Abstract
A comprehensive vaccine for human immunodeficiency virus type 1 (HIV-1) would block HIV-1 acquisition as well as durably control viral replication in breakthrough infections. Recent studies have demonstrated that Env is required for a vaccine to protect against acquisition of simian immunodeficiency virus (SIV) in vaccinated rhesus monkeys, but the antigen requirements for virologic control remain unclear. Here, we investigate whether CD8+ T lymphocytes from vaccinated rhesus monkeys mediate viral inhibition in vitro and whether these responses predict virologic control following SIV challenge. We observed that CD8+ lymphocytes from 23 vaccinated rhesus monkeys inhibited replication of SIV in vitro. Moreover, the magnitude of inhibition prior to challenge was inversely correlated with set point SIV plasma viral loads after challenge. In addition, CD8 cell-mediated viral inhibition in vaccinated rhesus monkeys correlated significantly with Gag-specific, but not Pol- or Env-specific, CD4+ and CD8+ T lymphocyte responses. These findings demonstrate that in vitro viral inhibition following vaccination largely reflects Gag-specific cellular immune responses and correlates with in vivo virologic control following infection. These data suggest the importance of including Gag in an HIV-1 vaccine in which virologic control is desired.
INTRODUCTION
Avaccine for human immunodeficiency virus type 1 (HIV-1) would ideally block HIV-1 acquisition as well as control viral replication. Recent studies showed that vaccine regimens expressing simian immunodeficiency virus (SIV) Gag, Pol, and Env reduced SIV infection risk in rhesus monkeys and that the inclusion of Env was required for protection against SIV acquisition (1, 16, 17). These vaccine trials in monkeys also demonstrated that optimal vaccines reduced mean set point SIV viral loads following challenge (1, 17). It remains unclear, however, which antigens or immune responses are most important for virologic control.
A wealth of literature has shown that CD8+ T lymphocytes can mediate control of viremia in HIV-1-infected humans (2, 7, 10, 11, 22, 25) and SIV-infected rhesus monkeys (8, 9, 23) and that T lymphocytes specific for HIV-1 Gag epitopes are associated with virologic control in vivo (4, 5, 13, 15, 26, 28). Vaccine trials in nonhuman primates have also indicated an association between Gag-specific cellular immune responses and virologic control in vaccinated rhesus monkeys following SIV challenge (1, 12, 14, 18, 27). In vitro CD8+ T cell-mediated inhibition of HIV-1 has been shown to correlate with in vivo virologic control in HIV-1-infected subjects (6, 13, 22, 24). However, the in vitro viral inhibition assay has not been extensively studied in vaccinated or SIV-infected rhesus monkeys, and no previous study has shown a correlation between in vitro SIVMAC251 inhibition and in vivo virologic control.
In the present study, we demonstrate that CD8+ lymphocytes from vaccinated rhesus monkeys inhibit SIVMAC251 in vitro in a viral inhibition assay that was originally developed for humans (6, 24) and adapted for use in rhesus monkeys (12, 19). We also show that CD8+ cell-mediated viral inhibition in vitro correlates with Gag-specific, but not Pol- or Env-specific, CD4+ and CD8+ T lymphocyte responses and that in vitro inhibition prior to challenge inversely correlates with in vivo virologic control following SIV challenge.
MATERIALS AND METHODS
Animals, immunizations, and challenges.
Cryopreserved peripheral blood mononuclear cells (PBMC) from rhesus monkeys of a previously published SIV vaccine study were utilized (1). All monkeys were immunized by the intramuscular route with the following vaccine regimens expressing SIVSME543 Gag-Pol and Env immunogens (n = 7 per group for this analysis): (i) DNA prime, modified vaccinia Ankara (MVA) boost; (ii) MVA prime, MVA boost; (iii) adenovirus serotype 26 (Ad26) prime, MVA boost; (iv) MVA prime, Ad26 boost; (v) sham controls. Monkeys were primed at week 0, boosted at week 24, and challenged at week 52. All monkeys were challenged intrarectally with the heterologous virus, SIVMAC251. All animal studies were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
SIV infection of PBMC in vitro.
PBMC obtained at week 20 prechallenge from 7 vaccinated animals were used to determine the minimum multiplicity of infection (MOI) of SIVMAC251 required to establish consistent productive infection in vitro. Cryopreserved PBMC were thawed and rested overnight in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% heat-inactivated fetal calf serum (FCS), 1% l-glutamine, 1% HEPES, and 1% penicillin-streptomycin (R10). On day −1 of the assay, CD8+ lymphocytes were depleted from PBMC by using a magnetically activated cell sorting nonhuman primate CD8 MicroBead kit (Miltenyi Biotec), according to the manufacturer's instructions, and the CD8-depleted fraction was incubated with concanavalin A (ConA; 5 μg/ml) in R10 containing 50 U of interleukin-2/ml (R10-50) at 37°C for 18 to 24 h. On day 0, aliquots of 106 CD8-depleted PBMC were resuspended in 150 μl of R10-50 and incubated with SIVMAC251 at MOIs of 0.01, 0.1, 1, 5, 10, and 100 in 1.5-ml Eppendorf tubes at 37°C for 2 h. After incubation with virus, CD8-depleted PBMC were washed twice and resuspended at 106 cells/ml of R10-50. Then, 0.5 × 106 CD8-depleted PBMC were cultured in duplicate in 1 ml of R10-50 in 48-well plates and incubated at 37°C for 7 days. Half of the supernatant was replaced with R10-50 on days 4 and 7. Supernatant SIV p27 content was measured qualitatively on day 7 by enzyme-linked immunosorbent assay (ELISA; Zeptometrix). Wells with p27 levels above 20,000 pg/ml (the upper limit of detection at a 1:10 dilution) were considered positive for productive infection. Based on this titration, an MOI of 10 and 7 days of culture were chosen to establish consistent infections of CD8-depleted PBMC from vaccinated rhesus monkeys prechallenge.
Viral inhibition assay.
Cryopreserved PBMC from weeks 28 to 34 prechallenge (reflecting peak vaccine immunity) were available from 23 of 29 vaccinated monkeys and 3 of 8 control monkeys that became SIV-infected following challenge. Cryopreserved PBMC from week 20 and freshly obtained PBMC from weeks 32 to 44 postchallenge (reflecting the viral set point/chronic infection) were available from 28 of 29 vaccinated, SIV-infected monkeys and from 7 of 7 surviving control, SIV-infected monkeys.
The in vitro HIV-1 inhibition assay has been described by Fauce et al. (6) and was modified for use with rhesus monkey PBMC. On day −1 of the assay, CD8+ lymphocytes were separated from thawed/rested or fresh PBMC, and the CD8-depleted fraction was incubated with ConA in R10-50 at 37°C for 18 to 24 h. The remaining fraction of CD8+ lymphocytes was incubated overnight in R10 alone. On day 0, 106 CD8-depleted PBMC were resuspended in 150 μl of R10-50 and incubated with SIVMAC251 at MOIs of 0, 5, 10, and 100 in 1.5-ml Eppendorf tubes at 37°C for 2 h. Experimental duplicates were typically performed. After incubation with virus, CD8-depleted PBMC were washed twice and resuspended at 106 cells/ml of R10-50. Then, 0.5 × 106 CD8-depleted PBMC were cultured with or without 0.5 × 106 autologous CD8+ lymphocytes in 1 ml of R10-50 in 48-well plates and incubated at 37°C for 14 days. Half of the well supernatant was replaced with R10-50 on days 4, 7, 11, and 14. Supernatant SIV p27 content was measured quantitatively on days 7 and 14 by ELISA. An additional CD8+ lymphocyte titration experiment was performed on three SIV-infected animals with a low in vivo SIV viral load (<3.0 log plasma SIV RNA; “SIV controllers”) based on CD8+/CD8− ratios of 0:1, 0.5:1, 1:1, and 2:1.
Statistical analyses.
CD8+ cell-mediated inhibition was expressed as the log reduction in p27 content of cocultures of CD8-depleted/CD8+ PBMC, compared with CD8-depleted PBMC alone. Negative values were treated as zero inhibition. Mean viral inhibition in each vaccine group was compared to sham controls by using Wilcoxon signed rank tests. All correlations with immunologic variables were performed using Spearman rank correlation tests. Gag-, Pol-, and Env-specific enzyme-linked immunosorbent spot assay (ELISPOT) and intracellular cytokine staining (ICS) data have been described previously (1). Two-tailed P values were calculated for all tests. P values of <0.05 were considered significant, except in the case of multiple comparisons when P values were adjusted using Bonferroni corrections.
RESULTS
Development of the in vitro SIV inhibition assay.
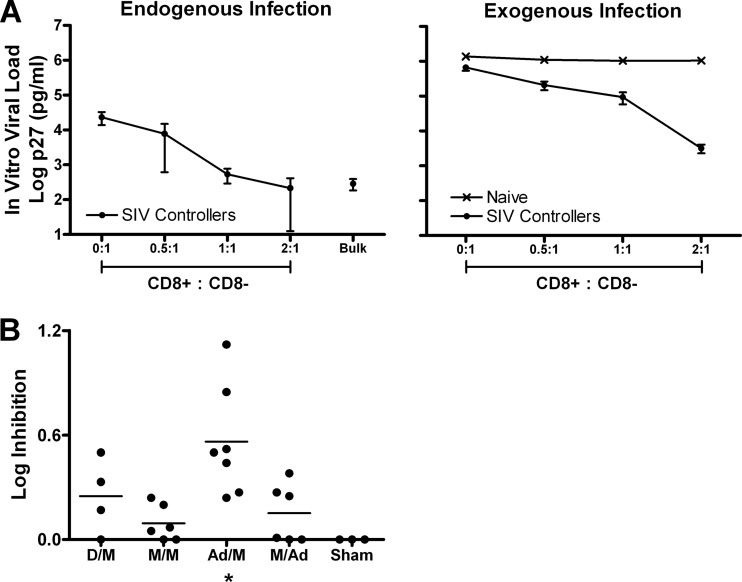
The in vitro viral inhibition assay was originally developed for use with HIV-1-infected human PBMC (6, 24). Here we adapted this assay to assess in vitro CD8+ cell-mediated inhibition of SIVMAC251 using PBMC from rhesus monkeys. We first added CD8+ lymphocytes to cultures of CD8-depleted PBMC from three SIV-infected rhesus monkeys that spontaneously controlled viral replication (SIV controllers) and one naïve uninfected animal. We measured endogenous SIV replication in cell cultures with increasing numbers of CD8+ lymphocytes, and we observed that p27 levels in culture supernatant decreased with increasing numbers of autologous CD8+ lymphocytes (Fig. 1A, left). We also assessed the capacity of CD8+ lymphocytes to suppress exogenous SIVMAC251 infection of CD8-depleted PBMC at a multiplicity of infection of 100. As a negative control, we observed that CD8+ lymphocytes from a naïve uninfected monkey did not suppress viral replication in PBMC, as expected (Fig. 1A, right). In contrast, CD8+ lymphocytes from SIV controllers effectively suppressed exogenous viral replication in CD8-depleted PBMC in a dose-dependent manner (Fig. 1A, right).
Fig 1.
In vitro SIV inhibition assay results. (A) Mean SIVMAC251 log p27 levels (in pg/ml) at day 7 of culture in PBMC from SIV-infected rhesus monkeys with excellent in vivo viral control (SIV controllers; n = 3) and one naïve monkey. In vitro viral load is plotted for endogenous infection (left) and exogenous infection at a multiplicity of infection of 100 (right) in cocultures of CD8+/CD8-depleted PBMC using increasing numbers of CD8+ lymphocytes. Bars represent standard errors of the means. Bulk, unfractionated PBMC. (B) CD8+ cell-mediated viral inhibition in vaccinated rhesus monkeys is depicted for each vaccine group. *, P = 0.016, Wilcoxon signed rank test (Ad/M versus sham). The horizontal lines represent mean log inhibition levels. Ad, adenovirus serotype 26; D, DNA; M, MVA.
Viral inhibition in vaccinated animals prior to SIV challenge.
We next evaluated whether CD8+ lymphocytes from vaccinated but uninfected rhesus monkeys in our recently published vaccine study (1) could also suppress SIVMAC251 replication in vitro. In that study we evaluated the immunogenicity and protective efficacy of DNA/MVA, MVA/MVA, Ad26/MVA, and MVA/Ad26 vectors expressing SIVSME543 Gag-Pol and Env immunogens against SIVMAC251 challenge. Of the 32 vaccinated animals in the study, 29 became SIV infected following challenge; of these 29 animals, 23 had cryopreserved PBMC available for use in the viral inhibition assay. We also assessed in vitro viral inhibition in 3 of 8 control animals. CD8+ cell-mediated viral inhibition in vaccinated animals ranged from a 0.00 to 1.12 log reduction in p27 levels and was 0.00 for all three control animals. The magnitude of in vitro CD8+ cell-mediated viral inhibition was less in vaccinated animals than in SIV-infected controllers (Fig. 1B), although in vitro viral inhibition in vaccinated animals was still clearly detectable. In particular, there was maximal viral inhibition in the Ad26/MVA group (P = 0.016, Wilcoxon signed rank test compared with sham controls), which also showed the highest adaptive immune responses in the parent vaccine study (1). These results demonstrate that CD8+ lymphocytes from vaccinated rhesus monkeys suppress heterologous SIV in vitro.
In vitro viral inhibition correlates with Gag-specific cellular immune responses.
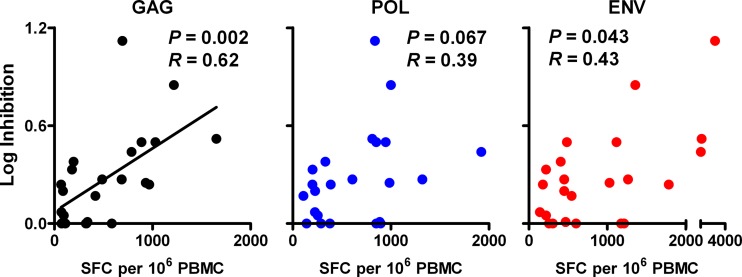
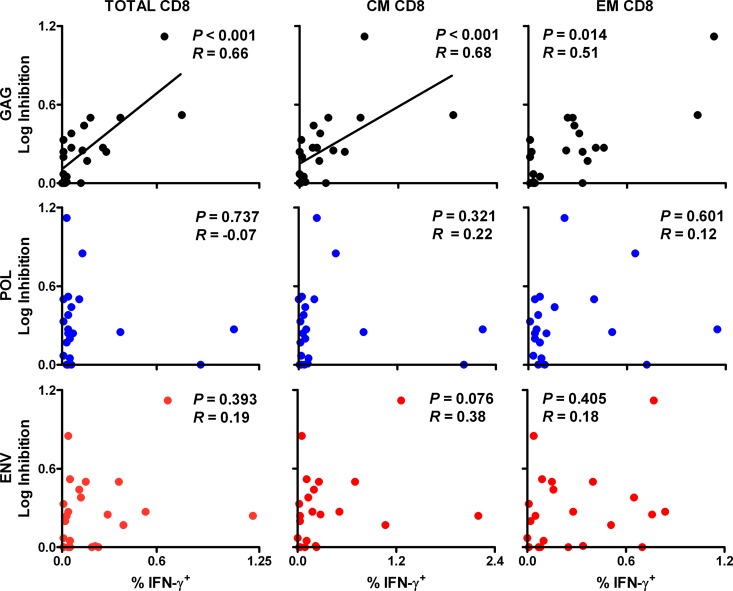
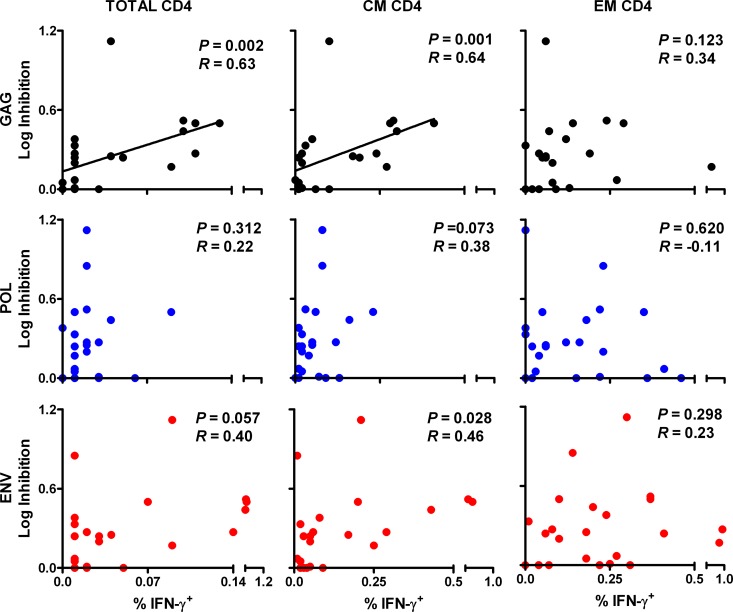
We next investigated the relationship between in vitro viral inhibition in vaccinated monkeys and cellular immune responses to various SIV antigens. We observed that in vitro CD8+ cell-mediated viral inhibition in vaccinated monkeys correlated significantly with Gag-specific, but not Pol- or Env-specific, cellular immune responses. In particular, viral inhibition correlated with Gag-specific ELISPOT responses (P = 0.002 and R = 0.62) (Fig. 2). In contrast, only a trend was observed between viral inhibition and Pol- and Env-specific responses (P = 0.067 and 0.043, respectively; a P value of <0.017 was required for significance due to multiple comparison adjustments). Moreover, viral inhibition correlated significantly with Gag-specific total CD8+ T lymphocyte responses (P < 0.001 and R = 0.66) (Fig. 3) and central/transitional memory CD8+ T lymphocyte responses (P < 0.001 and R = 0.68), as measured in multiparameter ICS assays. A trend was observed with Gag-specific effector memory CD8+ T lymphocyte responses (P = 0.014 and R = 0.51; P < 0.006 required for significance due to multiple comparison adjustments). However, there were no detectable associations between viral inhibition and Pol- or Env-specific CD8+ T lymphocyte responses. Viral inhibition in vaccinated animals also correlated significantly with Gag-specific total CD4+ T lymphocyte responses (P = 0.002 and R = 0.63) (Fig. 4) and central/transitional memory CD4+ T lymphocyte responses (P = 0.001 and R = 0.64), whereas there were no associations between viral inhibition and Pol- or Env-specific CD4+ T lymphocyte responses. These data demonstrate that in vitro viral inhibition is largely determined by Gag-specific cellular immune responses in vaccinated rhesus monkeys.
Fig 2.
Correlation of prechallenge CD8+ cell-mediated viral inhibition and peak ELISPOT responses in vaccinated animals. CD8+ cell-mediated viral inhibition in vaccinated rhesus monkeys (n = 23) is plotted versus cellular immune responses to SIVMAC239 Gag, Pol, and Env as determined in gamma interferon ELISPOT assays at week 26 prechallenge. P values reflect Spearman rank correlation tests. P values of <0.017 were required for significance after multiple comparisons adjustments. SFC, spot-forming cells.
Fig 3.
Correlation of prechallenge CD8+ cell-mediated viral inhibition and ICS responses among CD8+ T lymphocytes in vaccinated animals. CD8+ cell-mediated viral inhibition in vaccinated rhesus monkeys (n = 22) is plotted versus CD8+ total, central/transitional memory (CM; CD28+ CD95+), and effector memory (EM; CD28− CD95−) responses to Gag, Pol, and Env as determined in multiparameter gamma interferon ICS assays at week 26 prechallenge. ICS data are missing for one vaccinated animal. P values reflect Spearman rank correlation tests. P values of <0.006 were required for significance after multiple comparison adjustments.
Fig 4.
Correlation of prechallenge CD8+ cell-mediated viral inhibition and ICS responses among CD4+ T lymphocytes in vaccinated animals. CD8+ cell-mediated viral inhibition in vaccinated rhesus monkeys (n = 22) is plotted versus CD4+ total, central/transitional memory (CM; CD28+ CD95+), and effector memory (EM; CD28− CD95−) responses to Gag, Pol, and Env as determined by multiparameter gamma interferon ICS assays at week 26 prechallenge. ICS data are missing for one vaccinated animal. P values reflect Spearman rank correlation tests. P values of <0.006 were required for significance after multiple comparison adjustments.
In vitro SIV inhibition in vaccinated animals prior to challenge inversely correlates with in vivo virologic control following challenge.
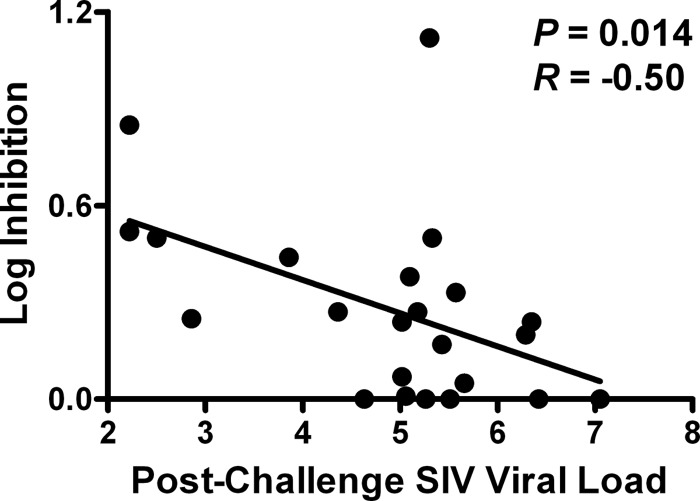
We next assessed whether in vitro viral inhibition in vaccinated rhesus monkeys predicted in vivo set point virologic control. We observed that in vitro CD8+ cell-mediated viral inhibition at weeks 28 to 34 prechallenge (reflecting peak vaccine immunity) inversely correlated with postchallenge in vivo set point plasma SIV RNA levels at week 12 following challenge (P = 0.014 and R = −0.50, Spearman rank-correlation test) (Fig. 5). To the best of our knowledge, this is the first study to demonstrate that in vitro SIVMAC251 inhibition following vaccination is significantly associated with in vivo virologic control following challenge.
Fig 5.
Correlation of prechallenge CD8+ cell-mediated viral inhibition with postchallenge in vivo set point plasma SIV RNA levels. CD8+ cell-mediated viral inhibition in vaccinated rhesus monkeys (n = 23) is plotted versus postchallenge set point plasma SIVMAC251 RNA levels (in log copies/ml). P values reflect Spearman rank correlation tests. P values of <0.05 were required for significance.
Viral inhibition in vaccinated animals after SIV challenge also correlates with Gag specificity and in vivo virologic control.
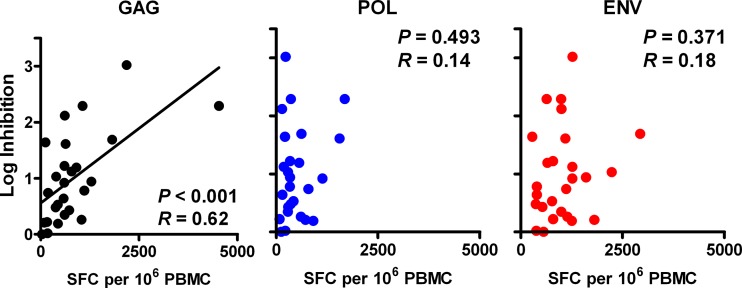
To elucidate further the relationship between in vitro viral inhibition and in vivo virologic control, we performed additional viral inhibition assays in these same animals 5 to 11 months after SIVMAC251 infection. CD8+ cell-mediated viral inhibition of endogenous virus in the 28 surviving vaccinated, SIV-infected monkeys ranged from 0.00 to 3.02 log reduction in p27 levels. In 7 control animals, viral inhibition ranged from 0.27 to 1.54 log reduction in p27 levels. These results show that CD8+ lymphocytes from vaccinated monkeys postchallenge suppress SIV replication in vitro. Moreover, viral inhibition correlated with Gag-specific ELISPOT responses (P < 0.001 and R = 0.62) (Fig. 6), but not Pol- or Env-specific responses (P = 0.493 and P = 0.371, respectively; P < 0.013 required for significance after multiple comparison adjustments). Interestingly, viral inhibition also weakly correlated with Nef-specific ELISPOT responses (P = 0.010), a protein not included in the vaccine (data not shown). These data suggest that Gag-specific cellular immune responses during chronic infection contribute substantially to in vitro viral inhibition, although responses to other SIV antigens may also contribute.
Fig 6.
Correlation of CD8+ cell-mediated viral inhibition and concurrent ELISPOT responses in vaccinated animals postchallenge. CD8+ cell-mediated viral inhibition in vaccinated rhesus monkeys at weeks 20 to 44 postchallenge (n = 27) is plotted versus concurrent cellular immune responses to SIVMAC239 Gag, Pol, and Env as determined in gamma interferon ELISPOT assays. P values reflect Spearman rank correlation tests. P values of <0.013 were required for significance after multiple comparison adjustments. SFC, spot-forming cells.
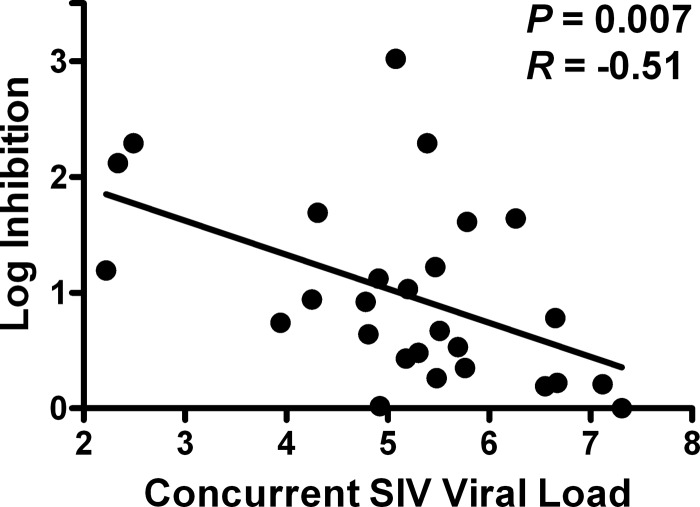
Finally, we evaluated whether CD8+ cell-mediated viral inhibition in vaccinated monkeys following challenge correlated with in vivo virologic control. Consistent with studies in HIV-1-infected humans (6, 22, 24), we found that viral inhibition was inversely correlated with concurrent in vivo plasma SIV RNA levels in these animals (P = 0.007 and R = −0.51) (Fig. 7). Taken together, these results demonstrate that Gag-specific cellular immune responses contribute to in vitro viral inhibition both before and after SIV challenge as well as in vivo virologic control in vaccinated rhesus monkeys.
Fig 7.
Correlation of postchallenge CD8+ cell-mediated viral inhibition with concurrent in vivo set point plasma SIV RNA levels. CD8+ cell-mediated viral inhibition in vaccinated rhesus monkeys at weeks 20 to 44 postchallenge (n = 27) is plotted versus concurrent plasma SIVMAC251 RNA levels (in log copies/ml). P values reflect Spearman rank correlation tests. P values of <0.05 were required for significance.
DISCUSSION
The antigenic determinants of in vitro viral inhibition and in vivo virologic control in vaccinated rhesus monkeys following SIV challenge have been unclear. In this study, we demonstrated that in vitro viral inhibition prior to challenge was inversely correlated with in vivo set point plasma SIV viral loads after challenge. Moreover, in vitro viral inhibition correlated significantly with total and central/transitional memory CD8+ and CD4+ T lymphocyte responses to Gag, but not to Pol or Env. These data demonstrated that the in vitro viral inhibition assay following vaccination largely reflected Gag-specific cellular immune responses and correlated with virologic control following challenge.
CD8+ T lymphocytes have been shown to be critical for the control of HIV-1 infection in persons with very low levels of plasma HIV-1 RNA in the absence of antiviral therapy (7, 10, 15, 22, 25). A similar observation has also been made in SIV-infected rhesus monkeys (8, 9, 12, 14). For example, Hansen et al. demonstrated in nonhuman primates that peak SIV-specific CD8+ T lymphocyte responses following immunization were associated with virologic control in rhesus monkeys immunized with rhesus cytomegalovirus vaccines that included Gag antigens (9). In HIV-1-infected elite controllers, multiple mechanisms have been proposed to account for the potency of CD8+ T lymphocytes, including increased perforin expression (11), preserved proliferative capacity (20), and increased granule exocytosis-mediated elimination of HIV-1-infected CD4+ T cells (21). Moreover, studies have shown that in vitro CD8+ T cell-mediated inhibition of viral replication correlates significantly with in vivo HIV-1 control and clinical status in HIV-1-infected subjects (2, 3, 22, 24). As a result, the in vitro viral inhibition assay has been proposed for use in the evaluation of HIV-1 vaccine candidates (24). Our findings extend these prior studies by adapting the in vitro viral inhibition assay for use in vaccinated rhesus monkeys. Specifically, we demonstrated that in vitro viral inhibition following vaccination correlates with virologic control following SIV challenge.
We observed that CD8+ cell-mediated viral inhibition was significantly associated with Gag-specific cellular immunity and not Pol- or Env-specific cellular immunity. This result is consistent with studies demonstrating the association of Gag-specific cellular immune responses with virologic control in HIV-1-infected individuals (4, 5, 13, 15, 26, 28) and SIV-infected rhesus monkeys (1, 12, 14, 18), as well as in vitro studies showing that Gag-specific cell lines and clones of cytotoxic T lymphocytes are more efficient than cell lines and clones with other specificities at killing exogenously peptide-loaded target cells (3, 27a, 28). In addition, our results are congruent with data from the parent vaccine trial that noted a correlation between Gag-specific ELISPOT magnitude and breadth and virologic control (1).
Our study also builds on previous work by Iwamoto et al. (12), who demonstrated that in a group of vaccinated rhesus monkeys (n = 8) with excellent SIV control after challenge, CD8+ T lymphocytes from the prechallenge phase were able to suppress homologous SIV in vitro. Our data extend these findings with larger numbers of animals, as well as by using a heterologous strain of SIV and assessing for correlations with postchallenge viral loads. In contrast, Martins et al. (19) reported that vaccine-induced CD8+ T lymphocytes from a small group of rhesus monkeys (n = 7) suppressed SIV replication in vitro but did not predict virologic control in vivo. We suspect that these differences may reflect the larger sample size in our study (n = 23) as well as differences in the assays utilized, such as the use of a carefully defined limiting amount of SIV to infect cells in our present study.
Despite its potential utility, the in vitro viral inhibition assay also has limitations in terms of potential clinical applications. The magnitude of viral inhibition in vaccinated animals was lower than in SIV-infected animals, suggesting that only relatively large vaccine-elicited viral inhibition responses would have been detectable. Moreover, this assay would be difficult to scale up for high-throughput analysis. In addition, it is not clear whether the in vitro viral inhibition assay has superior predictive power compared to Gag-specific ELISPOT responses. More studies are therefore warranted to confirm and compare these immune correlates of virologic control. Finally, it is likely that in vitro viral inhibition is only one determinant of in vivo virologic control and that other factors may also play a role.
In summary, our data demonstrate that CD8+ lymphocytes from vaccinated rhesus monkeys inhibited replication of a heterologous strain of SIV in vitro. Moreover, the magnitude of in vitro viral inhibition prior to challenge was inversely correlated with in vivo plasma viral loads following challenge. In addition, CD8 cell-mediated viral inhibition correlated significantly with Gag-specific, but not Pol- or Env-specific, cellular immune responses. Taken together, the above findings suggest that Gag should be included in HIV-1 vaccine regimens if virologic control following infection is a goal.
ACKNOWLEDGMENTS
We acknowledge J. Lian, A. K. Piechocka-Trocha, J. Liu, and D. Lynch for generous advice, assistance, and reagents. Human recombinant interleukin-2 was obtained through the NIH AIDS Research and Reference Reagent Program.
We acknowledge support from the U.S. National Institutes of Health (AI07387 to K.E.S.; AI066305, AI066924, AI078526, and AI095985 to D.H.B.; AI030914 to B.D.W.), the Bill and Melinda Gates Foundation, and the Ragon Institute of MGH, MIT, and Harvard.
Footnotes
Published ahead of print 3 July 2012
REFERENCES
- 1. Barouch DH, et al. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blackbourn DJ, et al. 1996. Suppression of HIV replication by lymphoid tissue CD8+ cells correlates with the clinical state of HIV-infected individuals. Proc. Natl. Acad. Sci. U. S. A. 93:13125–13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, et al. 2009. Differential neutralization of human immunodeficiency virus (HIV) replication in autologous CD4 T cells by HIV-specific cytotoxic T lymphocytes. J. Virol. 83:3138–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dahirel V, et al. 2011. Coordinate linkage of HIV evolution reveals regions of immunological vulnerability. Proc. Natl. Acad. Sci. U. S. A. 108:11530–11535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edwards B, et al. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fauce SR, Yang OO, Effros RB. 2007. Autologous CD4/CD8 co-culture assay: a physiologically-relevant composite measure of CD8+ T lymphocyte function in HIV-infected persons. J. Immun. Methods 327:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frahm N, et al. 2006. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat. Immunol. 7:173–178 [DOI] [PubMed] [Google Scholar]
- 8. Greene JM, et al. 2010. Extralymphoid CD8+ T cells resident in tissue from simian immunodeficiency virus SIVmac239Δnef-vaccinated macaques suppress SIVmac239 replication ex vivo. J. Virol. 84:3362–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen SG, et al. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hersperger AR, Migueles SA, Betts MR, Connors M. 2011. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr. Opin. HIV AIDS 6:169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hersperger AR, et al. 2010. Perforin expression directly ex vivo by HIV-specific CD8+ T-cells is a correlate of HIV elite control. PLoS Pathog. 6:e1000917 doi:10.1371/journal.ppat.1000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwamoto N, et al. 2010. Broadening of CD8+ cell responses in vaccine-based simian immunodeficiency virus controllers. AIDS 24:2777–2787 [DOI] [PubMed] [Google Scholar]
- 13. Julg B, et al. 2010. Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. J. Virol. 84:5540–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawada M, et al. 2008. Gag-specific cytotoxic T-lymphocyte-based control of primary simian immunodeficiency virus replication in a vaccine trial. J. Virol. 82:10199–10206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kiepiela P, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 [DOI] [PubMed] [Google Scholar]
- 16. Lai L, et al. 2011. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J. Infect. Dis. 204:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Letvin NL, et al. 2011. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci. Transl. Med. 3:81ra36 doi:10.1126/scitranslmed.3002351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, et al. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martins MA, et al. 2010. T-cell correlates of vaccine efficacy after a heterologous simian immunodeficiency virus challenge. J. Virol. 84:4352–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Migueles SA, et al. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immun. 3:1061–1068 [DOI] [PubMed] [Google Scholar]
- 21. Migueles SA, et al. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saez-Cirion A, et al. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U. S. A. 104:6776–6781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmitz JE, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860 [DOI] [PubMed] [Google Scholar]
- 24. Spentzou A, et al. 2010. Viral inhibition assay: a CD8 T cell neutralization assay for use in clinical trials of HIV-1 vaccine candidates. J. Infect. Dis. 201:720–729 [DOI] [PubMed] [Google Scholar]
- 25. Streeck H, et al. 2009. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J. Virol. 83:7641–7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Streeck H, et al. 2007. Recognition of a defined region within p24 Gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J. Virol. 81:7725–7731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamamoto T, et al. 2012. Virus inhibition activity of effector memory CD8+ T cells determines simian immunodeficiency virus load in vaccinated monkeys after vaccine breakthrough infection. J. Virol. 86:5877–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a. Yang OO, et al. 2003. Impacts of avidity and specificity on the antiviral efficiency of HIV-1-specific CTL. J. Immunol. 171:3718–3724 [DOI] [PubMed] [Google Scholar]
- 28. Zuniga R, et al. 2006. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J. Virol. 80:3122–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]