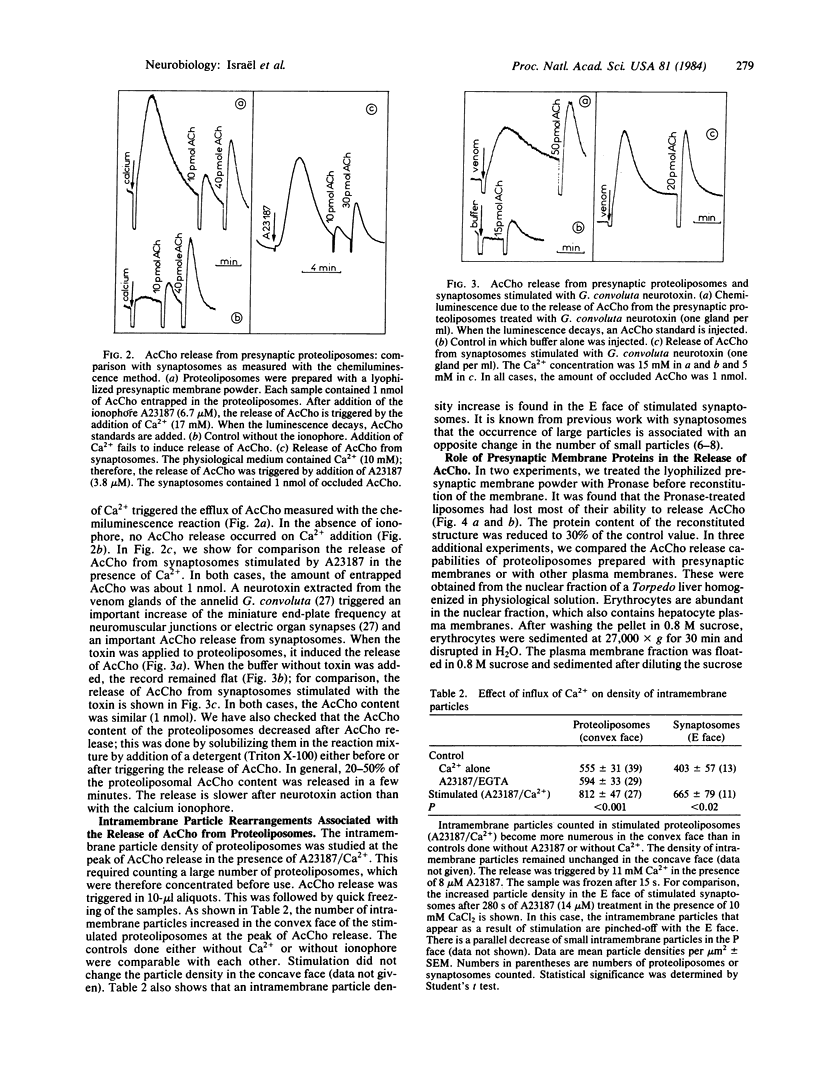
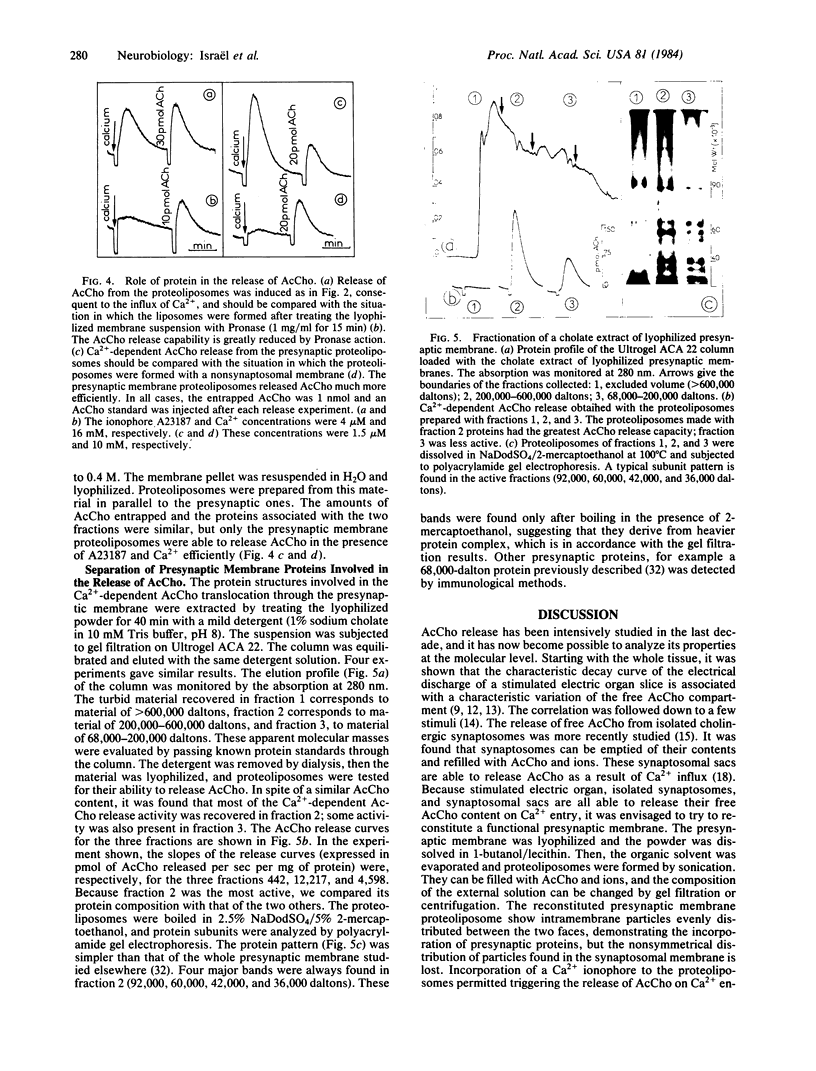
Reconstitution of a functional presynaptic membrane possessing calcium-dependent acetylcholine release properties has been achieved. The proteoliposomal membrane obtained gains its acetylcholine-releasing capabilities from presynaptic membrane proteins. At the peak of acetylcholine release, intramembrane particles became more numerous in one of the proteoliposomal membrane faces. This phenomenon resembles the intramembrane particle rearrangements found in stimulated synaptosomes. No visible structures capable of releasing acetylcholine as a result of the calcium influx were found inside the proteoliposomes. This supports the view that the release of free cytosolic acetylcholine from stimulated nerve terminals can be directly attributed to presynaptic membrane proteins. These proteins were extracted in a functional form from the synaptosomal membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunant Y., Gautron J., Israël M., Lesbats B., Manaranche R. Evolution de la décharge de l'organe électrique de la Torpille et variations simultanées de l'acétylcholine au cours de la stimulation. J Neurochem. 1974 Oct;23(4):635–643. doi: 10.1111/j.1471-4159.1974.tb04386.x. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Jones G. J., Loctin F. Acetylcholine measured at short time intervals during transmission of nerve impulses in the electric organ of Torpedo. J Physiol. 1982 Apr;325:441–460. doi: 10.1113/jphysiol.1982.sp014161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesce R., Grohovaz F., Hurlbut W. P., Ceccarelli B. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. III. A morphometric analysis of the number and diameter of intramembrane particles. J Cell Biol. 1980 May;85(2):337–345. doi: 10.1083/jcb.85.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Choline acetyltransferase binding to and release from membranes. Biochem J. 1968 Sep;109(3):389–398. doi: 10.1042/bj1090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulik-Krzywicki T., Costello M. J. The use of low temperature X-ray diffraction to evaluate freezing methods used in freeze-fracture electron microscopy. J Microsc. 1978 Jan;112(1):103–113. doi: 10.1111/j.1365-2818.1978.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Israel M., Dunant Y., Manaranche R. The present status of the vesicular hypothesis. Prog Neurobiol. 1979;13(3):237–275. doi: 10.1016/0301-0082(79)90017-0. [DOI] [PubMed] [Google Scholar]
- Israel M., Lesbats B., Manaranche R., Morel N. Acetylcholine release from proteoliposomes equipped with synaptosomal membrane constituents. Biochim Biophys Acta. 1983 Mar 9;728(3):438–448. doi: 10.1016/0005-2736(83)90516-3. [DOI] [PubMed] [Google Scholar]
- Israël M., Dunant Y. On the mechanism of acetylcholine release. Prog Brain Res. 1979;49:125–139. doi: 10.1016/S0079-6123(08)64627-0. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B. Application to mammalian tissues of the chemiluminescent method for detecting acetylcholine. J Neurochem. 1982 Jul;39(1):248–250. doi: 10.1111/j.1471-4159.1982.tb04727.x. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B. Continuous determination by a chemiluminescent method of acetylcholine release and compartmentation in Torpedo electric organ synaptosomes. J Neurochem. 1981 Dec;37(6):1475–1483. doi: 10.1111/j.1471-4159.1981.tb06317.x. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B. Détection continue de la libération d'acétylcholine de l'organe électrique de la Torpille à l'aide l'une réaction de chimiluminescence. C R Seances Acad Sci D. 1980 Oct 27;291(8):713–716. [PubMed] [Google Scholar]
- Israël M., Lesbats B., Manaranche R. ACh release from osmotically shocked synaptosomes refilled with transmitter. Nature. 1981 Dec 3;294(5840):474–475. doi: 10.1038/294474a0. [DOI] [PubMed] [Google Scholar]
- Israël M., Lesbats B., Manaranche R., Morel N., Gulik-Krzywicki T., Dedieu J. C. Rearrangement of intramembrane particles as a possible mechanism for the release of acetylcholine. J Physiol (Paris) 1982;78(4):348–356. [PubMed] [Google Scholar]
- Israël M., Manaranche R., Mastour-Frachon P., Morel N. Isolation of pure cholinergic nerve endings from the electric organ of Torpedo marmorata. Biochem J. 1976 Oct 15;160(1):113–115. doi: 10.1042/bj1600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël M., Manaranche R., Morel N., Dedieu J. C., Gulik-Krzywicki T., Lesbats B. Redistribution of intramembrane particles related to acetylcholine release by cholinergic synaptosomes. J Ultrastruct Res. 1981 May;75(2):162–178. doi: 10.1016/s0022-5320(81)80132-3. [DOI] [PubMed] [Google Scholar]
- King R. G., Marchbanks R. M. The incorporation of solubilized choline-transport activity into liposomes. Biochem J. 1982 May 15;204(2):565–576. doi: 10.1042/bj2040565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manaranche R., Thieffry M., Israel M. Effect of the venom of Glycera convoluta on the spontaneous quantal release of transmitter. J Cell Biol. 1980 May;85(2):446–458. doi: 10.1083/jcb.85.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. M., Cooper J. R. High affinity choline uptake and calcium-dependent acetylcholine release in proteoliposomes derived from rat cortical synaptosomes. J Neurosci. 1983 May;3(5):987–994. doi: 10.1523/JNEUROSCI.03-05-00987.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. Free and bound acetylcholine in frog muscle. J Physiol. 1982 Dec;333:189–199. doi: 10.1113/jphysiol.1982.sp014448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N., Israel M., Manaranche R., Mastour-Frachon P. Isolation of pure cholinergic nerve endings from Torpedo electric organ. Evaluation of their metabolic properties. J Cell Biol. 1977 Oct;75(1):43–55. doi: 10.1083/jcb.75.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N., Manaranche R., Gulik-Krzywicki T., Israel M. Ultrastructural changes and transmitter release induced by depolarization of cholinergic synaptosomes. A freeze-fracture study of a synaptosomal fraction from torpedo electric organ. J Ultrastruct Res. 1980 Mar;70(3):347–362. doi: 10.1016/s0022-5320(80)80017-7. [DOI] [PubMed] [Google Scholar]
- Morel N., Manaranche R., Israël M., Gulik-Krzywicki T. Isolation of a presynaptic plasma membrane fraction from Torpedo cholinergic synaptosomes: evidence for a specific protein. J Cell Biol. 1982 May;93(2):349–356. doi: 10.1083/jcb.93.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S. Action of brown widow spider venom and botulinum toxin on the frog neuromuscular junction examined with the freeze-fracture technique. J Physiol. 1977 Dec;273(2):443–457. doi: 10.1113/jphysiol.1977.sp012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano Y., Kamiya H. Effect of ionic environment on densities of membrane-associated particles in presynaptic membranes observed in freeze-fractured synaptosomes. Experientia. 1979 Aug 15;35(8):1076–1078. doi: 10.1007/BF01949951. [DOI] [PubMed] [Google Scholar]
- Tauc L. Non vesicular release of neurotransmitter. Physiol Rev. 1982 Jul;62(3):857–893. doi: 10.1152/physrev.1982.62.3.857. [DOI] [PubMed] [Google Scholar]
- Tokunaga A., Sandri C., Akert K. Increase of large intramembranous particles in the presynaptic active zone after administration of 4-aminopyridine. Brain Res. 1979 Oct 5;174(2):207–219. doi: 10.1016/0006-8993(79)90845-x. [DOI] [PubMed] [Google Scholar]
- Venzin M., Sandri C., Akert S. K., Wyss U. R. Membrane associated particles of the presynaptic active zone in rat spinal cord. A morphometric analysis. Brain Res. 1977 Jul 22;130(3):393–404. doi: 10.1016/0006-8993(77)90104-4. [DOI] [PubMed] [Google Scholar]
- Weiler M., Roed I. S., Whittaker V. P. The kinetics of acetylcholine turnover in a resting cholinergic nerve terminal and the magnitude of the cytoplasmic compartment. J Neurochem. 1982 May;38(5):1187–1191. doi: 10.1111/j.1471-4159.1982.tb07889.x. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]