Abstract
We analyzed the varicella-zoster virus (VZV) transcriptome in 43 latently infected human trigeminal ganglia (TG) with postmortem intervals (PMIs) ranging from 3.7 to 24 h. Multiplex reverse transcriptase PCR (RT-PCR) revealed no VZV transcripts with a PMI of <9 h. Real-time PCR indicated a significant increase (P = 0.02) in VZV ORF63 transcript levels but not the virus DNA burden with longer PMI. Overall, both the breadth of the VZV transcriptome and the VZV ORF63 transcript levels in human cadaver TG increased with longer PMI.
TEXT
Primary varicella-zoster virus (VZV) infection causes varicella, after which the virus becomes latent in ganglia along the entire neuraxis; reactivation decades later results in herpes zoster (2). Studies of VZV latency have been restricted to analysis of human ganglia obtained at autopsy and have reported discrepant numbers and abundance of VZV transcripts (3–7, 9–11, 14, 16). The discrepancies may be due to methodological differences and/or the undefined (9–11), inexact (<24 h) (3, 5, 6, 14), and long (average, 17 to 25 h) (4, 15) postmortem intervals (PMI) in obtaining cadaver ganglia. Herein, we searched for VZV transcripts in human trigeminal ganglia (TG) acquired not only at the usual intervals (PMI of 9 to 24 h) but also at earlier time points after death (PMI of 3 to 9 h).
Multiplex RT-PCR reveals no VZV transcripts in human TG with a PMI of <9 h.
Our recent use of multiplex reverse transcriptase (RT-PCR) to examine human TG with an average PMI of 17 h revealed a wide variability among the VZV genes transcribed in human TG (16, 17). Here, we extended the TG cohort with 17 additional samples obtained early after death (mean PMI of 7.75 h; range of 3.7 to 23.25 h) (Table 1), resulting in a total of 43 TG from 25 donors with PMIs ranging from 3.7 to 24 h (Table 2). Poly(A) mRNA was extracted from ganglia, and 100 ng mRNA was reverse transcribed and amplified in each of five multiplex PCRs covering all 68 VZV open reading frames (ORFs) (16). No VZV transcripts were detected with a PMI of <9 h, while the cellular control transcripts GAPdH, neurofilament (NF), and β-actin were detected in all samples by reverse transcriptase-linked real-time quantitative PCR (RT-qPCR) (Table 2 and data not shown). In TG with a PMI of 9 h or more, transcripts mapping to VZV immediate-early ORFs 63 (65%), 4 (42%), and 62 (39%); early ORF 29 (32%); or late ORFs 11 (23%), 68 (19%), 40 (13%), 43 (6%), 41 (3%), and 57 (3%) were detected (Table 2). The number of VZV genes transcribed correlated significantly with longer PMI (Fig. 1A) (r = 0.59, P < 0.0001; Pearson's correlation test).
Table 1.
Clinical features of human trigeminal ganglia donors
| Subject | Age; sexb | Cause of death | PMIa |
|---|---|---|---|
| A | 87; M | Cachexia and dehydration | 03:40 |
| B | 91; F | Dementia | 03:45 |
| C | 83; F | Dehydration | 04:55 |
| D | 90; F | Cachexia and dehydration | 05:35 |
| E | 62; M | Brain tumor | 07:43 |
| F | 74; M | Suicide | 07:45 |
| G | 44; M | Cachexia and sedation | 10:15 |
| H | 51; M | Unknown | 11:00 |
| I | 44: M | Pneumonia | 12:00 |
| J | 59; M | Cardiac arrest | 12:30 |
| K | 90; F | Unknown | 23:16 |
PMI, postmortem interval (hours:minutes).
M, male; F, female.
Table 2.
Detection of VZV transcripts in human trigeminal gangliaa
| PMIc | Subjectd | Locatione | Multiplex RT-PCR result |
RT-qPCR result |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IEb |
Earlyb |
Lateb |
|||||||||||
| 63f | 4 | 62 | 29 | 11 | 68 | 40 | 43 | 41 | 57 | ORF63 | |||
| 03:40 | A | R | − | − | − | − | − | − | − | − | − | − | + |
| A | L | − | − | − | − | − | − | − | − | − | − | + | |
| 03:45 | B | R | − | − | − | − | − | − | − | − | − | − | − |
| B | L | − | − | − | − | − | − | − | − | − | − | − | |
| 04:55 | C | R | − | − | − | − | − | − | − | − | − | − | + |
| C | L | − | − | − | − | − | − | − | − | − | − | + | |
| 05:35 | D | R | − | − | − | − | − | − | − | − | − | − | − |
| D | L | − | − | − | − | − | − | − | − | − | − | + | |
| 07:43 | E | R | − | − | − | − | − | − | − | − | − | − | + |
| E | L | − | − | − | − | − | − | − | − | − | − | + | |
| 07:45 | F | R | − | − | − | − | − | − | − | − | − | − | − |
| F | L | − | − | − | − | − | − | − | − | − | − | − | |
| 9:00 | 12 | R | − | + | − | − | − | + | − | − | − | − | + |
| 12 | L | − | − | − | − | − | + | − | − | + | − | + | |
| 10:15 | G | L | − | + | + | − | − | − | − | − | − | − | + |
| 11:00 | 9 | R | − | − | − | − | − | − | − | − | − | − | + |
| 11:00 | H | L | − | + | + | − | − | − | − | − | − | − | + |
| 12:00 | 6 | R | + | − | + | + | − | + | − | + | − | − | + |
| 6 | L | + | − | − | − | − | + | − | + | − | − | + | |
| 12:00 | I | L | − | + | + | − | − | − | − | − | − | − | + |
| 12:30 | J | L | + | + | + | − | − | − | − | − | − | − | + |
| 14:00 | 3 | R | − | − | − | − | − | − | − | − | − | − | + |
| 3 | L | − | − | − | − | − | − | − | − | − | − | + | |
| 14:00 | 13 | R | + | − | + | − | + | − | − | − | − | − | − |
| 13 | L | + | − | + | − | + | − | − | − | − | − | − | |
| 15:00 | 1 | R | − | − | − | − | − | − | − | − | − | + | + |
| 1 | L | − | − | − | − | − | − | − | − | − | − | + | |
| 17:00 | 14 | R | + | − | + | − | + | − | − | − | − | − | + |
| 14 | L | + | + | + | − | − | − | − | − | − | − | + | |
| 18:00 | 4 | R | + | − | − | − | − | − | − | − | − | − | − |
| 4 | L | + | − | − | + | − | − | + | − | − | − | + | |
| 18:00 | 7 | R | + | + | − | + | − | − | + | − | − | − | + |
| 7 | L | + | + | − | + | − | − | + | − | − | − | + | |
| 18:00 | 8 | R | + | + | + | − | + | − | − | − | − | − | − |
| 8 | L | + | + | + | + | + | − | − | − | − | − | − | |
| 22:00 | 2 | R | − | − | − | − | − | − | − | − | − | − | − |
| 23:00 | 10 | R | + | − | − | + | + | + | − | − | − | − | + |
| 10 | L | + | + | − | + | − | − | − | − | − | − | + | |
| 23:00 | 11 | R | + | − | − | + | − | + | − | − | − | − | + |
| 11 | L | + | + | − | + | − | − | − | − | − | − | − | |
| 23:16 | K | L | + | + | + | − | − | − | − | − | − | − | + |
| 24:00 | 5 | R | + | − | − | + | + | − | − | − | − | − | + |
| 5 | L | + | − | − | − | − | − | − | − | − | − | + | |
Viral transcripts were determined by VZV transcriptome-wide multiplex RT-PCR and VZV ORF63-specific real-time quantitative PCR (RT-qPCR; last column) on cDNA generated from human TG-derived RNA. +, transcript detected; −, transcript not detected.
Putative kinetic class of the VZV open reading frame with respect to HSV-1 (2, 18). IE, immediate early.
PMI, postmortem interval (hours:minutes).
See reference 16 for VZV multiplex RT-PCR data on subjects 1 to 14.
Left (L) or right (R) trigeminal ganglion.
VZV open reading frame.
Fig 1.
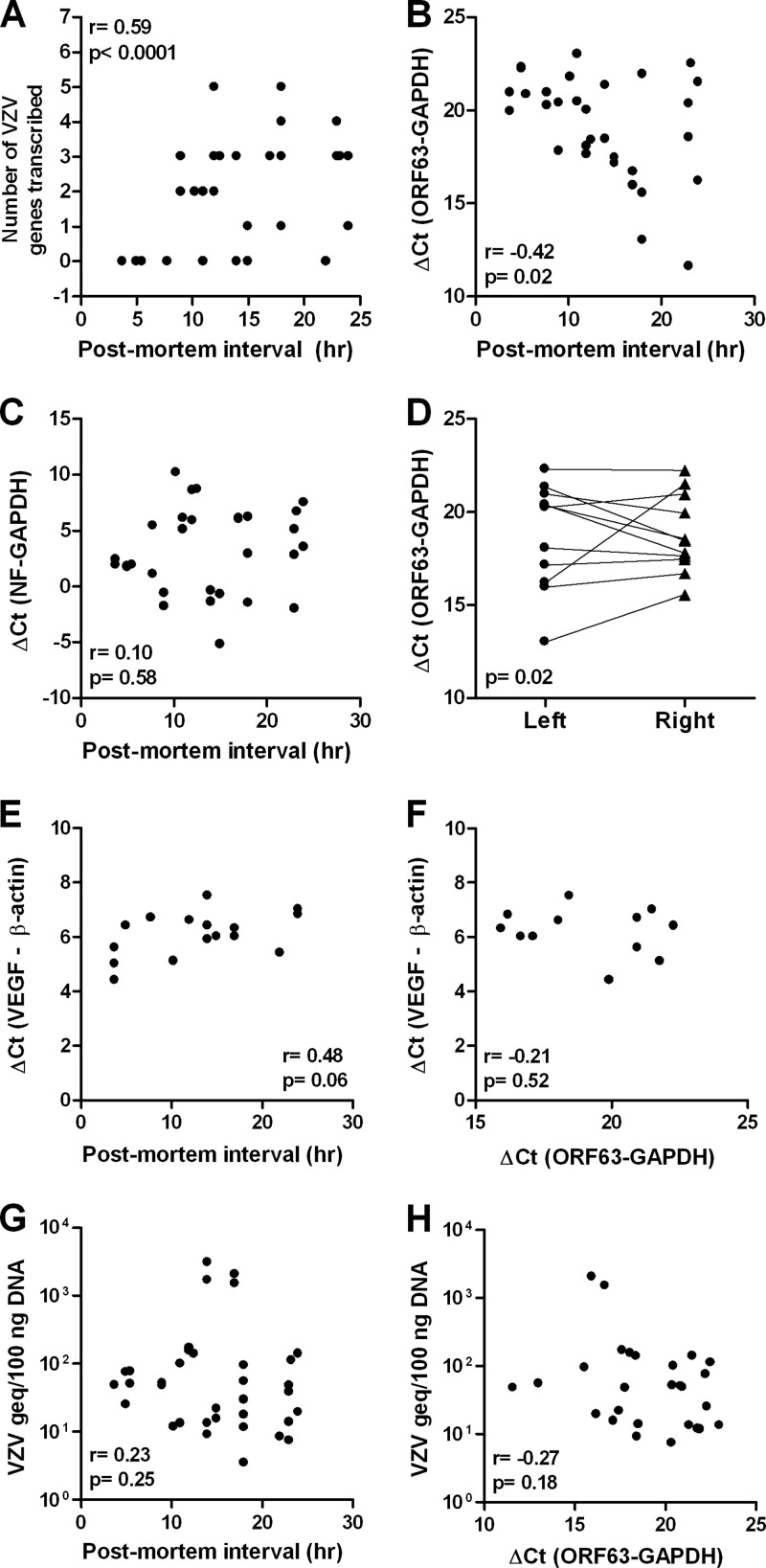
VZV ORF63 transcript levels, but not VZV DNA load, in human trigeminal ganglia (TG) correlate with the postmortem interval (PMI). (A) Correlation between PMI and number of VZV transcripts detected by multiplex RT-PCR; (B) correlation between PMI and abundance of ORF63 transcripts; (C) no correlation between PMI and abundance of neurofilament (NF) transcripts; (D) correlation of the abundance of ORF63 transcripts in paired left and right TG; (E) no correlation between vascular endothelial growth factor (VEGF) transcript levels and PMI; (F) no correlation between VEGF and ORF63 transcript levels; (G) no correlation between ganglionic VZV DNA load and PMI; or (H) abundance of ORF63 transcripts. Statistical analyses were performed using Pearson's correlation test (A to C, E, and F to H) and Wilcoxon matched-pair signed-rank test (D). Panel A and panels E and F contain VZV transcript data and VZV genome load levels, respectively, that in part have been published previously (see Table 2 and reference 16).
VZV ORF63 transcript levels in human TG increased with longer PMI.
VZV ORF63 is the most frequent and abundant VZV gene transcribed in latently infected human TG (3, 4, 9–11, 16). To determine whether VZV transcript abundance was affected by the PMI, cDNA was synthesized from 100 ng poly(A) mRNA extracted from all 43 TG and subjected to RT-qPCR analysis using primers and probes specific for VZV ORF63 along with cellular GAPdH and NF (4, 16). GAPdH and NF were amplified in all ganglia but not when reverse transcriptase was omitted from the cDNA synthesis reaction (data not shown). Consistent with earlier findings (3, 4, 9–11, 16), ORF63 was the most prevalent VZV transcript detected in latently infected human TG (Table 2). VZV ORF63 transcripts were detected in 31 of 43 (72%) ganglia (Table 2, last column), including those obtained when PMI was <9 h, and the abundance of VZV ORF63 transcripts increased significantly with longer PMIs (Fig. 1B) (r = −0.42, P = 0.02; Pearson's correlation test). There was no significant difference (P = 0.27; Fisher's exact test) in the number of TG that contained VZV ORF63 transcripts in donors with a PMI of <9 h (7 of 12 TG; 58%) compared to a PMI of ≥9 h (24 of 31 TG; 77%) (Table 2). The NF transcript levels were not affected by the PMI (Fig. 1C). There was no significant difference in the abundance of VZV ORF63 transcription in paired left and right TG from individual donors (Fig. 1D) (P = 0.02; Wilcoxon matched-pair signed-rank test), suggesting that the PMI-related increase of VZV ORF63 transcription found in paired TG reflects a general host response, which may involve cellular stress due to hypoxia, nutrient deprivation, or hypothermia (1). Vascular endothelial growth factor (VEGF) is a cellular gene product whose transcription is induced by hypoxia (13). Analysis of cDNA synthesized from 16 surplus TG mRNAs (12 donors) by RT-qPCR showed that the number of VEGF transcripts did not correlate with longer PMIs (Fig. 1E) (r = 0.48, P = 0.06; Pearson's correlation test) or with VZV ORF63 transcription (Fig. 1F) (r = −0.21, P = 0.52; Pearson's correlation test), indicating that the increase in VZV gene transcription is virus specific and not likely to be due to hypoxia.
No correlation of VZV DNA load in human TG with longer PMI.
The increased number of VZV transcripts (multiplex RT-PCR) and ORF63 transcript abundance (RT-qPCR) with longer PMI suggests postmortem VZV reactivation. Quantitative PCR analysis confirmed the presence of VZV DNA in 42 of 43 ganglia (VZV DNA was not detected in subject I), but the viral DNA load did not correlate with the PMI (Fig. 1G and data not shown). Thus, if VZV reactivated after death, reactivation had not yet advanced to virus DNA replication within the time frame investigated. Finally, the lack of correlation between VZV ORF63 transcript levels and ganglionic VZV DNA load (Fig. 1H) indicated that ORF63 transcription was independent of the latent VZV DNA burden.
This study is the first to investigate the entire VZV transcriptome in human ganglia with a short PMI. We detected no VZV transcription early (<9 h) after death by multiplex RT-PCR but found multiple VZV transcripts in TG at ≥9 h postmortem as well as an increased abundance of VZV ORF63 transcripts with longer PMI. Compared to multiplex RT-PCR, the RT-qPCR assay was more sensitive to detect ORF63 transcripts (Table 2; data not shown). The sensitivity of the multiplex RT-PCR assay is 1 to 10 copies for 82% of analyzed VZV transcripts and 100 to 500 copies for the remaining 18% of VZV transcripts (16, 17). Among the VZV transcripts detected in the TG analyzed in our current study (Table 2), 7 (70%) are detectable at 1 copy per sample and 2 (20%) up to 10 copies, and one transcript has been described to be detected from 100 copies/sample (Table 2) (16). Thus, differential VZV gene sensitivity of the multiplex RT-PCR assay did not account for the set of VZV transcripts detected in the TG analyzed. The data contrast with a previous study reporting no difference in simian varicella virus (SVV) transcription in ganglia removed immediately and 30 h after death, although ganglia from just 2 monkeys were analyzed for 7 transcripts and only at 2 postmortem intervals (12).
The current study raises an important question regarding ongoing low-level VZV gene transcription during latency or de novo VZV gene transcription after death. Until an appropriate in vitro culture model of VZV latency is developed, studies of VZV latency must be restricted to human ganglia (3–7, 9–11, 14, 16). While our data are consistent with continued VZV ORF63 transcription during latency with transcription of other VZV genes initiated only after death, a definitive answer would require analysis of human ganglia obtained during life, a situation not possible. The SVV nonhuman primate model of varicella latency provides an experimental setting to definitively determine the extent of virus transcription during latency (15).
Our data showed that the VZV immediate early genes are the most frequently transcribed in human TG (Table 2). However, the absence of increasing VZV DNA loads with longer PMI argues against viral replication that would be expected during VZV reactivation (Fig. 1E). No immediate explanation for the detection of multiple VZV transcripts at ≥9 h postmortem, but not apparent at earlier time points, can be provided but may reflect the epigenetic state of the VZV genome. We have previously shown that late VZV genes 14 and 36 are epigenetically silenced, whereas genes 63 and 62 are maintained in a euchromatic configuration in human TG (8). Future studies will address VZV genome-wide epigenetic modifications along with the detection and role of specific VZV transcripts, including ORF63, in human ganglia with a short versus long PMI.
ACKNOWLEDGMENTS
This work was supported in part by the Public Health Service grants AG032958 (W.J.D.O., D.G., R.J.C., G.M.G.M.V.), AG006127 (D.G.), and NS067070 (M.A.N.) from the National Institutes of Health.
The TG specimens, provided by the Netherlands Brain Bank (Netherlands Institute for Neuroscience; Amsterdam), were collected from donors from whom a written informed consent for brain autopsy and the use of the material and clinical information for research purposes had been obtained.
Footnotes
Published ahead of print 27 June 2012
REFERENCES
- 1. Birdsill AC, Walker DG, Lue L, Sue LI, Beach TG. 2011. Postmortem interval effect on RNA and gene expression in human brain tissue. Cell Tissue Bank 12:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen JI, Straus SE, Arvin AM. 2007. Varicella-zoster virus replication, pathogenesis, and management, p 2773–2818 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 3. Cohrs RJ, Barbour M, Gilden DH. 1996. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J. Virol. 70:2789–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohrs RJ, Gilden DH. 2007. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J. Virol. 81:2950–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PG. 2003. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J. Virol. 77:6660–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohrs RJ, et al. 1994. Varicella-zoster virus (VZV) transcription during latency in human ganglia: construction of a cDNA library from latently infected human trigeminal ganglia and detection of a VZV transcript. J. Virol. 68:7900–7908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Croen KD, Ostrove JM, Dragovic LJ, Straus SE. 1988. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc. Natl. Acad. Sci. U. S. A. 85:9773–9777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gary L, Gilden DH, Cohrs RJ. 2006. Epigenetic regulation of varicella-zoster virus open reading frames 62 and 63 in latently infected human trigeminal ganglia. J. Virol. 80:4921–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kennedy PG, Grinfeld E, Bell JE. 2000. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J. Virol. 74:11893–11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kennedy PG, Grinfeld E, Gow JW. 1999. Latent varicella-zoster virus in human dorsal root ganglia. Virology 258:451–454 [DOI] [PubMed] [Google Scholar]
- 11. Kennedy PG, Grinfeld E, Gow JW. 1998. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc. Natl. Acad. Sci. U. S. A. 95:4658–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahalingam R, et al. 2010. Effect of time delay after necropsy on analysis of simian varicella-zoster virus expression in latently infected ganglia of rhesus macaques. J. Virol. 84:12454–12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marti HH, Risau W. 1998. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc. Natl. Acad. Sci. U. S. A. 95:15809–15814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meier JL, Holman RP, Croen KD, Smialek JE, Straus SE. 1993. Varicella-zoster virus transcription in human trigeminal ganglia. Virology 193:193–200 [DOI] [PubMed] [Google Scholar]
- 15. Messaoudi I, et al. 2009. Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans. PLoS Pathog. 5:e1000657 doi:10.1371/journal.ppat.1000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagel MA, et al. 2011. Varicella-zoster virus transcriptome in latently infected human ganglia. J. Virol. 85:2276–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagel MA, Gilden D, Shade T, Gao B, Cohrs RJ. 2009. Rapid and sensitive detection of 68 unique varicella zoster virus gene transcripts in five multiplex reverse transcription-polymerase chain reactions. J. Virol. Methods 157:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roizman B, Knipe DM, Whitley RJ. 2007. Herpes simplex viruses, p 2501–2601 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]