Abstract
Influenza A virus (IAV) infection of epithelial cells activates NF-κB transcription factors via the canonical NF-κB signaling pathway, which modulates both the antiviral immune response and viral replication. Since almost nothing is known so far about a function of noncanonical NF-κB signaling after IAV infection, we tested infected cells for activation of p52 and RelB. We show that the viral NS1 protein strongly inhibits RIG-I-mediated noncanonical NF-κB activation and expression of the noncanonical target gene CCL19.
TEXT
Influenza A viruses (IAV) primarily infect lung epithelial cells of the upper and lower respiratory tract of humans. Activation of NF-κB transcription factors was identified to be one of the major regulators of the innate immune defense, as it participates in cytokine and chemokine production and regulation of apoptotic processes (24). NF-κB proteins are a family of dimeric transcription factors composed of five members, including RelA (p65), RelB, c-Rel, NF-κB1 (p50 and its precursor p105), and NF-κB2 (p52 and its precursor p100) (30), that are controlled by two distinct signaling pathways. The classical or canonical NF-κB pathway proceeds via degradation of inhibitors of κB proteins (IκBs) and leads to the release of p65/p50 heterodimers, whereas the alternative or noncanonical pathway regulates the proteolytic processing of NF-κB2/p100 to form p52. Like IκBs, p100 possesses C-terminal ankyrin repeats, binding predominantly RelB in unstimulated cells. Upon activation, noncanonical signaling proceeds via activation of IκB kinase 1 (IKK1) by the NF-κB-inducing kinase (NIK) (29, 35, 36). Recruitment of p100 to a complex of NIK and IKK1 allows phosphorylation of p100 by IKK1, subsequent ubiquitinylation and partial C-terminal degradation of p100 (35), and release of active p52/RelB dimers.
The important role of the canonical NF-κB signaling pathway in the context of IAV infection has been the subject of intense research. Findings from genome-wide gene expression array assays demonstrated a major role of NF-κB for cytokine responses induced by the highly pathogenic IAV H5N1 (28). Expression of the viral NS1 protein counteracts activation of NF-κB (31) and NF-κB promoter activity (21). However, it has been shown that IAV exploits the remaining NF-κB activity to ensure efficient replication (22). The unexpected viral dependency on the canonical NF-κB pathway paved the path for novel antiviral approaches (16, 15). A first proof of concept that NF-κB inhibitors can serve as anti-influenza agents was demonstrated using acetylsalicylic acid which efficiently acted in an antiviral manner in vitro and in vivo (37, 18). The beneficial function of canonical NF-κB activity was shown to be at least in part due to NF-κB-dependent expression of proapoptotic factors, such as tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) or FasL (33), which promoted caspase activation that in turn resulted in enhanced release of viral RNP complexes from the nucleus (34). Another recently described virus supporting action of NF-κB is the counteraction of the type I interferon (IFN)-stimulated gene (ISG) expression via induction of the suppressor of cytokine signaling-3 (SOCS-3) (25) and by suppression of ISG promoter regions (32). Furthermore, it was suggested that NF-κB differentially regulates viral RNA synthesis (12). So far, the noncanonical pathway has been linked mainly to the regulation of adaptive immunity and development of secondary lymphoid organs (reviewed in reference 4), but nothing is known about its activation and function in IAV-infected cells. Thus, in this study, we aimed to elucidate a possible function of noncanonical NF-κB signaling in IAV-infected epithelial A549 cells.
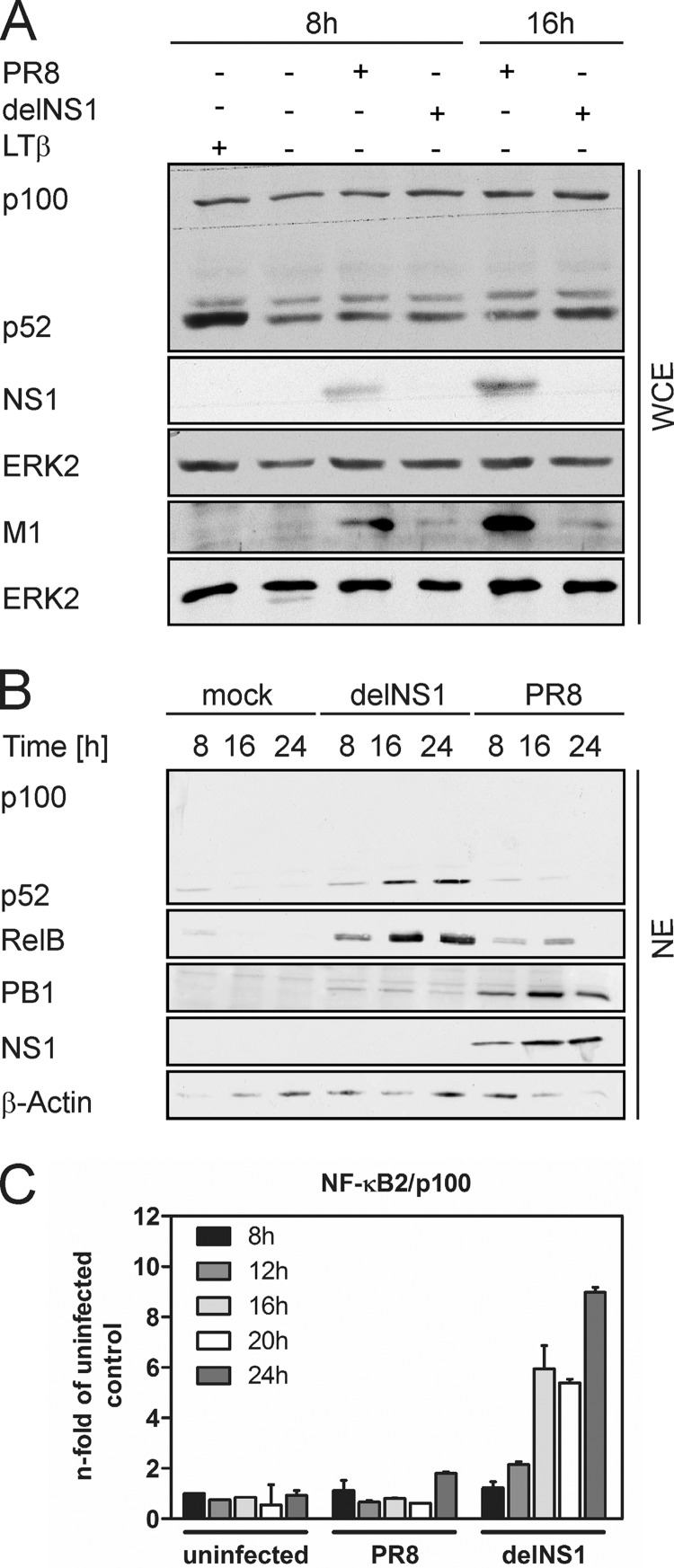
Western blot analysis was used to assess activation of the noncanonical signaling pathway via detection of the 100-kDa precursor protein p100 and the smaller 52-kDa processed form p52. A549 cells were infected with the human H1N1 strain influenza A/Puerto-Rico/8/34 (PR8) or a mutant PR8 virus lacking the viral NS1 protein (delNS1) (10) for different time periods at a multiplicity of infection (MOI) of 1. Subsequently, p52 protein accumulation in whole-cell extracts (WCE) was determined. As a control, cells were stimulated for 8 h with 100 ng/ml lymphotoxin-α1-β2 (LTβ), a strong activator of the noncanonical signaling pathway (17, 20). As shown in Fig. 1A, LTβ treatment readily resulted in increased generation of p52. In response to PR8 infection, no marked increase in p52 amounts was observed. In contrast, delNS1 induced a modest formation of p52 at later time points of infection visible at 16 h postinfection (hpi). Due to the attenuated phenotype of NS1-deficient viruses in interferon-competent systems, viral protein expression was strongly reduced in delNS1-infected cells compared to wild-type (wt)-infected cells, as indicated by reduced M1 protein accumulation. Thus, although the delNS1 mutant replicates less effectively than the wt virus, it is still capable of inducing p52 generation. We next aimed to elucidate whether IAV-induced processing of p100 would also lead to nuclear translocation of the noncanonical factors p52 and RelB. Therefore, cells were infected as described previously and nuclear extracts (NE) were prepared and subjected to Western blots. In accordance with the poor induction of p100 processing by PR8, both p52 and RelB were hardly detected in the nuclei of infected cells (Fig. 1B). In contrast, delNS1 infection induced a time-dependent increase of nuclear p52 as well as RelB accumulation already detectable at 8 hpi. Nuclear extracts contained no cytoplasmic contaminations, as p100, which resides in the cytosol, was completely absent in those samples. Together, these data indicate that, in IAV-infected cells, noncanonical NF-κB signaling is efficiently suppressed due to the action of the viral NS1 protein. This is evident in the absence of NS1, where processing of p100 and nuclear translocation of p52- and RelB-containing dimers readily occurs. Processing of NF-κB2/p100 was described as a cotranslational event that requires de novo protein synthesis of NF-κB2/p100 (20). For this reason, mRNA expression of the precursor protein was investigated at 8, 12, 16, 20, and 24 hpi by quantitative real-time PCR (qRT-PCR) (Fig. 1C). Indeed, a time-dependent increase in NF-κB2/p100 mRNA up to 9-fold at 24 hpi was provoked by delNS1. PR8 infection, however, did not lead to significant upregulation of NF-κB2/p100 expression. In summary, we showed that the processing of the precursor protein NF-κB2/p100, its de novo synthesis, and the nuclear translocation of p52 and RelB are induced late during IAV infection in the absence of NS1. These findings are in agreement with previous reports that define the noncanonical pathway to be activated with rather slow kinetics, in part due to the requirement of de novo protein synthesis of NF-κB2/p100 (2, 20).
Fig 1.
IAV-induced p100 processing and p52/RelB nuclear translocation in A549 cells. (A) Western immunoblots of WCE prepared from A549 cells infected with 1 MOI of either PR8 or delNS1 at the indicated times are shown. As a control for noncanonical pathway activation, cells were treated with 100 ng/ml LTβ for 8 h. Protein levels of p100 and p52 as well as viral proteins NS1 and M1 were detected with specific antibodies. NS1 and M1 were detected on separate nitrocellulose membranes due to their similar molecular weights. ERK2 was probed as a loading control. (B) Time course of IAV-induced nuclear translocation of p52 and RelB. A549 cells were infected with PR8 or delNS1 (MOI = 1) at the indicated times, and nuclear extracts were subjected to Western blot analysis. Blots were probed with anti-p100/p52, anti-RelB, anti-PB1, or anti-NS1, and β-actin was probed as a loading control. (C) Virus-induced expression of NF-κB2/p100 was analyzed by qRT-PCR. A549 cells were infected with PR8 or delNS1 (MOI = 1) for 8, 12, 16, 20, or 24 h. mRNA levels of NF-κB2/p100 were determined using the threshold cycle (2−ΔΔCT) method (14) and the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as an internal standard. Mean values ± the standard deviations (SD) relative to uninfected control samples from one representative experiment of two independent experiments are shown.
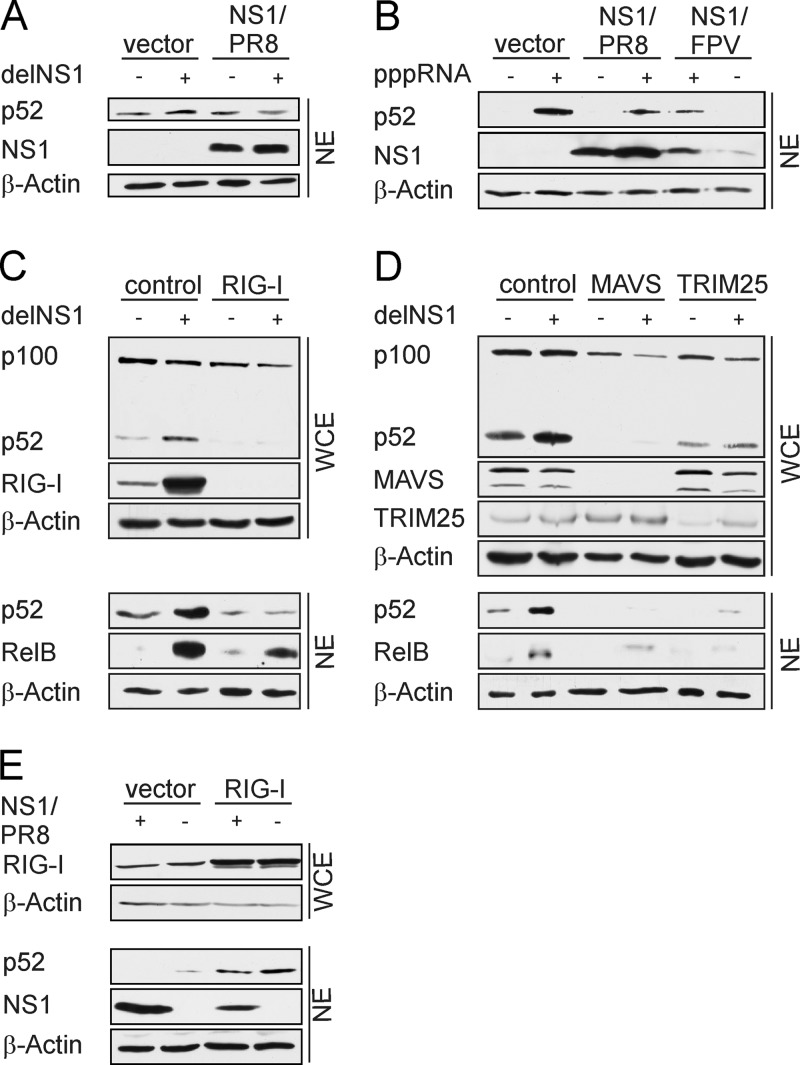
In the context of delNS1 infection, parameters other than the absence of NS1 may influence noncanonical NF-κB activation, and thus we examined whether engineered expression of NS1 is sufficient to abrogate delNS1 virus-induced nuclear accumulation of p52. In contrast to infected control cells, no increase of p52 levels was observed in NS1-expressing cells (Fig. 2A), demonstrating that NS1 actively suppresses noncanonical NF-κB activation.
Fig 2.
NS1 suppresses RIG-I/MAVS-mediated noncanonical NF-κB activation. (A) A549 cells were transfected with 0.5 μg pcDNA3-NS1/PR8 or 0.5 μg empty vector. After 24 h, cells were infected with delNS1 (MOI = 1) for 16 h. (B) A549 cells were transfected with 0.5 μg of expression plasmids encoding NS1 of either PR8 or FPV. Twenty-four hours posttransfection, cells were stimulated by transfection of in vitro-synthesized RNA bearing 5′ triphosphates for 8 h. (C and D) A549 cells were transfected with 50 pmol siRNA specific for RIG-I, MAVS, or TRIM25. Nonspecific control siRNA served as the control. Forty-eight hours (C) or 72 h (D) after transfection, cells were infected with delNS1 (MOI = 1) for another 20 h. (E) A549 cells were cotransfected with 0.5 μg pCAGGS-RIG-I expression plasmids, together with 0.5 μg pcDNA3 NS1/PR8 or 0.5 μg of pcDNA3 empty vector for 24 h. Cells were lysed and whole-cell lysates (WCE) and/or nuclear extracts (NE) were analyzed by Western blotting. Specific antibodies verified knockdown or overexpression of RIG-I, MAVS, TRIM25, and NS1. Nuclear accumulation of p52 and RelB was analyzed using anti-p100/p52 or anti-RelB antibody. β-Actin was probed as a loading control. The results shown here are representative of at least two independent experiments.
To gain more insight into how the noncanonical NF-κB pathway is activated and how NS1 inhibits its activation, we aimed to identify the viral stimulus and the cellular sensor that mediates activation. The cytoplasmic RNA helicase retinoic acid-inducible gene-I (RIG-I) was shown to be the major cellular sensor detecting viral RNA intermediates produced during IAV replication. Further, NS1 efficiently inhibits RIG-I-mediated interferon induction and canonical NF-κB activation (19, 31). Recently, NS1 was shown to interfere with RIG-I activation by the binding of the ubiquitin ligase TRIM25, which is crucial for full RIG-I activation (9). Previous studies showed that NIK, which acts upstream of IKK1 in the noncanonical NF-κB pathway, associates with RIG-I and its downstream adaptor MAVS. MAVS was also identified to directly interact with IKK1 after respiratory syncytial virus (RSV) infection (13). Hence, we hypothesized that noncanonical pathway activation in response to IAV infection is induced by accumulating viral RNA species via activation of RIG-I and its downstream signaling. Transfection of epithelial cells with viral RNA isolated from PR8-infected A549 cells (5 MOI, 5 h) led to the activation of the noncanonical NF-κB pathway, as measured by the detection of p100 processing and p52 formation in the Western blot analysis (data not shown). In addition, in vitro-synthesized RNA bearing 5′-triphosphate groups (pppRNA) provoked strong accumulation of p52 in nuclear extracts of transfected cells (Fig. 2B). pppRNA-induced p52 activation could be suppressed by overexpression of NS1 proteins from different virus strains, such as PR8 or the avian isolate A/FPV/Rostock/34 (H7N1) (NS1/FPV). This clearly demonstrates that NS1 proteins from different IAV strains display similar activities concerning the blocking of noncanonical NF-κB activation. To further test the hypothesis of RIG-I being the cellular sensor mediating activation of noncanonical signaling, we interfered with RIG-I signaling by small interfering RNA (siRNA) approaches. Cells transfected with scrambled control siRNA or RIG-I-, MAVS-, or TRIM25-specific siRNA were infected with delNS1 for 20 h, and p52 processing from p100 was analyzed by Western blotting of WCE. Accumulation of p52 and RelB in the nucleus was analyzed in NE of infected cells. As expected in control siRNA-transfected cells, RIG-I expression was strongly upregulated 20 h after delNS1 infection, but RIG-I was absent in RIG-I siRNA-transfected cells (Fig. 2C). Importantly, both the basal and the delNS1-induced p52 accumulation were decreased in RIG-I-silenced cells. Consistent with that finding, delNS1 infection provoked nuclear translocation of p52 and RelB in control siRNA-transfected cells, whereas levels of p52 were strongly reduced in RIG-I-silenced cells. Although less pronounced, the nuclear abundance of RelB was also decreased in cells transfected with RIG-I siRNA. Furthermore, similar to RIG-I depletion, downregulation of MAVS or TRIM25 by siRNA also reduced basal p52 amounts and nuclear translocation of p52 and RelB was reduced compared to control cells (Fig. 2D). In order to further verify that NS1 inhibits RIG-dependent noncanonical NF-κB activation, NS1 was expressed together with RIG-I as a nonviral stimulus. In accordance with our observations from the siRNA approach, expression of RIG-I provoked enhanced p52 protein accumulation in vector-transfected cells, whereas NS1 inhibits nuclear accumulation of p52 in cotransfected cells (Fig. 2E). Taken together, these results verified that RIG-I/MAVS signaling mediates IAV-induced noncanonical NF-κB signaling and that NS1 counteracts this activation. Furthermore, the observed requirement of TRIM25 for full induction of the noncanonical pathway might also be a direct link to the inhibiting function of NS1 on its activation.
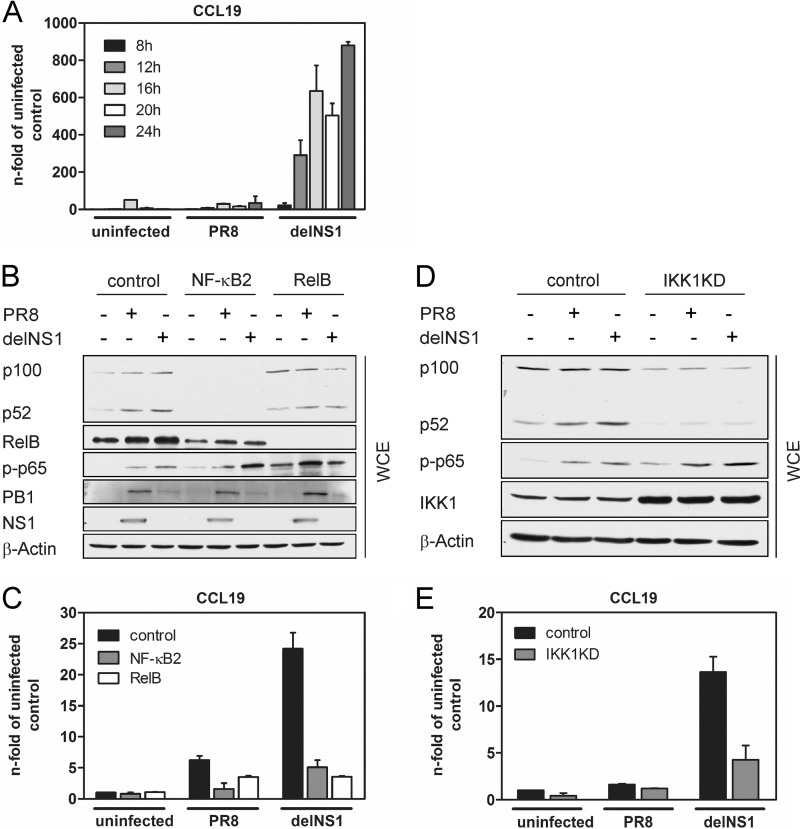
Previous studies identified a subset of chemokine genes, including CCL19, CCL21, CXCL13, and CXCL12, to be dependent on IKK1 and contain an NF-κB binding site that is preferentially recognized by p52/RelB dimers (1). These chemokines are normally expressed in secondary lymphoid organs, where they direct the steady-state recruitment and placement of lymphocytes and dendritic cells (3). Via the use of mice deficient in p52 (NF-κB/p52−/− mice), it was demonstrated that the noncanonical NF-κB pathway is required for the T-cell-mediated immune response against lymphocytic choriomeningitis virus (LCMV) and vesicular stomatitis virus (VSV) (6). These mice failed to initiate an adequate immune response and clearance of LCMV infection due to a disturbed splenic microarchitecture which is associated with low expression of the T-cell chemoattractant CCL21 (6, 8). Apart from their function in secondary lymphoid tissues, CCL19, CCL21, and CXCL13 were also shown to be inducibly expressed after IAV infection in nonlymphoid tissues such as the lung and may recruit responsive immune cells to the site of infection (7, 27). Here, we focused on CCL19 gene expression in A549 epithelial cells to investigate the impact of noncanonical NF-κB activation on virus-induced gene expression. A549 cells were infected with PR8 or delNS1 virus for 8, 12, 16, 20, or 24 h. Subsequently, total RNA was isolated and reverse transcribed. When performing qRT-PCR with CCL19-specific primers, a strong upregulation of CCL19 mRNA starting at 12 hpi with delNS1 virus was observed (Fig. 3A). In contrast, PR8 infection only weakly induced the expression of CCL19. To evaluate the contribution of noncanonical NF-κB factors to CCL19 mRNA expression, we used two different approaches. First, we transfected A549 cells with scrambled control siRNA and NF-κB2- or RelB-specific siRNA for 48 h and infected cells with PR8 or delNS1 for 20 h. Efficient knockdown of NF-κB2 and RelB in siRNA-transfected cells was verified by Western blotting (Fig. 3B). As demonstrated in Fig. 1A, delNS1 provokes a stronger induction of p100 processing than the wt virus. We also observed that NF-κB2 knockdown affects virus-induced expression of RelB and that formation of p52 was reduced in RelB-depleted cells, illustrating that these transcription factors influence their own expression. Interestingly, phosphorylation of the canonical NF-κB subunit p65 at serine 536 was not inhibited but rather slightly enhanced in NF-κB2 siRNA-transfected cells. This shows that canonical NF-κB signaling is still intact in cells in which noncanonical signaling is inhibited via NF-κB2 knockdown. Expression of viral proteins was equal in control and NF-κB2 or RelB siRNA-transfected cells (Fig. 3B), and viral replication was not altered by knockdown of either NF-κB2 or RelB, as determined by a standard plaque assay (data not shown). In the same experiment, we analyzed virus-induced expression of CCL19 by qRT-PCR. Levels of CCL19 mRNA were increased in IAV-infected cells compared to uninfected cells at 20 hpi, and delNS1 virus provoked a significantly higher induction of CCL19 expression than PR8, as seen in Fig. 3C. Most important, depletion of either NF-κB2 or RelB resulted in a significant reduction of CCL19 mRNA transcription. Thus, activation of the noncanonical NF-κB pathway resulting in nuclear abundance of transcriptionally active p52- and RelB-containing dimers is crucial for CCL19 gene expression in response to IAV infection.
Fig 3.
Activation of the noncanonical NF-κB pathway regulates expression of CCL19. (A) Induction of CCL19 mRNA expression by IAV infection. A549 cells were infected with PR8 or delNS1 (MOI = 1) for 8, 12, 16, 20, or 24 h. mRNA levels of CCL19 were determined using the 2−ΔΔCT method (14), normalized to the GAPDH internal control, and plotted as means ± the SD relative to uninfected control samples. Results from one representative of two independent experiments are shown. (B) A549 cells were transfected with 50 pmol of NF-κB2- or RelB-specific siRNA or 50 pmol of nonsilencing control siRNA. After incubation for 48 h, cells were infected with PR8 or delNS1 (MOI = 1) or left uninfected for 20 h. Whole-cell lysates were prepared, and efficient downregulation of NF-κB2 and RelB protein was verified by Western blotting. Phosphorylated p65 (S536), PB1, and NS1 were detected using specific antibodies. (C) A549 cells were treated as described for panel B, and total RNA was isolated to perform qRT-PCR. The influence of NF-κB2 and RelB knockdown on CCL19 expression was analyzed using CCL19-specific primers. mRNA levels of CCL19 were determined using the 2−ΔΔCT method (14), normalized to the GAPDH internal control, and plotted as means ± the SD relative to uninfected control samples. Results from one representative experiment of three independent experiments are shown. (D) IAV-induced p100 processing requires IKK1 activity. A549 cells that overexpress a dominant negative form of IKK1 (IKK1KD) were generated by retroviral transduction. Empty vector-transduced A549 cells served as a control. Control and IKK1KD cells were infected with PR8 or delNS1 (MOI = 1) for 20 h, and WCE were prepared. Overexpression of IKK1 and protein amounts of p100/p52 were detected by Western blotting using specific antibodies. (E) Control and IKK1KD cells were infected, and qRT-PCR was performed as described for panel C. A representative of three independent experiments is shown.
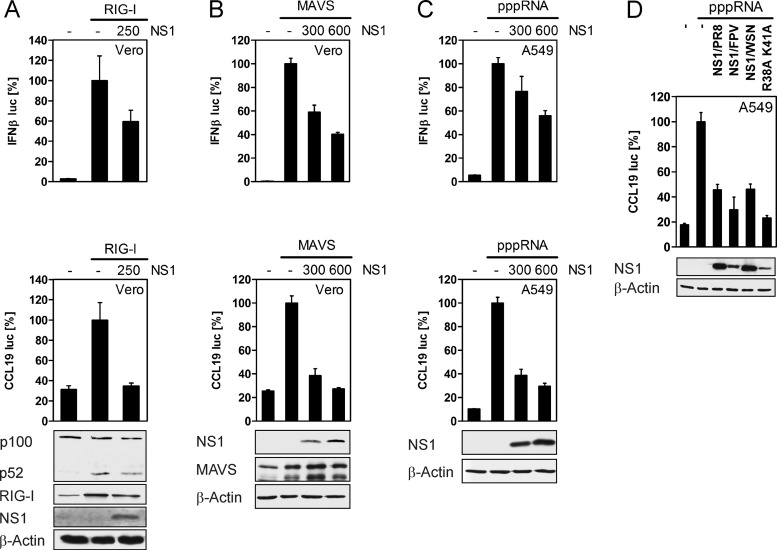
To verify our findings, in an independent approach, we generated A549 cells that overexpress a dominant negative form of IKK1 (IKK1KD) via retroviral transduction. Consequently, activation of the noncanonical NF-κB pathway is disrupted in these cells due to the loss of the ATP binding ability of the mutant IKK1KD. The A549 cells constitutively expressing IKK1KD were infected with PR8 and delNS1 for 20 h, NF-κB2 proteins were analyzed in WCE by Western blotting (Fig. 3D), and mRNA expression of CCL19 was evaluated by qRT-PCR (Fig. 3E). As seen in Fig. 3D, overexpression of IKK1KD was detectable with an IKK1-specific antibody. Interestingly, basal p100 and p52 protein amounts were reduced in IKK1KD cells compared to those in control cells, demonstrating the importance of noncanonical signaling for NF-κB2/p100 expression. As IKK1 is also part of the kinase complex mediating canonical NF-κB activation, we also investigated phosphorylation of the canonical NF-κB factor p65 in IKK1KD cells. In accordance with the data obtained from NF-κB2 knockdown cells (Fig. 3B), phosphorylation of p65 at serine 536 was also not impaired in IKK1KD cells but rather slightly enhanced. Determination of viral titers by plaque assay revealed that viral replication of PR8 and delNS1 was similar in control and IKK1KD cells (data not shown). As determined by qRT-PCR, delNS1-induced CCL19 expression was strongly reduced in IKK1KD cells, whereas delNS1 provoked an upregulation of CCL19 mRNA in control cells. These findings support our previous data using NF-κB2- and RelB-specific siRNAs and clearly demonstrate that activation of noncanonical NF-κB signaling by delNS1 IAV, resulting in nuclear translocation of p52, is crucial for induction of CCL19 chemokine expression. To further confirm our observation that NS1 blocks RIG-I/MAVS-mediated activation of noncanonical signaling and expression of CCL19, we conducted expression-based reporter analysis in epithelial Vero and A549 cells. Expression of single components of the RIG-I pathway was previously shown to activate the IFN-β promoter which is efficiently suppressed by the coexpressed NS1 protein (11, 23). Thus, cells were transfected with an IFN-β or CCL19 reporter plasmid (26), together with expression vectors encoding RIG-I or NS1, and luciferase activities were assessed. In addition, Western blot analysis was performed to confirm noncanonical NF-κB activation in RIG-I-expressing cells (Fig. 4A, lower panel). Both reporter genes were activated in cells that were transfected with RIG-I compared to empty control vector-transfected cells (Fig. 4A, upper panel). In contrast, NS1 inhibited activation of the IFN-β and CCL19 promoters by coexpressed RIG-I. Induction of p100 processing was detected in RIG-I-expressing cells; however, activation of the noncanonical NF-κB pathway was reduced upon expression of NS1. Moreover, we examined the stimulation of IFN-β and CCL19 reporter gene activities by transfection of a MAVS expression plasmid or by transfection of pppRNA in the absence or presence of the viral NS1 (Fig. 4B and C). While both stimuli raised the level of reporter activation, NS1 impaired IFN-β and CCL19 promoter activation in a dose-dependent manner. Taken together, these experiments support the data obtained by RNA interference (RNAi) and suggest that RIG-I and MAVS mediate CCL19 induction at least in part via activation of the noncanonical NF-κB pathway. Next, we analyzed the interference of different NS1 proteins, including NS1 of PR8, FPV, and influenza A/WSN/33 (H1N1), in pppRNA-induced CCL19 promoter activation. In order to gain some insight into how NS1 blocks the noncanonical pathway and CCL19 expression, cells were also transfected with NS1/WSN R38A K41A, an NS1 mutant unable to bind RNA which exhibits defects in the counteraction of the IFN response. Expression of both NS1 proteins from H1N1 strains PR8 and WSN displayed a similar inhibitory effect on CCL19 promoter activation, while NS1/FPV showed a slightly enhanced inhibition of luciferase expression driven by the CCL19 promoter (Fig. 4D). While NS1/WSN R38A K41A was previously shown to be less effective in the inhibition of IFN-β reporter gene activation (5), the mutation of the RNA binding domain does not seem to have an impact on the regulation of the CCL19 promoter.
Fig 4.
The NS1 proteins inhibit CCL19 promoter activation by the RIG-I pathway. (A and B) Vero cells were cotransfected with 50 ng IFN-β or CCL19 reporter plasmid and 0.5 μg of either pCAGGS-RIG-I or pcDNA3-MAVS, respectively, together with the indicated amounts of pcDNA3-NS1/PR8 given in nanograms. The amounts of transfected DNA were kept equal by using empty vector. After 24 h, luciferase activities were measured. The reporter values for transfected cells expressing only RIG-I or MAVS were arbitrarily set to 100% and compared with reporter activation in cells that had received the indicated amounts of the NS1 expression plasmid. p100 processing induced by RIG-I expression was demonstrated by Western blot analysis of the same cell lysates, and expression of RIG-I, MAVS, or NS1 was detected by specific antibodies. β-Actin was probed as a loading control. (C) A549 cells were transfected with IFN-β or CCL19 reporter plasmid and indicated amounts of pcDNA3-NS1/PR8 or empty vector given in nanograms. Twenty-four hours later, cells were transfected with in vitro-synthesized RNA bearing triphosphates at their 5′ end (pppRNA) (0.5 μg per 12 wells) for 8 h. Reporter activation was analyzed by luciferase assay. The reporter values for cells transfected with pppRNA were set to 100% and compared with reporter activation in NS1-expressing cells. Expression of NS1 was detected by Western blotting. (D) A549 cells were cotransfected with 50 ng CCL19 reporter plasmid and 600 ng of expression vectors encoding NS1/PR8, NS1/FPV, NS1/WSN, NS1/WSN/R38A K41A, or empty vector for 24 h. Cells were stimulated by transfection with 0.5 μg pppRNA and lysed 8 h after transfection. Luciferase activities were assessed and analyzed as described for panel C. Data shown are representative of at least two experiments, with transfections performed in triplicate.
In conclusion, our data provide evidence that the noncanonical NF-κB signaling pathway is activated by influenza viral RNA via a RIG-I/IKK1-dependent mechanism in respiratory epithelial cells, leading to p100 processing and expression of p52/RelB-regulated genes. Unlike the canonical pathway, which is only partially suppressed by NS1, the noncanonical pathway does not seem to influence cell intrinsic processes that would affect viral replication. Therefore, it is reasonable that in IAV-infected cells, NS1 strongly antagonizes RNA-induced activation of the noncanonical pathway and thereby prevents expression of genes such as CCL19. Thus, our findings further suggest a role for virus-induced noncanonical NF-κB signaling in chemokine release from epithelial cells. Expression of CCL19 from infected epithelial cells might affect recruitment of immune cells, thereby contributing to the onset of an effective immune response to infection. Since this may hamper efficient influenza virus spread, the virus may have evolved efficient countermeasures to prevent expression of CCL19 and other p52/RelB-dependent genes.
ACKNOWLEDGMENTS
We thank Thomas Muster (AVIR Green Hills Biotechnology AG, Vienna, Austria) and Adolfo García-Sastre (Mount Sinai School of Medicine, NY) for kindly providing the influenza virus A/Puerto-Rico/8/34 (H1N1) and the NS1-deficient mutant A/Puerto-Rico/8/34/delNS1.
This work was supported by grant Lu477/16-1 from the Deutsche Forschungsgemeinschaft (DFG), by the BMBF zoonosis network “FluResearchNet,” and by IZKF grant Lud2/032/06 from the University of Muenster Medical School.
Footnotes
Published ahead of print 11 July 2012
REFERENCES
- 1. Bonizzi G, et al. 2004. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. EMBO J. 23:4202–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coope HJ, et al. 2002. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 21:5375–5385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cyster JG. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098–2102 [DOI] [PubMed] [Google Scholar]
- 4. Dejardin E. 2006. The alternative NF-κB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem. Pharmacol. 72:1161–1179 [DOI] [PubMed] [Google Scholar]
- 5. Donelan NR, Basler CF, García-Sastre A. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 77:13257–13266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Droebner K, et al. 2010. The alternative NF-kappaB signalling pathway is a prerequisite for an appropriate immune response against lymphocytic choriomeningitis virus infection. Viral Immunol. 23:295–308 [DOI] [PubMed] [Google Scholar]
- 7. Fleming-Canepa X, et al. 2011. Expression of duck CCL19 and CCL21 and CCR7 receptor in lymphoid and influenza-infected tissues. Mol. Immunol. 48:1950–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franzoso G, et al. 1998. Mice deficient in nuclear factor (NF)-κB/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J. Exp. Med. 187:147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gack MU, et al. 2009. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5:439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. García-Sastre A, et al. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330 [DOI] [PubMed] [Google Scholar]
- 11. Guo Z, et al. 2007. NS1 protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. Am. J. Respir. Cell Mol. Biol. 36:263–269 [DOI] [PubMed] [Google Scholar]
- 12. Kumar N, Xin Z-T, Liang Y, Ly H, Liang Y. 2008. NF-κB signaling differentially regulates influenza virus RNA synthesis. J. Virol. 82:9880–9889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu P, Li K, Garofalo RP, Brasier AR. 2008. Respiratory syncytial virus induces RelA release from cytoplasmic 100-kDa NF-kappa B2 complexes via a novel retinoic acid-inducible gene-I{middle dot}NF-kappa B-inducing kinase signaling pathway. J. Biol. Chem. 283:23169–23178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 15. Ludwig S. 2011. Disruption of virus-host cell interactions and cell signaling pathways as an anti-viral approach against influenza virus infections. Biol. Chem. 392:837–847 [DOI] [PubMed] [Google Scholar]
- 16. Ludwig S, Planz O. 2008. Influenza viruses and the NF-κB signaling pathway—towards a novel concept of antiviral therapy. Biol. Chem. 389:1307–1312 [DOI] [PubMed] [Google Scholar]
- 17. Matsushima A, et al. 2001. Essential role of nuclear factor (NF)-κB–inducing kinase and inhibitor of κB (IκB) kinase α in Nf-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J. Exp. Med. 193:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazur I, et al. 2007. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell. Microbiol. 9:1683–1694 [DOI] [PubMed] [Google Scholar]
- 19. Mibayashi M, et al. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81:514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mordmüller B, Krappmann D, Esen M, Wegener E, Scheidereit C. 2003. Lymphotoxin and lipopolysaccharide induce NF-κB-p52 generation by a co-translational mechanism. EMBO Rep. 4:82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. M, Zohari S, Berg M. 2011. Non-structural protein 1 of avian influenza A viruses differentially inhibit NF-κB promoter activation. Virol. J. 8:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nimmerjahn F, et al. 2004. Active NF-kappaB signalling is a prerequisite for influenza virus infection. J. Gen. Virol. 85:2347–2356 [DOI] [PubMed] [Google Scholar]
- 23. Opitz B, et al. 2007. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell. Microbiol. 9:930–938 [DOI] [PubMed] [Google Scholar]
- 24. Pahl HL. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853–6866 [DOI] [PubMed] [Google Scholar]
- 25. Pauli E-K, et al. 2008. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 4:e1000196 doi:10.1371/journal.ppat.1000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pietilä TE, et al. 2007. Multiple NF-κB and IFN regulatory factor family transcription factors regulate CCL19 gene expression in human monocyte-derived dendritic cells. J. Immunol. 178:253–261 [DOI] [PubMed] [Google Scholar]
- 27. Rangel-Moreno J, Carragher D, Randall TD. 2007. Role of lymphotoxin and homeostatic chemokines in the development and function of local lymphoid tissues in the respiratory tract. Inmunologia 26:13–28 [PMC free article] [PubMed] [Google Scholar]
- 28. Schmolke M, Viemann D, Roth J, Ludwig S. 2009. Essential impact of NF-kappaB signaling on the H5N1 influenza A virus-induced transcriptome. J. Immunol. 183:5180–5189 [DOI] [PubMed] [Google Scholar]
- 29. Senftleben U, et al. 2001. Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 293:1495–1499 [DOI] [PubMed] [Google Scholar]
- 30. Siebenlist U, Franzoso G, Brown K. 1994. Structure, regulation and function of NF-kappa B. Annu. Rev. Cell Biol. 10:405–455 [DOI] [PubMed] [Google Scholar]
- 31. Wang X, et al. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566–11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei L, et al. 2006. NFkappaB negatively regulates interferon-induced gene expression and anti-influenza activity. J. Biol. Chem. 281:11678–11684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wurzer WJ, et al. 2004. NF-kappaB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J. Biol. Chem. 279:30931–30937 [DOI] [PubMed] [Google Scholar]
- 34. Wurzer WJ, et al. 2003. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 22:2717–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiao G, Fong A, Sun S-C. 2004. Induction of p100 processing by NF-κB-inducing kinase involves docking IκB kinase α (IKKα) to p100 and IKKα-mediated phosphorylation. J. Biol. Chem. 279:30099–30105 [DOI] [PubMed] [Google Scholar]
- 36. Xiao G, Harhaj EW, Sun S-C. 2001. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell 7:401–409 [DOI] [PubMed] [Google Scholar]
- 37. Yin M-J, Yamamoto Y, Gaynor RB. 1998. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 396:77–80 [DOI] [PubMed] [Google Scholar]