Abstract
Adenovirus (Ad) vaccine vectors have proven highly immunogenic in multiple experimental models, but the innate immune responses induced by these vectors remain poorly characterized. Here we report innate cytokine responses to 5 different Ad vectors in 26 rhesus monkeys. Vaccination with adenovirus serotype 35 (Ad35), Ad26, and Ad48 induced substantially higher levels of antiviral (gamma interferon [IFN-γ], 10-kDa gamma interferon-induced protein [IP-10]) and proinflammatory (interleukin 1 receptor antagonist [IL-1RA], IL-6) cytokines than vaccination with Ad5 on day 1 following immunization. In vitro studies with capsid chimeric vectors and receptor-blocking monoclonal antibodies suggested that fiber-receptor interactions, as well as other capsid components, were critical for triggering these innate responses. Moreover, multiple cell populations, including dendritic cells, monocytes/macrophages, and T lymphocytes, contributed to these innate cytokine profiles. These data demonstrate that Ad35, Ad26, and Ad48, which utilize CD46 as their primary cellular receptor, induce significantly greater innate cytokine responses than Ad5, which uses the coxsackievirus and adenovirus receptor (CAR). These differences in innate triggering result in markedly different immunologic milieus for the subsequent generation of adaptive immune responses by these vaccine vectors.
INTRODUCTION
Adenovirus (Ad) vectors are widely used for vaccination due to their immunogenicity, relatively large transgene coding capacity, and multiple available serotypes with diverse biological properties. While considerable data have been generated regarding adaptive immune responses elicited by Ad vectors, much less is known about innate immune responses induced by these vectors. As innate immune induction is critical for understanding both reactogenicity and adaptive immunity, it is important to define the innate pathways triggered by Ad vectors from various serotypes. Accumulating evidence suggests that different serotype Ad vectors induce qualitatively different adaptive immune response phenotypes (1, 23, 27, 42). In particular, vaccine studies using the simian immunodeficiency virus (SIV) infection model in rhesus monkeys have shown qualitative differences in adaptive immune responses elicited by various serotype Ad vectors (27) which translated into different levels of protective efficacy against SIV challenges (6, 7, 28). However, innate immune profiles of different alternative serotype Ad vectors have not previously been studied in nonhuman primates.
Adenoviruses are a diverse group of double-stranded DNA viruses with at least 65 known human serotypes, which are subdivided into species A to G based upon sequence homology (10, 26, 30, 49). Vectors constructed using these viruses have been shown to differ significantly in terms of primary receptor usage (1, 9, 13, 40, 50), intracellular trafficking patterns (14, 22, 31, 32), transduction and activation of dendritic cells (2, 11, 20, 29, 36, 53), utilization of secondary receptors (15, 48), cellular tropism (3, 16, 33, 44, 46, 47), and interaction with pattern recognition receptors (PRR) (12, 18, 35). The species C adenovirus serotype 5 (Ad5), the species B2 adenovirus serotype 35 (Ad35), and the species D adenovirus serotype 26 (Ad26) are currently being evaluated as vaccine candidates in clinical trials, yet relatively little is known about the possible differences in innate immunity induced by these vectors. Notably, Ad5 utilizes the coxsackievirus and adenovirus receptor (CAR) as its primary cellular receptor, whereas Ad35, Ad26, and Ad48 utilize CD46 (24).
In this study, we describe the innate cytokine profiles induced in vivo by Ad vectors from 5 serotypes in 26 rhesus monkeys. We then assessed the mechanism of differential viral triggering of these innate responses using capsid chimeric vectors and receptor-blocking monoclonal antibodies (MAbs) in vitro in human peripheral blood mononuclear cells (PBMC). Our studies demonstrate that Ad35, Ad26, and Ad48 vectors that utilize CD46 as their primary cellular receptor trigger innate cytokine profiles characterized by higher levels of antiviral and proinflammatory cytokines and chemokines than those triggered by Ad5 vectors that utilize CAR.
MATERIALS AND METHODS
Viruses.
E1/E3-deleted vectors Ad5, Ad35, Ad26, Ad48, and chimeric Ad5 with the hexon hypervariable regions (HVRs) replaced with those of Ad48 (Ad5HVR48) expressing SIV antigens and no transgene were produced as previously described (1, 34). Briefly, vectors were produced by recombination in E1-complementing PER.55K cells and were purified by CsCl density centrifugation.
Cells.
Normal human blood was collected in the presence of sodium heparin and processed by the Ficoll-Hypaque gradient method as previously described (8). Cells were resuspended in R10 medium (RPMI, 10% fetal calf serum [FCS], 50 U/ml penicillin, 50 μg/ml streptomycin) at a concentration of 1 × 106 cells/ml and utilized in in vitro assays. All studies involving human subjects were approved by the Beth Israel Deaconess Medical Center Institutional Review Board (IRB).
Antibodies.
The anti-CAR MAb RmcB (Millipore, Billerica, MA) and anti-CD46 MAbs 13/42 (LifeSpan Biosciences, Seattle, WA) and M177 (Hycult Biotechnology, Plymouth Meeting, PA), as well as the anti-KLH mouse IgG isotype control (R&D Systems, Minneapolis, MN), were washed 3× with 1 ml unsupplemented Dulbecco's phosphate-buffered saline (DPBS) and concentrated to 1 μg/μl by centrifugation at 3,000 rpm in Amicon Ultra-4 centrifugal filters (molecular mass, 30 kDa) (Millipore, Billerica, MA) and stored at 4°C for immediate use. Flow cytometry antibody panels included CD3-allophycocyanin (UCHT1), CD16-fluorscein isothiocyanate (3G8), CD123-peridinin chlorophyll protein-Cy5.5 (9F5), CD11c-phycoerythrin (B-ly6), CD56-phycoerythrin-Cy7 (B159), CD19-V450 (HlB19), CD14-allophycocyanin-Cy7 (MφP9) (BD Biosciences, San Diego, CA), and HLA-DR-AlexaFluor700 (LN3) (eBioscience, San Diego, CA).
In vitro cytokine stimulation assay.
A total of 1 × 106 PBMC were incubated with 1,000 viral particles (vp)/cell of the indicated adenovirus vector. In certain experiments, cells were preincubated with MAbs for 1 h prior to infection. Cells were cultured at 37°C, and supernatants were harvested 24 h after infection by centrifugation at 1,400 rpm for 5 min. Supernatants were analyzed using the Millipore Milliplex Map Human Cytokine/Chemokine Magnetic Luminex (Millipore, Billerica, MA) and the Life Technologies Cytokine Human 30-plex panel (Life Technologies, Grand Island, NY) according to the manufacturers' protocols. Luminex data were analyzed on a BioPlex 200 instrument running BioPlex Manager 4.1 (Bio-Rad, Hercules, CA) with an 80% to 120% standard acceptance range. Data were graphed using GraphPad Prism v5.0 (GraphPad Software, Inc., La Jolla, CA). Means between groups were compared using Kruskal-Wallis tests with Dunn's correction for multiple comparisons and plotted as means and standard errors of the mean (SEM).
Magnetic cell separation.
PBMC were depleted of CD3-, CD14-, CD19-, CD56-, CD1c-, or BDCA-1-positive cell fractions by use of magnetic MicroBeads according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). Depleted populations were confirmed by flow cytometry on LSRII and BD FACSDiva v.6.1.1 (BD Biosciences). Results were analyzed using FlowJo v.8.8.6 (Tree Star, Inc., Ashland, OR).
Nonhuman primate Ad vector vaccination.
Rhesus monkeys (n = 4 to 8 per group) were immunized with 3 × 1010 vp of Ad5, Ad35, Ad26, Ad48, or Ad5HVR48 expressing SIV Gag/Pol/Env antigens. Serum was collected on days 0, 1, 3, 7, 14, and 28 following vaccination, thawed on ice, and inactivated with 0.05% Tween 20 for 15 min at room temperature. Samples were then run on Milliplex Nonhuman Primate 23-plex premix (Millipore) or Invitrogen Monkey Cytokine 28-plex (Life Technologies), a VeriKine cynomolgus/rhesus IFN-α serum enzyme-linked immunosorbent assay (ELISA) (PBL InterferonSource, Piscataway, NJ), and a human CXCL10/IP-10 Quantikine ELISA (R&D Systems, Minneapolis, MN) according to the manufacturers' protocols, analyzed, and graphed as described above. All studies involving rhesus monkeys were approved by the Harvard Medical School Institutional Animal Care and Use Committee (IACUC).
Data analysis and statistical methods.
Concentrations of cytokines and chemokines were obtained from Luminex assays using a 5-parameter logistic model. Results of in vitro PBMC studies were compared by Kruskal-Wallis tests with Dunn's correction for multiple comparisons. Total cytokine induction in vitro was assessed by the group average fold induction versus medium controls. Cytokine induction in vivo was assessed as the fold change of the cytokine over the average baseline level of all monkeys. The group fold change over the averaged baseline was compared to that for monkeys vaccinated with Ad5 by Mann-Whitney U tests.
RESULTS
Ad35, Ad26, and Ad48 induce more potent antiviral and proinflammatory cytokines and chemokines than Ad5 following vaccination of rhesus monkeys.
We initiated studies by assessing serum cytokine levels in rhesus monkeys following vaccination with 5 different serotype Ad vectors in 26 rhesus monkeys. Rhesus monkeys (n = 4 to 8 per group) were immunized intramuscularly (i.m.) with 3 × 1010 vp of Ad5, Ad35, Ad26, Ad48, or Ad5HVR48 expressing SIV Env/Gag/Pol (1, 39). All vectors were replication-incompetent E1/E3-deleted Ad vectors that were prepared similarly and that exhibited comparable characteristics, specific infectivity, and purity (1). Sera were collected on days 0, 1, 3, 7, 10, 14, and 28 following vaccination, and cytokine levels were assessed by Luminex assays.
Longitudinal analysis of cytokine responses following vaccination with Ad5 revealed only low levels of antiviral and proinflammatory cytokine and chemokine induction on day 1 following vaccination (Fig. 1A and B). In contrast, animals vaccinated with Ad35, Ad26, and Ad48 displayed greater induction of multiple cytokines and chemokines on day 1 following vaccination. Increased induction by Ad35, Ad26, and Ad48 compared with that by Ad5 was observed for the inflammatory markers interleukin 1 receptor antagonist (IL-1RA) (17.7-, 8.2-, and 26.2-fold greater induction than Ad5, respectively; P = 0.006, P = 0.009, and P = 0.02, respectively; Mann-Whitney U test) and IL-6 (8.3-, 4.0-, and 3.4-fold greater induction than Ad5, respectively; P = not significant [NS], P = 0.04, and P = 0.03, respectively). Additionally, significant induction of gamma interferon (IFN-γ) (1.4-, 1.7-, and 1.5-fold greater induction than Ad5; P = NS, P = NS, and P = 0.02, respectively) and its downstream-signaled chemokines 10-kDa gamma interferon-induced protein (IP-10) (8.3-, 6.0-, and 4.9-fold greater induction than Ad5; P = 0.03, P = 0.009, and P = 0.03, respectively) and interferon-inducible T-cell alpha chemoattractant (I-TAC) (2.8-, 1.1-, and 3.6-fold greater induction than Ad5; P = 0.03, P = NS, and P = 0.03, respectively) was observed in animals that received Ad35, Ad26, or Ad48. These data demonstrate that Ad35, Ad26, and Ad48, which utilize CD46 as their cellular receptor, trigger substantially greater antiviral and proinflammatory cytokine responses than does Ad5, which utilizes CAR as its cellular receptor, following vaccination of rhesus monkeys.
Fig 1.
Serum concentrations of cytokines and chemokines in rhesus monkeys following vaccination with Ad vectors. Rhesus monkeys (n = 4 to 8 per group) were injected i.m. with 3 × 1010 vp of Ad5, Ad35, Ad26, Ad48, or Ad5HVR48 vector. Sera were collected on days 0, 1, 3, 7, 10, 14, and 28 following vaccination, and systemic levels of cytokines and chemokines were measured by Luminex and ELISAs. (A) Systemic levels of selected cytokines are shown with colored lines, with means shown with black lines. Groups are compared by fold changes over the baseline compared with Ad5. *, P < 0.05; **, P < 0.01. (B) Mean fold induction of all cytokine responses relative to the baseline by the various Ad vectors in rhesus monkeys 1 day (left) and 7 days (right) following immunization. Data are fold changes over the averaged baseline. GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; HGF, hepatocyte growth factor; FGF, fibroblast growth factor; MIF, macrophage migration inhibitory factor; MDC, macrophage-derived chemokine; MIG, monokine induced by gamma interferon; TGFα, transforming growth factor alpha.
Interestingly, on day 7 following vaccination, animals that received Ad48 or the chimeric Ad5HVR48 vector also displayed a temporally distinct peak of cytokine induction characterized by the proinflammatory cytokines IL-1RA, IL-6, IL-1β, and TNF-α (Fig. 1A and B). This cytokine peak was not observed with the Ad5, Ad35, or Ad26 vector. Since Ad48 and Ad5HVR48 share only common hexon HVR sequences, we speculate that this day 7 cytokine peak may be triggered by hexons rather than fiber-receptor interactions. These data suggest that both fibers and hexons may contribute to triggering innate immune responses.
Cytokine induction following a heterologous boost immunization.
We next assessed whether innate cytokine responses following an Ad26 boost immunization would be impacted by previous priming with a heterologous Ad vector. Systemic cytokine levels were assessed in rhesus monkeys (n = 4 per group) that were previously primed with Ad35, Ad48, or Ad5HVR48 vector as described above and then boosted 24 weeks later i.m. with 3 × 1010 vp of Ad26 expressing SIV Env/Gag/Pol. Sera were collected and analyzed as described above. Longitudinal analysis of animals revealed cytokine profiles on day 1 following the boost immunization (Fig. 2) that appeared similar to the cytokine profile induced following priming with Ad26 (Fig. 1A), including induction of IFN-γ, IL-6, IP-10, and IL-1RA (Fig. 2). These data suggest that the innate cytokine profiles following a boost immunization are dictated primarily by the vector utilized for the boost and are not substantially imprinted by the heterologous vector utilized for priming.
Fig 2.
Serum concentrations of cytokines and chemokines in rhesus monkeys following a boost vaccination with Ad26 vector. Rhesus monkeys (n = 4 per group) were boosted i.m. with 3 × 1010 vp of Ad26 vector 24 weeks after priming with 3 × 1010 vp of Ad35, Ad48, or Ad5HVR48 vector. Sera were collected on days 0, 1, 3, 7, 14, and 28 following vaccination, and systemic levels of cytokines and chemokines were measured by Luminex assays. Systemic levels of selected cytokines are shown with colored lines, with group means shown with black lines.
Ad35, Ad26, and Ad48 induce higher levels of antiviral and proinflammatory cytokines and chemokines than Ad5 in vitro.
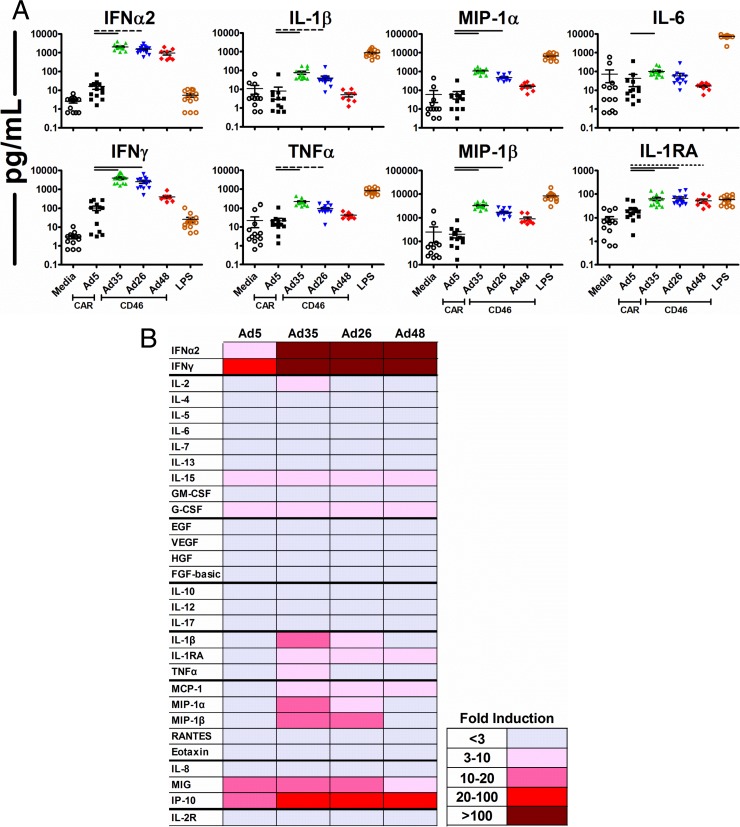
We next sought to probe the mechanism by which different serotype Ad vectors elicit differential innate cytokine profiles. We therefore assessed the capacity of Ad5, Ad35, Ad26, and Ad48 to trigger secretion of innate cytokines in vitro in freshly isolated human PBMC. Human PBMC (n = 8 to 13 per group) were stimulated with Ad5, Ad35, Ad26, or Ad48 at a multiplicity of infection (MOI) of 103 viral particles (vp) per cell. Cytokine and chemokine responses were measured in culture supernatant 24 h postinfection by Luminex assays.
Transduction of PBMC with Ad35 and Ad26 induced substantially higher levels of IFN-α2 (128- and 96-fold higher levels, respectively; P < 0.001 and P < 0.01, respectively; Kruskal-Wallis test with Dunn's correction for multiple comparisons), IFN-γ (38- and 24-fold higher levels, respectively; P < 0.001), IL-1β (10- and 5-fold higher levels, respectively; P < 0.001 and P < 0.01, respectively), and tumor necrosis factor alpha (TNF-α) (10- and 4-fold higher levels, respectively; P < 0.001 and P < 0.01, respectively) than transduction of PBMC with Ad5 (Fig. 3A). Ad35 and Ad26 also induced higher levels of macrophage inflammatory protein 1 alpha (MIP-1α) (18- and 8-fold, respectively; P < 0.001), MIP-1β (16- and 9-fold, respectively; P < 0.001), IL-6 (2.4- and 1.4-fold higher levels, respectively; P < 0.001 and P = NS, respectively), and IL-1RA (3-fold higher levels for both; P < 0.001) (Fig. 3A). The species D vector Ad48 displayed an intermediate phenotype of cytokine and chemokine induction relative to that of Ad5 and those induced by Ad35 and Ad26. A complete analysis of all cytokines and chemokines measured in the Luminex assays demonstrated that Ad35, Ad26, and Ad48 induced higher levels of multiple antiviral and proinflammatory cytokines as well as chemokines, including MCP-1 and IP-10, than did Ad5 (Fig. 3B). These in vitro data are largely consistent with the in vivo data from vaccinated rhesus monkeys with a few notable exceptions, such as the high levels of IFN-α2 induced in vitro compared to those induced in vivo.
Fig 3.
Cytokine and chemokine responses induced in vitro in human PBMC following Ad vector stimulation. (A) Individual PBMC (n = 8 to 13 per group) were stimulated with 103 vp/cell of Ad5, Ad35, Ad26, or Ad48 vector, and cytokine responses were measured after 24 h by Luminex assays. Lipopolysaccharide (LPS) (1 ng/ml) was used as the positive control. Data are individual measurements, with mean responses and the SEM indicated by solid lines. Bars indicate P values of <0.05 (dotted lines), P values of <0.01 (dashed lines), or P values of <0.001 (solid lines) using Kruskal-Wallis tests with Dunn's correction for multiple comparisons. (B) Mean group fold induction of all cytokine responses relative to those in medium by various Ad vectors in human PBMC.
Both fiber and capsid components contribute to innate immune stimulation by Ad vectors.
We next explored the role of the key structural proteins of the adenovirus capsid on innate immune stimulation. We utilized a panel of chimeric Ad5/Ad35 (34) vectors and stimulated human PBMC (n = 8 per group) as described above. Chimeric Ad vectors included Ad5 with the Ad35 fiber (Ad5f35) or Ad35 fiber and penton (Ad5p35f35) and Ad35 with the Ad5 fiber (Ad35f5) or Ad5 fiber knob (Ad35k5). Replacement of the Ad5 fiber with the Ad35 fiber or Ad35 fiber and penton resulted in an increased induction of multiple cytokines, including IFN-α2 (126- and 184-fold higher levels, respectively; P < 0.01 and P < 0.001, respectively), IFN-γ (16- and 9-fold higher levels, respectively; P < 0.05 and P < 0.01, respectively), IL-1β (2.5- and 1.6-fold higher levels, respectively; P = NS), TNF-α (12- and 11-fold higher levels, respectively; P < 0.001), MIP-1α (52- and 40-fold higher levels, respectively; P < 0.01 and P < 0.001, respectively), MIP-1β (30- and 25-fold higher levels, respectively; P < 0.001), IL-6 (7- and 7-fold higher levels, respectively; P < 0.001), and IL-1RA (4.4- and 3.5-fold higher levels, respectively; P < 0.05 and P < 0.01, respectively), relative to that by Ad5 (Fig. 4).
Fig 4.
Cytokine and chemokine responses elicited by chimeric Ad5/Ad35 vectors in human PBMC. Normal human PBMC (n = 8 per group) were stimulated for 24 h in the presence of chimeric Ad5/Ad35 vectors, and cytokine and chemokine levels were measured by Luminex assays. LPS (1 ng/ml) was included as the positive control. Data are individual measurements, with mean responses and the SEM indicated by solid lines. Bars indicate P values of <0.05 (dotted lines), P values of <0.01 (dashed lines), or P values of <0.001 (solid lines) using Kruskal-Wallis tests with Dunn's correction for multiple comparisons.
A corresponding decrease in stimulatory capacity was observed with Ad35 vectors containing the Ad5 fiber or fiber knob compared to the stimulatory capacity with Ad35, suggesting the importance of the Ad35 fiber for innate cytokine stimulation. For example, Ad35f5 and Ad35k5 induced lower levels of IFN-γ (4.7- and 7.1-fold lower levels, respectively; P < 0.05 and P < 0.01, respectively), IL-1β (2.7- and 10.6-fold lower levels, respectively; P < 0.05 and 0 < 0.001, respectively), TNF-α (5.5- and 7.1-fold lower levels, respectively; P < 0.01), MIP-1α (13.0- and 23.6-fold lower levels, respectively; P < 0.01 and P < 0.001, respectively), MIP-1β (6.0- and 9.4-fold lower levels, respectively; P < 0.05), and IL-6 (3.4- and 5.2-fold lower levels, respectively; P < 0.05 and 0 < 0.01, respectively) than did Ad35 (Fig. 4). However, Ad35f5 and Ad35k5 still induced most cytokines to a greater level than did Ad5, indicating that other capsid components in addition to fiber also likely contribute to innate cytokine stimulation. Taken together, these results are consistent with the model in which both fibers and hexons contribute to innate immune triggering.
Fiber-receptor interactions are required for Ad35 and Ad26 stimulation of human PBMC.
We next assessed whether fiber interactions with the CD46 receptor were critical for the robust innate immune stimulation by Ad35 and Ad26 vectors. Human PBMC (n = 4 to 8 per group) were preincubated with 10 μg/ml of the anti-CAR monoclonal antibody RmcB (17) or the anti-CD46 monoclonal antibodies 13/42 and M177 (43, 45) and were then stimulated with Ad35 and Ad26 as described above. As expected, IFN-α2 and IFN-γ levels induced by Ad35 and Ad26 vectors were not affected by RmcB. In contrast, Ad35 induced lower levels of IFN-α2 and IFN-γ in the presence of 13/42 (P < 0.05) and Ad26 elicited dramatically lower levels of IFN-α2 and IFN-γ following preincubation of cells with either 13/42 (P < 0.05) or M177 (P < 0.05) (Fig. 5). No interferon responses were observed in control experiments following incubation of cells with the monoclonal antibodies alone (data not shown). These results support previous reports from our laboratory and others showing that CD46 is a primary cellular receptor for Ad35 and Ad26 (1, 13), and these data demonstrate that fiber-receptor binding contributes substantially to the innate immune stimulation by these vectors. The greater effect of a CD46 blockade on innate responses triggered by Ad26 than on those triggered by Ad35 may reflect subtle differences in primary receptor interactions and/or secondary receptor usage by these vectors.
Fig 5.
Interferon induction by Ad35 and Ad26 in the presence of anti-CAR or anti-CD46 blocking MAbs. Normal human PBMC (n = 4 to 6 per group) were preincubated with 10 μg/ml anti-CAR (RmcB) or anti-CD46 (13/42 or M177) for 1 h and then stimulated with 103 vp/cell of Ad35 or Ad26 vector. IFN-α2 and IFN-γ levels were measured 24 h following stimulation by Luminex assays. LPS (1 ng/ml) was included as the positive control. Data are mean responses and the SEM. Bars indicate P values of <0.05 (dotted lines), P values of <0.01 (dashed lines), or P values of < 0.001 (solid lines) using Kruskal-Wallis tests with Dunn's correction for multiple comparisons. Unstim., unstimulated.
Multiple cellular subsets are stimulated by Ad35 and Ad26.
To explore the contributions of various cellular subsets to innate cytokine secretion following Ad35 and Ad26 stimulation, we depleted specific cell subsets from healthy human PBMC (n = 6 per group) and repeated the cytokine assays as shown in Fig. 3. T cells, B cells, NK cells, monocytes/macrophages (MonoMac), myeloid dendritic cells (mDC), or plasmacytoid dendritic cells (pDC) were depleted by magnetic bead separation, and effective depletion was confirmed by flow cytometry (data not shown). PBMC depleted of these various cell subsets were then incubated with Ad35 or Ad26, and cytokine and chemokine responses were assessed. Depletion of T cells resulted in complete abrogation of IFN-γ induction and a partial reduction of IL-1RA, whereas depletion of pDC led to a substantial reduction of IFN-α2, TNF-α, and IL-1RA (Fig. 6). Depletion of monocytes and macrophages resulted in a pronounced reduction of the proinflammatory markers IL-1RA, MIP-1α, and MIP-1β (Fig. 6). These data suggest that multiple cellular subsets contribute to the overall milieu of innate cytokines triggered by Ad vectors.
Fig 6.
Cytokine and chemokine responses elicited by Ad vectors in normal human PBMC depleted of various cell populations. Normal human PBMC (n = 6 per group) were depleted of the indicated cell subsets by magnetic bead separation, and depletion was confirmed by flow cytometry (data not shown). PBMC were then stimulated with 103 vp/cell of Ad35 or Ad26 vector, and cytokine and chemokine responses were measured 24 h after stimulation by Luminex assays. LPS (1 ng/ml) was included as the positive control. Data are means and the SEM, and unseparated and depleted cell populations were compared. Bars indicate P values of <0.05 (dotted lines), P values of <0.01 (dashed lines), or P values of <0.001 (solid lines) using Kruskal-Wallis tests with Dunn's correction for multiple comparisons.
DISCUSSION
The innate immune profiles induced by various serotype Ad vaccine vectors remain poorly understood and have not previously been characterized in nonhuman primates. Here we demonstrate that the alternative serotype vectors Ad35, Ad26, and Ad48 induce substantially greater innate cytokine responses than Ad5 following vaccination of rhesus monkeys. In particular, Ad35, Ad26, and Ad48 induce more antiviral and proinflammatory cytokines, characterized by higher levels of IFN-γ, IP-10, I-TAC, IL-1RA, and IL-6, than Ad5.
Previous reports have characterized innate cytokine profiles of Ad vectors in mice (3, 4, 53) and in vitro (2, 18, 19, 20, 29, 36, 52). For example, several studies have reported that Ad35 induces higher levels of the costimulatory markers CD80 and CD40 and interferons than does Ad5 in vitro in human DC (29) as well as higher levels of IL-10 and reduced proliferation in T cells cocultured with DCs (2). Studies in mice have suggested increased proinflammatory cytokine induction by Ad5 relative to CD46-utilizing vectors (11), a finding which contrasts with our data from rhesus monkeys. Moreover, a recent study demonstrated that Ad35 and Ad28 induce high levels of IFN-α following immunization of mice (20). In contrast, we observed only minimal IFN-α secretion by all Ad vectors, including Ad35 and Ad26, in rhesus monkeys. Taken together, these results demonstrate the importance of studying innate immune profiles of Ad vectors in primates rather than mice, which lack the functional cellular receptor CD46 that is utilized by the alternative serotype Ad vectors (24). This is particularly important since our data suggest that the innate cytokine responses elicited by these vectors are triggered largely by receptor binding. Indeed, both our in vitro and in vivo studies suggest that Ad fiber binding to CD46 strongly influences elicited cytokine profiles (Fig. 1, 4, and 5), and thus, the absence of functional CD46 in mice may account for the differences observed between the murine and primate experimental models.
Fiber-receptor interactions appear to be critical but do not fully explain the observed differences in innate immune triggering by the various serotype Ad vectors. In addition to its role as a receptor to certain species B and D adenoviruses, CD46 has a role in binding complement components C3b and C4b (5, 25) and, due to differential splicing, may be immunosuppressive or immunostimulatory upon ligand binding (37, 38). Additionally, the degree of CD46 binding may influence ligand endocytic trafficking fate as well as the induction of autophagy (21, 41). As such, Ad35, Ad26, and Ad48 binding to CD46 may target them to endosomal compartments rich in pattern recognition receptors that may then influence innate immune responses differently from those induced by Ad5. The mechanism by which CD46 binding influences Ad vector innate stimulation thus warrants further exploration. Our results also suggest that Ad capsid components, such as hexon, contribute to innate stimulation. In particular, we observed a distinct day 7 peak of cytokine secretion in monkeys that received Ad48 and Ad5HVR48, which share only common hexon HVRs. The ability of the hexon HVRs to influence innate immune stimulation may reflect their role in binding serum factors (48) or endocytic localization (51).
In summary, our data demonstrate that the innate cytokine responses triggered by Ad35, Ad26, and Ad48 are substantially greater than those induced by Ad5 in rhesus monkeys. Specifically, the alternative serotype Ad vectors that utilize CD46 as their primary cellular receptor induce more potent antiviral and proinflammatory cytokine responses following vaccination than Ad5, which utilizes CAR. The in vivo characterization of these innate cytokine profiles contributes to our understanding of these Ad vectors that are currently being evaluated as candidate vaccine vectors in human clinical trials. Further studies assessing the impact of these profoundly different innate immunologic milieus on the subsequent generation of adaptive immune responses are therefore warranted.
ACKNOWLEDGMENTS
We thank P. Abbink, C. Berger, E. Borducci, A. Carville, S. Clark, J. Goudsmit, M. Lifton, D. Lynch, A. LaPorte, K. Mansfield, M. Pau, E. Rhee, N. Simmons, K. Smith, K. Stanley, and H. Streeck for advice, assistance, and reagents. We thank P. Penaloza and L. Maxfield for careful reading of the manuscript.
We acknowledge support from the National Institutes of Health (AI066924, AI078526, AI095985, and AI096040), the Bill and Melinda Gates Foundation, and the Ragon Institute of MGH, MIT, and Harvard (D.H.B), as well as a National Defense Science & Engineering Graduate Fellowship and the Herchel Smith Graduate Fellowship from Harvard University (J.E.T.).
Footnotes
Published ahead of print 11 July 2012
REFERENCES
- 1. Abbink P, et al. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams WC, et al. 2011. Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation. Proc. Natl. Acad. Sci. U. S. A. 108:7499–7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appledorn DM, et al. 2008. Wild-type adenoviruses from groups A-F evoke unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Ther. 15:885–901 [DOI] [PubMed] [Google Scholar]
- 4. Appledorn DM, et al. 2008. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 15:1606–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barilla-LaBarca ML, Liszewski MK, Lambris JD, Hourcade D, Atkinson JP. 2002. Role of membrane cofactor protein (CD46) in regulation of C4b and C3b deposited on cells. J. Immunol. 168:6298–6304 [DOI] [PubMed] [Google Scholar]
- 6. Barouch DH, et al. 2009. Protective efficacy of a single immunization of a chimeric adenovirus vector-based vaccine against simian immunodeficiency virus challenge in rhesus monkeys. J. Virol. 83:9584–9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barouch DH, et al. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bennett S, Breit SN. 1994. Variables in the isolation and culture of human monocytes that are of particular relevance to studies of HIV. J. Leukoc. Biol. 56:236–240 [DOI] [PubMed] [Google Scholar]
- 9. Bergelson JM. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320–1323 [DOI] [PubMed] [Google Scholar]
- 10. Berk Arnold J. 2007. Adenoviridae: the viruses and their replication, p 2355–2394 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 11. DiPaolo N, et al. 2006. Evaluation of adenovirus vectors containing serotype 35 fibers for vaccination. Mol. Ther. 13:756–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Paolo NC, et al. 2009. Virus binding to a plasma membrane receptor triggers interleukin-1a-mediated proinflammatory macrophage response in vivo. Immunity 31:110–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaggar A, Shayakhmetov DM, Lieber A. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408–1412 [DOI] [PubMed] [Google Scholar]
- 14. Gastaldelli M, et al. 2008. Infectious adenovirus type 2 transport through early but not late endosomes. Traffic 9:2265–2278 [DOI] [PubMed] [Google Scholar]
- 15. Greig JA, et al. 2009. Influence of coagulation factor X on in vitro and in vivo gene delivery by adenovirus (Ad) 5, Ad35, and chimeric Ad5/Ad35 vectors. Mol. Ther. 17:1683–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Havenga MJ, et al. 2002. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J. Virol. 76:4612–4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu K-H, Lonberg-Holm K, Alstein B, Crowell RL. 1988. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J. Virol. 62:1647–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iacobelli-Martinez M, Nemerow GR. 2007. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J. Virol. 81:1305–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iacobelli-Martinez M, Nepomuceno RR, Connolly J, Nemerow GR. 2005. CD46-utilizing adenoviruses inhibit C/EBPβ-dependent expression of proinflammatory cytokines. J. Virol. 79:11259–11268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson MJ, et al. 2012. Type I IFN induced by adenovirus serotypes 28 and 35 has multiple effects on T cell immunogenicity. J. Immunol. 188:6109–6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joubert P-E, et al. 2009. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe 6:354–366 [DOI] [PubMed] [Google Scholar]
- 22. Kälin S, et al. 2010. Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35. J. Virol. 84:5336–5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaufman DR, et al. 2008. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J. Immunol. 181:4188–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kemper C, et al. 2001. Membrane cofactor protein (MCP; CD46) expression in transgenic mice. Clin. Exp. Immunol. 124:180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liszewski MK, Post TW, Atkinson JP. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431–455 [DOI] [PubMed] [Google Scholar]
- 26. Liu EB, et al. 2011. Genetic analysis of a novel human adenovirus with a serologically unique hexon and a recombinant fiber gene. PLoS One 6:e24491 doi:10.1371/journal.pone.0024491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, et al. 2008. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82:4844–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, et al. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loré K, et al. 2011. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J. Immunol. 179:1721–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsushima Y, et al. 2012. Novel human adenovirus strain, Bangaledesh. Emerg. Infect. Dis. 18:846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meier O, et al. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyazawa N, Crystal RG, Leopold PL. 2001. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 75:1387–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mizuguchi H, Hayakawa T. 2002. Adenovirus vectors containing chimeric type 5 and type 35 fiber proteins exhibit altered and expanded tropism and increase the size limit of foreign genes. Gene 285:69–77 [DOI] [PubMed] [Google Scholar]
- 34. Nanda A, et al. 2005. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J. Virol. 79:14161–14168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nociari M, Ocheretina O, Schoggins JW, Falck-Pedersen E. 2007. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J. Virol. 81:4145–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ophorst OJ, et al. 2004. An adenoviral type 5 vector carrying a type 35 fiber as a vaccine vehicle: DC targeting, cross neutralization, and immunogenicity. Vaccine 22:3035–3044 [DOI] [PubMed] [Google Scholar]
- 37. Post BTW, et al. 1991. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J. Exp. Med. 174:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Purcell DFJ, et al. 1991. Alternatively spliced RNAs encode several isoforms of CD46 (MCP), a regulator of complement activation. Immunogenetics 33:335–344 [DOI] [PubMed] [Google Scholar]
- 39. Roberts DM, et al. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239–243 [DOI] [PubMed] [Google Scholar]
- 40. Roelvink PW. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568–1571 [DOI] [PubMed] [Google Scholar]
- 41. Sakurai F, et al. 2006. The short consensus repeats 1 and 2, not the cytoplasmic domain, of human CD46 are crucial for infection of subgroup B adenovirus serotype 35. J. Control. Release 113:271–278 [DOI] [PubMed] [Google Scholar]
- 42. Santra S, et al. 2009. Heterologous prime/boost immunizations of rhesus monkeys using chimpanzee adenovirus vectors. Vaccine 27:5837–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schneider-Schaulies J, Dunster LM, Schwartz-Albiez R, Krohne G, ter Meulen V. 1995. Physical association of moesin and CD46 as a receptor complex for measles virus. J. Virol. 69:2248–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schnell M, et al. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3:708–722 [DOI] [PubMed] [Google Scholar]
- 45. Seya T, Hara T, Matsumoto M, Akedo H. 1990. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J. Immunol. 145:238–245 [PubMed] [Google Scholar]
- 46. Sharma A, Bangari DS, Tandon M, Hogenesch H, Mittal SK. 2010. Evaluation of innate immunity and vector toxicity following inoculation of bovine, porcine or human adenoviral vectors in a mouse model. Virus Res. 153:134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Varnavski AN, et al. 2002. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J. Virol. 76:5711–5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Waddington SN, et al. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409 [DOI] [PubMed] [Google Scholar]
- 49. Walsh MP, et al. 2010. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J. Clin. Microbiol. 48:991–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang H, et al. 2011. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 17:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. 2005. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 79:1992–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu W, et al. 2010. Human lung innate immune cytokine response to adenovirus type 7. J. Gen. Virol. 91:1155–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Y, et al. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697–707 [DOI] [PubMed] [Google Scholar]