Abstract
Simian immunodeficiency viruses infecting western lowland gorillas (SIVgor) are closely related to HIV-1 and are most likely the ancestors of HIV-1 groups O and P. At present, limited data are available on genetic diversity, transmission, viral evolution, and pathogenicity of SIVgor in its natural host. Between 2004 and 2011, 961 putative gorilla fecal samples were collected at the Campo Ma'an National Park, Cameroon. Among them, 16% cross-reacted with HIV-1 antibodies, corresponding to at least 34 infected gorillas. Combining host genotyping and field data, we identified four social groups composed of 7 to 15 individuals each, with SIV rates ranging from 13% to 29%. Eleven SIVgor-infected gorillas were sampled multiple times; two most likely seroconverted during the study period, showing that SIVgor continues to spread. Phylogenetic analysis of partial env and pol sequences revealed cocirculation of closely related and divergent strains among gorillas from the same social group, indicating SIVgor transmissions within and between groups. Parental links could be inferred for some gorillas infected with closely related strains, suggesting vertical transmission, but horizontal transmission by sexual or aggressive behavior was also suspected. Intrahost molecular evolution in one gorilla over a 5-year period showed viral adaptations characteristic of escape mutants, i.e., V1V2 loop elongation and an increased number of glycosylation sites. Here we show for the first time the feasibility of noninvasive monitoring of nonhabituated gorillas to study SIVgor infection over time at both the individual and population levels. This approach can also be applied more generally to study other pathogens in wildlife.
INTRODUCTION
Chimpanzees and gorillas are the only nonhuman primates known to harbor viruses closely related to HIV-1 (22, 40, 56). Phylogenetic analyses showed that gorillas acquired the simian immunodeficiency virus SIVgor from chimpanzees (50), and SIVcpz/SIVgor strains have been transmitted to humans on at least four occasions, leading to HIV-1 groups M, N, O, and P. West Central African chimpanzees (Pan troglodytes troglodytes) infected with SIVcpzPtt in southern Cameroon are recognized as the reservoir of the ancestors of pandemic HIV-1 group M and of HIV-1 group N (22). SIVgor from western lowland gorillas (Gorilla gorilla gorilla) is closely related to the two other HIV-1 lineages, i.e., group O and group P (34, 56). Group O represents 1% of HIV-1 infections in West Central Africa, and group P was recently shown to infect two Cameroonian patients (39, 42, 52, 57). The direct ancestors of HIV-1 group O have not been identified yet, but the short genetic distance between HIV-1 group P and SIVgor suggests that a zoonotic transmission of SIV from gorillas to humans has occurred (34, 42).
SIVgor infection was reported for the first time in 2006, when it was isolated from fecal samples from three gorillas living about 400 km apart in Cameroon (56). Between 2006 and 2008, a comprehensive survey was conducted to determine the geographic distribution and prevalence of SIVgor in wild gorilla populations in southern Cameroon (34). This study was conducted on fecal samples from more than 1,200 gorillas from 21 locations and showed that SIVgor infection is much less widespread than SIVcpzPtt infection in chimpanzees. SIVgor infection was found at only 3 sites, whereas SIVcpzPtt infection was identified at 10 locations. Moreover, the overall SIV prevalence in gorillas was 1.6% (ranging from 0% to 4.6%), which is significantly lower than the average prevalence of 5.9% (ranging from 0% to 32%) obtained for chimpanzees. However, a closer look at the locations where the SIVgor infection rate reached almost 5% showed that a quarter of the individuals belonging to selected social groups were infected with this virus.
Our knowledge of the consequences of SIV infection on the health of wild-living ape populations is limited to a few studies on chimpanzees, and at present we have no information on the impact of SIVgor infection on gorillas. Only one long-term study, initiated more than 10 years ago on a few habituated communities of East African chimpanzees (P. t. schweinfurthii) living in Gombe National Park, Tanzania, provided evidence that SIVcpzPts infection has a negative impact on the health, reproduction, and survival of chimpanzees in the wild and can cause the decline of chimpanzee populations (21, 44). SIVcpzPtt infecting P. t. troglodytes can also lead to an AIDS-like disease in this subspecies, as documented in a recent report of a naturally infected chimpanzee rescued in Cameroon (13). Since gorillas acquired SIV only recently, by cross-species transmission from chimpanzees (50), we can hypothesize that SIV infection may also have a negative health impact on lowland gorilla populations. However, there are no studies to date that have included G. g. gorilla habituation to humans and long-term health monitoring of these populations. Studies to characterize SIVgor infection in its natural host in more detail are highly needed but are particularly challenging in light of the elusive nature of this species, its endangered status, and the documented constant threat of poaching and human disturbance (63). During our previous exploratory surveys, we identified 13 SIVgor-infected gorillas in a relatively small territory of the Campo Ma'an National Park in southwestern Cameroon (34). We therefore decided to focus our efforts on the nonhabituated gorilla groups living in this area and to determine the feasibility of long-term monitoring of SIV infection in these apes by collecting fecal samples over time and genotyping the SIVgor-positive samples and a subset of negative ones at selected microsatellite loci. This follow-up study allowed us not only to characterize new viral strains but also to document potential routes of viral transmission within and between gorilla groups. Furthermore, sequential sampling of the same infected individuals enabled us to document viral evolution and adaptation. Finally, we show for the first time that it is possible to sample and resample the same gorillas in a noninvasive way and thus to study their viral infections. In the future, information obtained from such long-term studies will be crucial to understanding the evolution and pathogenicity of SIVgor, one of the precursors of HIV-1.
MATERIALS AND METHODS
Study site and sample collection.
The Campo-Ma'an National Park was created in 2000 and covers 2,640 km2 of primary and mangrove forests in southwestern Cameroon, spanning an area from the Atlantic coast to roughly 100 km inland (62). We conducted our follow-up study in an area of approximately 200 km2 at the center of the Campo-Ma'an National Park (CP), where previous surveys showed a relatively high rate of SIVgor infection in wild gorillas (34). In this follow-up study, the three previously described zones of collection (CP-GR, CP-OV, and CP-MV) were pooled together and were considered a single site, as they are located less than 10 km from each other and SIVgor-positive individuals were identified at all three locations. A total of 12 sampling sessions were conducted between April 2004 and November 2011, including four previously reported sessions from our prospective studies (34, 56). At each sampling session, three field teams collected gorilla fecal samples from locations around night nests, on the ground, at feeding sites, or directly on track. At each collection, the GPS position and estimated time of fecal deposition were recorded. Fecal samples collected on the same day, at the same GPS location, and with the same estimated time of deposition were defined as a cluster of collected samples and were assumed to be from the same gorilla group, especially at nesting sites (1). Sequential numbers were assigned to these clusters of collected samples during the 7.5-year study period (clusters 01 to n). The species origin was inferred in the field, according to cues such as the type of nest, footprints, texture, and odor of the samples. About 20 g of dung was collected in a 50-ml tube containing 20 ml of RNAlater (Ambion, Austin, TX), kept at ambient temperature at base camp for a maximum of 3 weeks, and then stored at −80°C.
Detection of SIVgor antibodies in fecal samples from gorillas.
All collected fecal samples were tested with the Inno-Lia HIV score confirmation test (Innogenetics, Ghent, Belgium) for the presence of HIV-1-cross-reactive antibodies. Briefly, 2 ml of fecal sample was incubated with 7 ml phosphate-buffered saline (PBS)–Tween 20 for 1 h at 60°C, centrifuged at 3,900 × g for 10 min to clarify the solution, and dialyzed against PBS overnight at 4°C. One milliliter of reconstituted extract was then subjected to cross-reactive immunoblot analysis (56).
Nucleic acid extraction from ape fecal samples.
Total nucleic acids were extracted from all antibody-positive fecal samples by use of a NucliSens magnetic extraction kit (bioMérieux, Craponne, France) which utilizes magnetic silica particles (4). Briefly, 1.5 ml of sample was mixed with 7 ml of NucliSens lysis buffer for 1 min and incubated at room temperature for 1 to 12 h before centrifugation at 3,900 × g for 30 min (34). The supernatant was filtered through gauze and centrifuged at 3,900 × g for 5 min. The magnetic extraction procedure was then followed according to the manufacturer's instructions to obtain a final elution volume of 50 μl fecal RNA. A QIAamp stool DNA miniprep kit (Qiagen, Valencia, CA) was used to extract fecal DNA for species confirmation and microsatellite analyses. Two milliliters of fecal sample was used to obtain a final elution volume of 100 μl of fecal DNA (22).
Species and subspecies determination.
In a previous study, we showed that the species determination in the field matched 97.3% of the time with host mitochondrial DNA (mtDNA) sequence results (34). Nevertheless, for all seropositive fecal samples and a subset of SIV-negative fecal samples, the species origin was confirmed by mtDNA analyses as described previously (22, 53, 56). Briefly, an ∼450- to 500-bp fragment spanning the hypervariable D-loop region was amplified using primers L15997 and H16498 and/or a 386-bp fragment spanning the 12S gene was amplified using primers 12S-L1091 and 12S-H1478. Phylogenetic analyses of these sequences allowed identification of gorilla samples. If both amplification strategies yielded no results, samples were considered degraded.
Identification of individuals.
To identify each SIV-positive individual and determine the number of SIVgor-infected gorillas, microsatellite analyses were performed on all seropositive samples (34, 56). Furthermore, negative samples collected at the same location as SIV-positive samples between April 2004 and November 2010 were also subjected to microsatellite analyses. Samples were genotyped at seven loci in two multiplex PCRs (amplifying D18s536, D4s243, D10s676, and D9s922 or D2s1326, D2s1333, and D4s1627), and for gender determination, a region of the amelogenin gene that contains a deletion in the X but not the Y chromosome (48) was amplified using a Taq DNA polymerase core kit (MP Biomedical, Irvine, CA) with 2 to 10 μl fecal DNA. Homozygous loci were amplified three to seven times to minimize allelic dropout (3, 33, 49), and when multiplex PCRs yielded poor results, fecal DNA was extracted again and a new set of PCRs was performed. We discarded all samples that did not provide successful results after five PCR attempts and two independent DNA extractions. All samples that displayed an incomplete allelic profile (fewer than four loci), a multiple-peak profile for the same locus, or discordant results in subsequent analyses were also discarded from further analyses. The allele size data from successful amplifications were compared against each other and with those for previously genotyped individuals (34, 56).
We used Cervus v3.0 (20) and Genecap v3.1 (60) to assess allele frequency, expected heterozygosity, polymorphic information content (PIC), Hardy-Weinberg (HW) equilibrium, and sibling identity, for parentage analysis, and to identify SIV-positive samples with matching genotypes (5, 19, 35). All genotypes mismatching at one locus were checked for data entry errors or allelic dropout possibilities (17). To guard for potential allelic dropout, we allowed an allelic mismatch at one locus, but only if it represented a missing allele (45). SIV-positive matching samples were given a consensus ID number and genotype if their P(ID)sib value was <0.05, corresponding to at least 95% confidence that two matching samples originated from the same individual (assuming that the individuals from the panel could be full siblings) (61). Both P(ID)HW and P(ID)sib were calculated for each individual (14, 38, 58). P(ID)HW is the probability that two individuals drawn at random from a population will have the same genotype at multiple loci, whereas P(ID)sib assumes that individuals are full siblings. In this case, P(ID)sib is more informative for samples from individuals with a possible full sibling (genetically linked individuals) and from nonrelated gorillas in the study; the P(ID) to consider is between P(ID)sib and P(ID)HW. We assumed that the loci we chose for these analyses are inherited independently (i.e., they are unlinked). For parentage analysis, we first calculated allele frequencies, which are critical to run simulations and for assessing the confidence of parentage assignment. Cervus calculates the likelihood ratio, which is the likelihood that the candidate parent is the true parent divided by the likelihood that the candidate parent is not the true parent. Cervus calculates this ratio for each candidate parent selected, taking into account possible typing errors. We estimated that we collected and successfully genotyped half of the offspring in every group, as well as half of the candidate parents. The frequency of typing errors and the error rate in likelihood calculations were set at 0.01. The minimum number of typed loci was set at four (of seven loci), and the proportion of loci typed was 0.83. Confidence in assignment is defined as the proportion of all candidate parents with limit-of-detection (LOD) scores exceeding a given LOD score that are true parents. Simulations of parentage analysis reported that any candidate parent with an LOD score exceeding a critical value of 2.94 could be assigned parentage with 95% confidence and that those with an LOD score of 2 could be assigned parentage with a relaxed confidence of 80%.
Amplification of partial SIVgor sequences in env and pol conserved regions from fecal RNA.
To amplify SIVgor fragments, reverse transcription-PCR (RT-PCR) was performed on fecal RNA, using SIVgor-specific and SIVgor/SIVcpz/HIV-1 consensus primers targeting env (gp41 ectodomain; 315 or 440 bp) and pol (245 or 330 bp) as previously reported (34). To increase the amplification success, a 200-bp fragment in gp41 was amplified with the following more-specific primers designed from an alignment of available gp41 sequences previously obtained from SIVgor strains infecting gorillas in the Campo Ma'an Park: F1, 5′-GCT WGC YRT AGA AAG BTA YCT A-3′; R1, 5′-TCC AAT TCY AGY ARG GCY YTT TCA T-3′; F2, 5′-TCC TRG GAC TRT GGG GHT G-3′; and R2, 5′-TCA TTM TYH TCC TGY TGT CCY TGT GC-3′. Reverse transcription was carried out with 10 μl of fecal RNA and 40 pmol of R1 primer, using Expand reverse transcriptase (Roche Diagnostics, Indianapolis, IN) or Superscript Retro-Transcriptase III (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions, with minor adjustments as previously described (34). PCRs were then performed with the Expand Long Template PCR system (Roche Diagnostics, Indianapolis, IN) or a Taq DNA polymerase core kit (MP Biomedical, Irvine, CA) following the manufacturer's instructions. Briefly, 10 μl of cDNA was used for first-round PCR amplification with the R1 and F1 primers, and 5 μl of the first-round reaction product was used for nested PCR with the second-round primers, i.e., F2 and R2. Thermocycling conditions could vary slightly, with annealing temperatures ranging from 45°C to 55°C, using a touch-down PCR strategy, and extension times were typically set at 1 min/kb. The resulting amplification products were purified using a Geneclean Turbo kit (Qbiogene, Inc., Carlsbad, CA) and sequenced directly using an automated sequencer (model 3130xl genetic analyzer; Applied Biosystems, Foster City, CA). If at least one sample from a given SIV-positive individual yielded RT-PCR-positive amplification, we considered the serological status of this individual to be confirmed by viral RNA amplification. We performed 2 to 12 independent RNA extractions for each SIV-positive individual, followed by 5 to 10 RT-PCR attempts for each RNA extract, with various conditions and reagents as described above.
Amplification and molecular cloning of SIVgor gp120 V1V4 region.
To document the viral diversity and evolution in the envelopes of SIVgor strains, we studied the V1V4 region of gp120 (env) of the virus infecting gorilla ID1 over a 5-year period. We amplified a fragment spanning the env V1V4 region (∼1,000 bp) by using specific primers on two samples: SIVgor-04CP684, infecting gorilla ID1 in 2004, and SIVgor-08CP3425, infecting the same animal in 2008. The primer sets were as follows: 684envR1 (5′-CCT TGT AGG GGT ACA ATT CAC TC-3′) and 684/3425envF1 (5′-TGG GAT CAG AGC TTA AAG CC-3′) for the first-round SIVgor-04CP684 env V1V4 PCR (∼1,150 bp), 684envR2 (5′-TCA GCC AGG GGT GTA TCT CTT T-3′) and 684/3425envF2 (5′-AGC CTT GTG TAA AAC TAA CAG TC-3′) for the second-round SIVgor-04CP684 env V1V4 PCR (∼1,020 bp), 3425envR1 (5′-ATA GGC CTG AGC CTA CTC TCA T-3′) and 684/3425envF1 for the first-round SIVgor-08CP3425 env V1V4 PCR (∼990 bp), and 3425envR2 (5′-ACC TGT TTT AGG CGG CAT GGA A-3′) and 684/3425envF2 for the second-round SIVgor-08CP3425 env V1V4 PCR (∼950 bp). The amplified and gel-purified products were cloned by use of the pGEM-T system following the manufacturer's instructions (pGEM-T Easy vector system II; Promega, Madison, WI). We sequenced 20 and 24 SIV clones for SIVgor-04CP684 and SIVgor-08CP3425, respectively, to analyze the viral envelope diversity and evolution.
Phylogenetic and genetic diversity analyses of SIVgor sequences.
All new SIVgor nucleotide sequences were compared to SIVgor, SIVcpz, and HIV-1 reference sequences available in the GenBank database. MEGA5 (51) was used to align sequences and to edit them where necessary. Ambiguously aligned sites were excluded from the analyses. We analyzed 220 nucleotides in the partial pol alignment and 263 bp and 195 bp in gp41 alignments. We used a codon nucleotide alignment of 918 bp for the env V1V4 region. Phylogenies were inferred by maximum likelihood, using PhyML (16) and 1,000 bootstrap replicates. According to Topali (32), the best evolution models were HKY85 with a gamma distribution across sites for the small pol and gp41 fragments and GTR+G for the V1V4 alignment. Sequences (GenBank accession numbers) for additional partial pol and gp41 sequences used in comparative analyses were as follows: for SIVgor, sequences DJ3795 (FN554935), DJ4099 (FN554936, FN554958), DJ4112 (FN554957), and BQ664 (AM296484, AM296488); for SIVcpzPtt, MB66 (DQ373063); for SIVcpzPts, TAN1 (AF447763); for HIV-1 group O, MVP5180 (L20571) and ANT70 (L20587); and for HIV-1 group P, RBF168 (GQ328744) and U14788 (HQ179987).
Viral diversity in the SIVgor-04CP684 and SIVgor-08CP3425 env V1V4 regions in 2004 and 2008, respectively, was determined by the mean pairwise nucleotide and amino acid distances between env clones at each time point, using MEGA5 with the maximum composite likelihood method; the minimum, maximum, and standard deviation (SD) were also calculated. The cumulative number of nonsynonymous and synonymous nucleotide substitutions (dN and dS, respectively) was estimated using SNAP (23). Amino acid sequence length and the number of putative N-linked glycosylation sites (PNGS) of SIVgor-04CP684 and SIVgor-08CP3425 V1V4 clones were calculated, and the means at each time point were compared. The Mann-Whitney U test was used to assess statistical differences in viral diversity, sequence length, and PNGS between 2004 and 2008.
Nucleotide sequence accession numbers.
All of the new SIVgor sequences are available at GenBank under accession numbers JQ924140 to JQ924164 for env V1V4 clones, JQ924165 to JQ924181 for env (gp41), and JQ924182 to JQ924187 for the pol fragment.
RESULTS
Detection of 34 SIV-infected wild-living gorillas during long-term noninvasive monitoring in the Campo Ma'an National Park.
In two previous studies, we identified 13 SIV-infected gorillas in the center of the Campo Ma'an National Park in southwestern Cameroon: 2 individuals were detected between April 2004 and March 2006 (56), and 11 were detected in subsequent field surveys between June 2006 and April 2008 (34). Given the relatively high SIVgor infection rate observed previously in some gorilla groups, we increased our sampling efforts in this region. Since April 2004, a total of 1,001 putative gorilla fecal samples were collected during 12 independent missions. Gorilla dung was collected as clusters of samples at nest sites (50 clusters of 2 to 28 fecal samples per cluster), at feeding sites, or on track (67 clusters of 2 to 20 fecal samples), and 46 individually isolated fecal samples were also collected. The host species was confirmed by mtDNA analysis of 437 (44%) of 1,001 putative gorilla fecal samples. Fifteen samples were degraded, and 25 were incorrectly assigned in the field (i.e., chimpanzee, monkey, African buffalo, deer, and elephant samples). The results showed 94% concordance between field observations and host mtDNA analysis.
A total of 152 samples (16% of the remaining 961 putative gorilla fecal samples) cross-reacted with HIV-1 antibodies in serological analyses; 34 of these came from previous studies (34, 56), and 118 were obtained during the follow-up missions that started at the end of 2008. To ascertain how many individuals were infected with SIVgor, we ran host microsatellite analyses of seven selected loci. We also determined the sex of the gorilla for each SIV-positive sample. Overall, the microsatellite loci used were polymorphic (mean PIC, 0.73), with a mean number of alleles per locus of 8.86 and an observed heterozygosity ranging from 0.76 to 0.9 (see Table S1 in the supplemental material). For 131 of 152 positive fecal samples, usable genotypes were obtained, resulting in an 86% success rate for these SIV-positive samples. We compared and combined the locus profiles of the 131 samples. For each SIV-infected gorilla, Table 1 shows the consensus genetic profile and the matching probabilities P(ID)HW and P(ID)sib, including those for the 13 previously reported individuals (56). We found that at least 34 SIV-positive gorillas were circulating in the studied area.
Table 1.
SIVgor-positive gorillas detected in the Campo Ma'an National Park from April 2004 to November 2011
| Individual (CPg ID) | Collection informationa |
Serology result (Inno-Lia HIV-1/2 test)b | SIV-specific PCR resultc |
Genetic profile (consensus multilocus genotype)d |
Sexe | P(ID) HWf | P(ID) Sibf | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group or cluster | Collection details | Collection date (mo/yr) | Collected sample(s) | Pol | Gp41 | Other | Locus D18S536 | Locus D4S243 | Locus D10S676 | Locus D9S922 | Locus D2S1326 | Locus D2S1333 | Locus D4S1627 | |||||
| ID1 | E | t | 04/04 | CP684, CP685 | + | + | + | FL | 142/146 | 185/193 | 180/192 | 280/280 | 251/263 | 298/318 | 234/274 | F | ** | ** |
| 5n | 12/08 | CP3417 | + | − | − | |||||||||||||
| t | 12/08 | CP3425, CP3426 | + | − | + | env (1,674 bp) | ||||||||||||
| t | 12/08 | CP3428 | + | − | + | |||||||||||||
| ID32 | E | t | 04/04 | CP686 | − | − | − | 146/154 | 193/193 | 180/192 | 280/280 | 251/271 | 298/334 | 234/− | M | * | * | |
| 5n | 12/08 | CP3416 | + | − | + | |||||||||||||
| ID2 | F1 (cf. ID36) | n | 04/06 | CP1434, CP1436 | + | − | + | gp41-nef (1,237 bp) | 146/154 | 173/193 | 196/200 | 276/280 | 255/267 | 298/298 | 234/242 | F | * | * |
| F3 | t | 10/10 | CP6101 | + | − | − | ||||||||||||
| ID70 | F3 | t | 10/10 | CP6097, CP6098, CP6099, CP6100 | + | + | + | 146/146 | 173/189 | 192/196 | 276/280 | 259/271 | 306/314 | 230/238 | M | ** | ** | |
| t | 10/10 | CP6102, CP6103 | + | − | − | |||||||||||||
| ID3 | 02/07 | CP2071 | + | − | − | 146/150 | 181/193 | 196/200 | 268/280 | − | 314/334 | 234/238 | F | |||||
| ID4 | A | n | 02/07 | CP2072, CP2074 | + | + | + | 146/146 | 185/193 | 196/200 | 260/268 | 267/275 | 322/334 | 234/274 | F | * | * | |
| 12n | 02/07 | CP2109, CP2117, CP2118 | + | + | + | |||||||||||||
| 8n | 04/10 | CP5760, CP5761 | + | − | − | |||||||||||||
| t | 04/10 | CP5806 | + | − | − | |||||||||||||
| t | 04/10 | CP5815 | + | − | − | |||||||||||||
| t | 04/10 | CP5851 | + | − | − | |||||||||||||
| 9n | 03/11 | CP6435, CP6437, CP6438 | + | − | − | |||||||||||||
| t | 03/11 | CP6451 | + | − | − | |||||||||||||
| t | 03/11 | CP6473, CP6477 | + | − | − | |||||||||||||
| t | 03/11 | CP6481 | + | − | − | |||||||||||||
| 8n | 03/11 | CP6485, CP6486, CP6495 | + | − | + | |||||||||||||
| 6n | 05/11 | CP6640, CP6641 | + | − | − | |||||||||||||
| t | 05/11 | CP6682 | + | − | + | |||||||||||||
| ID5 | A | 12n | 02/07 | CP2110, CP2111, CP2119, CP2123 | + | − | + | 146/150 | 177/185 | 192/200 | 260/280 | 251/267 | 322/326 | 234/246 | F | ** | ** | |
| 8n | 04/10 | CP5752, CP5803, CP5804, CP5807 | + | − | − | |||||||||||||
| t | 04/10 | CP5849 | + | − | − | |||||||||||||
| 9n | 03/11 | CP6442 | + | − | − | |||||||||||||
| t | 03/11 | CP6453 | + | − | − | |||||||||||||
| t | 03/11 | CP6465, CP6466 | + | − | + | |||||||||||||
| t | 03/11 | CP6476, CP6478 | + | − | − | |||||||||||||
| 8n | 03/11 | CP6488, CP6489 | + | − | − | |||||||||||||
| t | 05/11 | CP6685 | + | − | − | |||||||||||||
| ? | A | t | 05/11 | CP6684 | + | − | − | 146/− | − | − | 268/− | − | 298/− | − | − | |||
| ID65 | A | t | 04/10 | CP5816 | + | − | − | 146/146 | 185/193 | 196/196 | 268/280 | 247/275 | 322/334 | 234/274 | M | ** | ** | |
| t | 04/10 | CP5856, CP5857 | + | − | − | |||||||||||||
| 8n | 03/11 | CP6484 | + | − | − | |||||||||||||
| ID19 | A | 12n | 02/07 | CP2120, CP2121 | − | ND | ND | 146/154 | 181/185 | 192/196 | 264/264 | 255/271 | 318/318 | 242/242 | F | * | * | |
| t | 04/10 | CP5838, CP5839, CP5839bis, CP5848 | +/− | − | − | |||||||||||||
| ? | A | t | 04/10 | CP5802 | + | ND | ND | 146/154 | − | 196/− | 268/− | 251/− | − | − | − | |||
| ID6 | 11 | 12n | 02/07 | CP2094, CP2095 | + | + | − | 146/150 | − | 196/200 | 268/280 | 267/275 | 322/334 | 242/246 | F | * | * | |
| ID7 | 11 | 12n | 02/07 | CP2098 | + | − | − | 146/150 | 185/185 | 196/200 | − | 251/287 | − | 242/242 | F | |||
| ID8 | 11 | 12n | 02/07 | CP2087 | + | − | − | 146/150 | − | 196/200 | − | 267/271 | 354/374 | − | − | |||
| ID9 | C | t | 02/07 | CP2139 | + | + | + | FL | 146/146 | 177/189 | 192/200 | 276/280 | 251/271 | 318/334 | 242/242 | F | ||
| ID10 | C | t | 02/07 | CP2126, CP2132, CP2133, CP2134, CP2135, CP2141 | + | + | + | FL | 146/154 | 173/193 | 180/180 | 276/280 | 255/271 | 298/322 | 230/234 | F | ** | ** |
| 64 (=C?) | 17n | 11/11 | CP6990, CP6991, CP6998 | + | − | − | ||||||||||||
| ID75 | 64 (=C?) | 17n | 11/11 | CP6988 | + | − | − | 146/150 | 173/193 | − | 264/280 | − | 294/322 | 242/242 | F | |||
| ID76 | 64 (=C?) | 17n | 11/11 | CP6989, CP6992 | + | − | + | 146/150 | 193/193 | 196/200 | 280/280 | 267/271 | −/322 | 242/242 | F | |||
| ID77 | 64 (=C?) | 17n | 11/11 | CP7001, CP7002 | + | − | − | 146/158 | 177/181 | 196/200 | 268/272 | 251/271 | 334/334 | 242/274 | F | ** | ** | |
| ID11 | X | t | 11/07 | CP2740, CP2744, CP2746, CP2747, CP2749 | + | − | − | 146/150 | 181/189 | 196/200 | 264/280 | − | 314/318 | 234/234 | M | ** | ** | |
| ID12 | 15 | t | 04/08 | CP2994 | + | − | − | 150/154 | 185/185 | 184/196 | 268/280 | 267/267 | 298/306 | − | F | |||
| ID13 | 16 | 1n | 04/08 | CP3018, CP3019 | + | − | − | 150/154 | 185/185 | 180/180 | 268/280 | − | − | − | M | |||
| ID30 | 20 | 1n | 12/08 | CP3400, CP3401, CP3402, CP3403, CP3404, CP3405, CP3406 | + | + | + | 146/146 | 185/189 | 180/196 | 268/272 | 267/271 | − | 234/242 | M | * | * | |
| 3n | 12/08 | CP3407 | + | − | + | |||||||||||||
| ID31 | 20 | 3n | 12/08 | CP3408, CP3409, CP3410 | + | − | + | 146/146 | 173/189 | 180/196 | 276/280 | 255/267 | 318/322 | 242/274 | M | * | * | |
| t | 12/08 | CP3411 | + | − | + | |||||||||||||
| ID33 | G | t | 12/08 | CP3443 | + | + | + | 142/146 | 169/185 | 180/192 | 276/280 | 251/251 | 298/318 | 230/234 | F | |||
| ID34 | 24 | t | 12/08 | CP3468, CP3469 | + | − | + | gp41-nef (1,207 bp) | 146/150 | 193/193 | 192/200 | 264/276 | 251/255 | 298/334 | 230/242 | M | ** | ** |
| ID35 | F2 | t | 09/09 | CP4758 | + | − | + | 146/146 | 177/189 | 196/200 | 268/280 | 255/271 | 314/334 | 234/274 | M | * | * | |
| 61 (=F2?) | t | 11/11 | CP6970 | + | − | + | ||||||||||||
| ID73 | 61 (=F2?) | t | 11/11 | CP6971 | + | − | + | 146/146 | 177/181 | 196/200 | 264/280 | 267/275 | 322/334 | 242/246 | F | |||
| ID36 | F1 | t | 04/06 | CP1440 | − | − | − | 146/154 | 185/193 | 192/196 | 268/276 | 247/267 | 322/330 | 234/244 | F | ** | ** | |
| F2 | t | 09/09 | CP4759 | + | − | + | ||||||||||||
| ID37 | F2 | t | 09/09 | CP4760 | + | + | + | 146/154 | 177/193 | 192/196 | 264/268 | 251/267 | 334/334 | 234/234 | F | * | * | |
| t | 09/09 | CP4765, CP4766 | + | + | + | |||||||||||||
| 55 (=F2?) | t | 05/11 | CP6631, CP6635 | + | − | − | ||||||||||||
| ID38 | Single | 1n | 09/09 | CP4762, CP4763, CP4764 | + | + | + | 146/150 | 185/193 | 196/200 | 280/284 | 267/275 | 314/322 | 234/242 | M | ** | ** | |
| ? | 31 | 6n | 09/09 | CP4775 | + | − | − | 150/154 | − | − | 268/272 | − | − | 234/246 | M | |||
| ? | 31 | 6n | 09/09 | CP4776 | + | − | − | 142/146 | 189/189 | − | − | − | 298/322 | − | M | |||
| ? | 31 | 6n | 09/09 | CP4779 | + | − | + | 150/150 | − | − | − | − | −/334 | 242/242 | M | |||
| ID66 | 35 | 10n | 04/10 | CP5781 | + | − | + | 150/150 | 185/185 | 200/200 | 264/280 | 247/271 | 334/334 | 238/274 | M | * | * | |
| t | 04/10 | CP5813 | + | ND | ND | |||||||||||||
| t | 03/11 | CP6534 | + | − | + | |||||||||||||
| 11n | 03/11 | CP6555 | + | − | − | |||||||||||||
| ID72 | ? | t | 05/11 | CP6688 | + | − | − | 146/154 | 181/193 | 192/196 | 268/268 | 251/− | 298/298 | 234/274 | F | |||
| ID67 | 35 | t | 04/10 | CP5810 | + | − | − | 150/154 | 181/189 | 180/196 | 260/272 | 267/267 | 322/334 | − | − | |||
| ID60 | 38 | t | 04/10 | CP5827, CP5828, CP5830, CP5831, CP5832, CP5833, CP5834, CP5835, CP5836, CP5837 | + | − | − | 150/150 | 173/185 | 192/196 | 268/276 | 255/275 | 314/− | 242/246 | M | ND | ND | |
| Mixed | 39 | 3n | 10/10 | CP6090, CP6091, CP6092 | + | + | + | F | ||||||||||
| ? | 11/11 | CP6968 | + | ND | + | 146/154 | − | − | −/271 | − | − | M | ||||||
| ID74 | 62 | t | 11/11 | CP6977 | + | − | − | 146/− | 185/193 | 196/200 | 272/272 | −/275 | 322/334 | 234/− | F | |||
| ID78 | 66 | 7n | 11/11 | CP7010 | + | − | − | 146/154 | 181/185 | 188/192 | 268/280 | 271/275 | 306/314 | 242/242 | F | |||
For each gorilla, an ID number was given and the following collection information recorded: collected cluster (01 to 64) or social group (A to H), collection details (t, collected on track; 3n, collected on three nests; n, collected on a nesting site but nests were not counted), collection date, and number of collected samples (multiple samples may have been collected for one individual; SIV sequences are available for samples shown in bold). Eight seropositive samples from the April 2010 field trip that were too degraded to be assigned to an individual are not shown. Samples of poor quality (degraded RNA and/or DNA) are underlined.
+, positive in the Inno-Lia HIV-1/2 serological test; −, negative test result.
Samples confirmed by SIV amplification and sequencing are shown by SIV-specific RT-PCR results (Pol, pol fragment amplification; gp41, env fragment amplification; other, other regions in the genome could be amplified and sequenced; ND, not done; FL, full-length sequence [50]).
The consensus multilocus genotype profile of seven microsatellite loci is given for each gorilla, with two allele sizes per locus. −, repeatedly negative.
F, female; M, male.
The maximum P(ID)HW (**, P < 10−6 ; *, P < 10−3) and P(ID)Sib (**, P < 0.01; *, P < 0.05) values are given for each individual and confirm the matches between samples.
High SIVgor infection rates in gorilla groups.
To estimate infection rates in gorilla groups, we tried to assign individuals to social gorilla groups by combining microsatellite analysis and field data as previously described by Arandjelovic and colleagues (1). Overall, samples collected on the same day, at the same GPS location, and with the same time of deposition were assumed to belong to individuals from the same group. When two or more individuals from the same cluster of samples were consecutively found together, all of the individuals from the different collections were considered to be from the same gorilla social group. In total, we performed microsatellite analyses on all SIV-negative samples from 31 different clusters of collection in which SIV-positive samples were identified. Complete genotypes were obtained for all samples for only 19 clusters, while the remaining 12 clusters also included degraded or mixed samples.
Our analyses showed that one cluster of three samples collected around one night nest corresponded to a single SIVgor-positive male, ID38, probably a solitary adult male. We identified four social groups with SIVgor-positive gorillas: groups A and C, already reported in 2007 (34), and groups E and G, newly identified in this study (Fig. 1). In addition, we identified three putative social groups, groups F1 to F3; groups F2 and F3, collected in 2009 and 2010, respectively, each shared one individual with group F1, collected in 2006 (ID2 and ID36, respectively) (Fig. 1). However, we considered these three groups to be putative separate groups because they shared only one individual. Furthermore, given the 3- to 4-year gap in collection, the social structure of these groups may have changed.
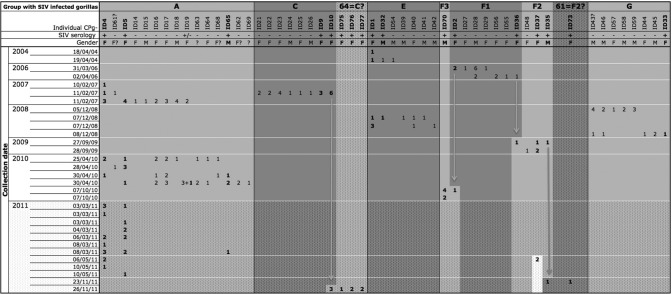
Fig 1.
Inferred composition of gorilla groups of interest at the Campo Ma'an field site over the 7.5-year study period, underlining the follow-up of SIVgor-positive individuals and social groups. For each studied group, data on sampled individuals are shown, with information on sex (F, female; M, male; ?, unknown) and SIV serology (data for seropositive gorillas appear in bold). The number of fecal samples for each gorilla sampled at a given collection location and date are noted. Putative group F1 is linked with group F3 and group F2 by two independent gorillas, i.e., ID2 and ID36, respectively (highlighted by vertical arrows). These three putative groups may be a single group, but they were analyzed separately because of the lack of certainty. Cluster 64, collected in 2011, is linked with group C by ID10, and cluster 61, collected in 2011, is linked with putative group F2 by ID35. These two individuals could have emigrated from their original groups, or clusters 64 and 61 may in fact be groups C and F2, respectively. Data from the collections carried out in 2011 are shown in the dotted area, since genotyping data were available only for positive samples.
As summarized in Fig. 1, among the four identified social groups, three were detected on multiple occasions, up to 17 times at different track or nest sites. The putative F1 to F3 social groups were each sampled twice during the same mission. Interestingly, group A was sampled in 2007, in 2010, and on two occasions in 2011, with at least seven linked individuals (ID4+, ID5+, ID61−, ID16−, ID17−, ID18−, and ID19−), and was composed of a total of 15 individuals between 2007 and 2010. Data on the number of total and linked individuals were not available for this group in 2011. Group E was detected in 2004 and 2008, with two linked individuals (ID1+ and ID32−/+) and a total of seven individuals. The minimum number of gorillas for groups A, C, E, and G ranged from 7 to 15 individuals, and the three putative social groups had between 2 and 7 individuals. The rate of SIV-infected individuals in identified social groups ranged from 13% (group G, with one female [ID33] infected among eight individuals sampled in this group) to 29% (group E, with two infected individuals among seven gorillas sampled). These SIV rates are estimates based on the detected gorillas; we cannot exclude the possibility that some individuals were not sampled during our survey. Despite a possible sampling underrepresentation, we observed a trend suggesting that the ratio of infected females to the total number of females in a social group was generally higher than the ratio of infected males to the total number of males (Fig. 1).
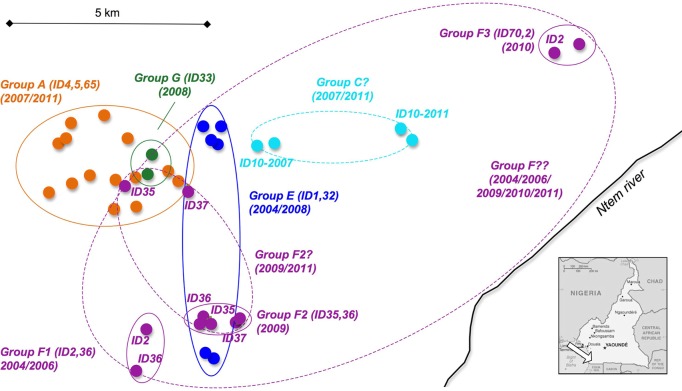
Group C was detected only once during our study. However, one SIVgor-positive individual from this group (ID10) was sampled again 5 years later (November 2011), at a nesting site with SIVgor-positive individuals (ID75, ID76, and ID77) not found in group C in 2006. Either animal ID10 moved to another group or we did not sample all individuals from this group in 2007 and 2011. Based on the GPS information collected in the field, we determined the minimum home range for gorilla groups that were sampled at least twice. As shown in Fig. 2, we found that the different gorilla groups with SIVgor-positive animals had overlapping home ranges.
Fig 2.
Inferred minimum home ranges for gorilla groups of interest, with overlapping ranges shown. From April 2004 to November 2011, an area of approximately 200 km2 was covered during successive field missions in the Campo Ma'an National Park (CP). (Inset) Map of Cameroon (adapted from The World Factbook, https://www.cia.gov/library/publications/the-world-factbook/geos/cm.html). The arrow indicates the CP field site. Minimum home ranges inferred from collection sites (small circles) are delineated by solid-line ellipses for identified social groups and for the three putative social groups (F1, F2, and F3) (Table 1; Fig. 1). The positive individuals and years of collection are given for each gorilla group. The hypothetical home ranges of the possible groups (group C?, group F2?, and group F??) with linked individuals are highlighted by dashed-line ellipses, and the corresponding linked IDs are noted (Fig. 1).
Capture and recapture of SIVgor-negative and -positive animals suggests ongoing infection and transmission in the wild.
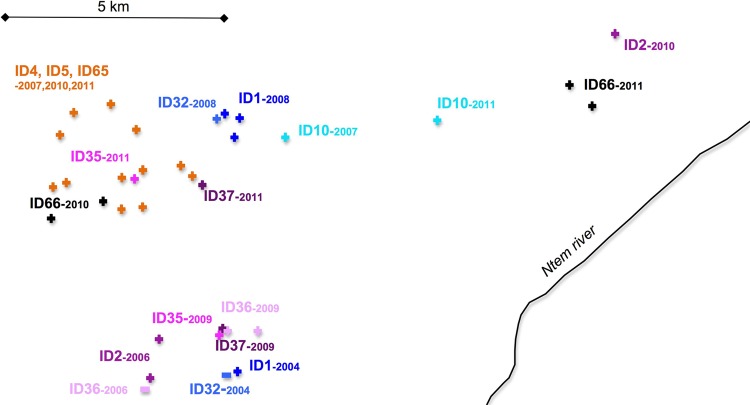
In addition to some of the SIVgor-infected gorillas from the above-mentioned groups, more gorillas were sampled on two or more occasions. First, during a single survey, between one and nine fecal samples from the same animal could be collected. More importantly, 11 of the 34 SIVgor-positive gorillas were sampled on at least two independent field missions (Fig. 3; Table 2). Samples from nine of these gorillas were collected and identified as SIV positive during a first collection trip and were resampled on subsequent field missions 1.5 to 5 years later, as summarized in Table 2. Two other SIV-positive gorillas were sampled in two independent field surveys, but SIV-cross-reactive antibodies were detected only in the follow-up sample: ID32 was SIV negative in April 2004 but SIV positive in December 2008 (almost 5 years later), and ID36 was SIV negative in April 2006 but SIV positive in September 2009 (3.5 years later) (Fig. 3; Table 2). These negative serological observations with the Inno-Lia test were confirmed for both individuals by Western blot analyses (56) and, importantly, by SIVgor-specific RT-PCR tests (Table 1). Repeated PCR attempts on the antibody-negative samples were negative, while the virus could be amplified and sequenced from the antibody-positive samples. These two gorillas most likely seroconverted during the study period, indicating that SIVgor continues to spread in the Campo Ma'an National Park.
Fig 3.
SIVgor-infected gorilla capture and recapture (via sampling) in the Campo Ma'an study area. From April 2004 to November 2011, an area of approximately 200 km2 was covered during successive field missions in the Campo Ma'an National Park. The 11 SIVgor-positive individuals that were captured and recaptured (via sampling) in at least two different collection missions (Table 2) were plotted and are identified by ID numbers, with a positive sign for those found to be SIV seropositive and a negative sign for those that were first found to be SIV negative (ID32 and ID36).
Table 2.
Capture and recapture (via sampling) of 11 SIV-positive nonhabituated gorillas in the Campo Ma'an Parka
| Individual | Group or cluster | Serology result | No. of collection missions | Collection dates (mo/yr) | Collection interval(s) (yr) |
|---|---|---|---|---|---|
| ID4 | A | + | 4 | 02/2007, 04/2010, 03/2011, 05/2011 | 3.2, 0.9, 0.2 (total, 4.3) |
| ID5 | A | + | 4 | 02/2007, 04/2010, 03/2011, 05/2011 | 3.2, 0.9, 0.2 (total, 4.3) |
| ID65 | A | + | 2 | 04/2010, 03/2011 | 0.9 |
| ID10 | C, 64 | + | 2 | 02/2007, 11/2011 | 4.8 |
| ID1 | E | + | 2 | 04/2004, 12/2008 | 4.7 |
| ID32 | E | −, + | 2 | 04/2004, 12/2008 | 4.7 |
| ID36 | F1, F2 | −, + | 2 | 04/2006, 09/2009 | 3.4 |
| ID2 | F1, F3 | + | 2 | 04/2006, 10/2010 | 4.5 |
| ID35 | F2, 61 | + | 2 | 09/2009, 11/2011 | 2.2 |
| ID37 | F2 | + | 2 | 09/2009, 05/2011 | 1.7 |
| ID66 | 35 | + | 2 | 04/2010, 03/2011 | 0.9 |
The locations of sample collections are depicted in Fig. 3. Data separated by commas are for different sampling occasions.
High genetic diversity of SIVgor strains circulating in gorillas from the Campo Ma'an National Park.
We tried to amplify fragments of SIVgor pol and gp41 regions from all SIV-seropositive samples by use of SIVgor consensus and specific primers. In total, we amplified and sequenced SIVgor fragments for 21 of the 34 identified SIVgor-positive individuals (shown in bold in Table 1). For the remainder, SIV amplifications were unsuccessful, possibly as a result of viral nucleic acid degradation and/or because of the low level of viral shedding in feces. SIV amplification rates were variable and ranged from 0% for antibody-positive gorillas sampled in November 2007 and April 2008 to 100% for SIV-positive gorilla fecal samples collected in April 2004, April 2006, December 2008, and September 2009.
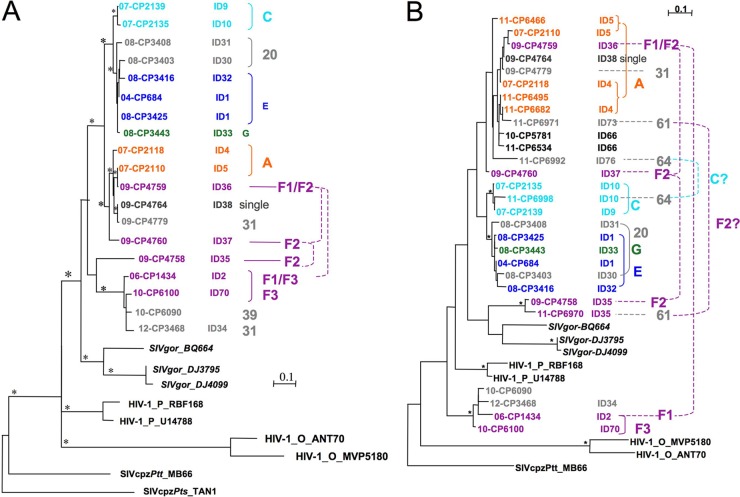
We compared phylogenetic relationships between new SIVgor strains and previously characterized SIVgor, SIVcpz, and HIV-1 strains by aligning sequences of pol (alignment of 202 bp for 11 SIVgor strains) and gp41 (alignments of 263 bp [without gaps] and 195 bp for 19 and 21 SIVgor strains, respectively). The phylogenetic tree for the 263-bp gp41 fragment is shown in Fig. 4A, and the phylogenetic tree for the 195-bp gp41 fragment (for the largest number of SIVgor strains) is shown in Fig. 4B. The phylogenetic tree for the partial pol alignment is shown in Fig. S1 in the supplemental material. The length of the fragment analyzed in Fig. 4B was too short to obtain well-supported clusters or to show any phylogeographic clustering, as previously reported for SIVgor strains from different locations in Cameroon (CP, DJ, and BQ) and as observed in Fig. 4A. Overall, we observed high genetic diversity among SIVgor strains infecting gorillas from the Campo Ma'an National Park, with a maximum genetic distance of 0.403 in the gp41 fragment. Generally, viruses infecting individuals belonging to the same collection cluster or gorilla group were closely related, with a short genetic distance (<0.05), as in the case for ID4 and ID5 from group A, ID9 and ID10 from group C, and ID1 and ID32 from group E (Fig. 4A and B). However, certain viruses obtained from animals from different groups were also closely related, as observed for individuals from groups E (ID1 and ID32) and G (ID33) or for individuals from group A (ID4 and ID5) and the putative groups F1 (ID36) and F2 (ID37) (supported with bootstrap values of >80% in Fig. 4A). Similarly, we also observed divergent SIVgor strains cocirculating in the same collection clusters; for example, in the putative group F2 (Fig. 4A), two females (ID2 and ID36) were infected with divergent SIVgor strains (genetic distance, >0.20), each clustering in well-separated clades supported by bootstrap values of >80%. Also, in 2011, one male (ID35) and one female (ID73) sampled at the same nesting site (cluster 61) were infected with divergent strains (genetic distance, >0.20) (Fig. 4B). Finally, ID10, infected with a strain closely related to SIVgor from ID9, sampled at the same nesting site in 2007 (social group C), was resampled in 2011 at a new nesting site (cluster 64), together with the female ID76, who was infected with a more distantly related strain (Fig. 4B).
Fig 4.
Phylogenetic analyses of partial env (gp41) (263 bp [A] and 195 bp [B]) nucleotide sequences of SIVgor strains. The trees were inferred by maximum likelihood phylogeny (PhyML) with previously characterized SIVcpzPtt/SIVgor/HIV-1 strains (in black). Stars represent support values above 800 (A) or 700 (B) for 1,000 maximum likelihood bootstraps. Bar, 0.1 substitution per site. Sample numbers and collection dates (i.e., 11-CP6466 is sample CP6466, collected in 2011), individual identification numbers (IDs), clusters (i.e., 20, 61, and 64 [in gray]), and social groups (A, C, G, E, and F1/2/3 [in color]) are noted and are used in Table 1. The color code for social groups is conserved in Fig. 2 to 4 and in Fig. S1 in the supplemental material. Solid brackets highlight individuals from the same group/cluster that were infected with closely related strains (very short genetic distance), whereas dashed links indicate individuals from the same group/cluster that were infected with divergent strains.
Suspected vertical and horizontal SIVgor transmissions within and between gorilla groups.
Combining phylogenetic analysis of the SIVgor strains and genotyping of the gorillas with field data allowed us to trace potential transmission routes. The phylogenetic trees showed closely related viruses that suggested potential epidemiologic links between the infected animals. As mentioned above, some of these viruses were derived from individuals from the same social group, but they could also concern animals from different groups. We looked in more detail at the microsatellite analyses of the individuals infected with closely related viruses and performed statistical analyses to examine whether parentage relations existed. In the clade of viruses derived from animals ID4, ID5, ID36, ID38, ID73, and ID66 (Fig. 4A [bootstrap value of >80%] and B), we observed that certain individuals shared one allele at each locus, and a parent-offspring relationship was inferred between ID4 and ID5 (both females) (LOD = 2.66; 80% confidence) and between ID5 and ID73 (both females) (LOD = 3.12; 95% confidence). Similarly, in the clade of viruses derived from animals ID9, ID10, ID1, ID30, ID31, ID32, and ID33 (Fig. 4A [bootstrap value of >80%]), parent-offspring relationships were seen between ID1 and ID32 (female and male, respectively) (LOD = 2.32; 80% confidence), between ID1 and ID33 (both females) (LOD = 4.93; 95% confidence), and between ID10 and ID32 (female and male, respectively) (LOD = 2.98; 95% confidence). Thus, some of these linked viruses could be the result of vertical transmission from mother to offspring. However, it is important that given the fact that we do not know the ages of the individuals, we cannot assess in which direction the parentage link was formed. Furthermore, it is possible that some linked viruses represent other transmission routes, since closely related viruses were also seen among animals without parental links. The individuals with closely related strains and no parental link acquired their infection by horizontal transmission routes, which could be sexual or also a consequence of aggressive or grooming behavior. Sexual transmission could be suspected between male ID38 and female ID36 and between male ID34 and female ID2, with each pair infected with closely related viruses, as they did not display any significant level of parental linkage. Two male pairs (ID30-ID32 and ID34-ID70) without a parental link who were infected with closely related viruses belonged to different groups, suggesting horizontal transmission by blood contact during fights or sexual transmission from the same SIVgor-infected female.
Viral adaptation of the SIVgor envelope variable loops over 5 years of natural infection in a nonhabituated gorilla.
To study SIVgor evolution over time in its natural host, we focused on the SIV-positive individuals sampled in independent field trips (Table 2). Unfortunately, we could not characterize the virus infecting ID65 and we amplified an SIV for ID2, ID10, and ID37 at one time point only (Table 1), probably because of sample degradation. Samples from animals ID1, ID4, ID5, ID35, and ID66 were amplified from collections in different follow-up surveys (Table 1). The gp41 fragments of the SIVs infecting each of these animals 1 to 5 years apart were highly similar, as demonstrated by the phylogenetic analyses (Fig. 4) and by the short genetic distance separating them, based on the gp41 fragment (0.010 to 0.075). We did not observe any evidence of superinfection in these gorillas, but considering the low viral loads in fecal samples, variants circulating at low concentrations could have been undetected.
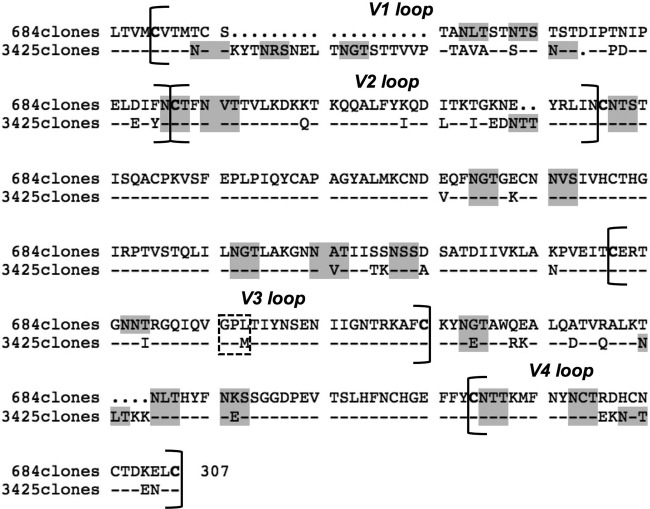
To study the diversity and evolution of the hypervariable region of the viral envelope over the course of infection, we amplified and sequenced 20 and 24 molecular clones of the gp120 env V1V4 region (∼1,000 bp) of the viruses infecting ID1 in April 2004 and November 2008, i.e., SIVgor-04CP684 and SIVgor-08CP3425, respectively. The 20 clones of the SIVgor-04CP684 V1V4 region were 100% similar along the 1,020-bp fragment. The 24 clones obtained from SIVgor-08CP3425 displayed very little variability, with a nucleotide diversity of 0.005 (minimum, 0; maximum, 0.011; SD, 0.001) and an amino acid diversity of 0.010 (minimum, 0; maximum, 0.027; SD, 0.002). These observations reflect the limits of detection of low viral loads in fecal samples more than the actual viral diversity of viruses infecting gorillas. The calculation of the dN/dS ratio did not show evidence of positive selection (dN/dS < 0) but was not considered informative because the clones displayed low or no genetic diversity as a consequence of the low viral loads in fecal sample RNA extracts. The comparison of the SIVgorID1 V1V4 regions after almost 5 years of infection revealed an extensive elongation of 24 amino acids (aa) (from the first cysteine of V1 to the last cysteine of V4, i.e., 273 aa in 2004 versus 297 aa in 2008; P < 0.0001) (Fig. 5). This considerable amino acid elongation was due mainly to an 18-aa extension of the V1 loop. The number of PNGS in the V1V4 region increased significantly between 2004 and 2008, from 17 to 20 PNGS (P < 0.0001). Actually, four additional PNGS in SIVgor-08CP3425 (2008) were located in the V1 loop (+2 PNGS), the V2 loop (+1 PNGS), and the C3 region (+1 PNGS), while one PNGS was lost in the V3 loop (−1 PNGS) (Fig. 5). The latter loop was relatively conserved over the years, except for the loss of one PNGS and a V3 crown switch from a GPL to a GPM motif. In an attempt to deduce the phenotypes of these two strains, we used the 11/25 rule (no K or R at positions 11 and 25) and the net charge rule designed for HIV strains (the net charge in 2004 and 2008 was 2, which is under the threshold of 5). Both methods suggest that the characterized viruses infecting ID1 in 2004 and 2008 have the R5 phenotype, but further analyses are needed.
Fig 5.
Amino acid evolution of the SIVgorID1 Env hypervariable region between April 2004 (SIVgor-04CP684) and December 2008 (SIVgor-08CP3425). The hypervariable loops V1, V2, V3, and V4 and the C2 and C3 regions were analyzed. The SIVgor-04CP684 sequence (the 20 clones found for SIVgor-04CP684 were 100% similar), indicated at the top (684 clones), was compared with the consensus of all clonal sequences from SIVgor-08CP3425 (3,425 clones). Dots stand for gaps, and dashes stand for the same amino acids as those in the sequence above. The putative N-linked glycosylation consensus motifs (NXT/S) are highlighted in gray, important cysteines are shown in bold, and the V3 crown is shown in a dashed rectangle, stressing the switch from GPL to GPM.
DISCUSSION
Our goal was to study SIVgor infection over time in nonhabituated wild-living gorillas in an area with a relatively high density of SIVgor-infected animals. We were able to identify at least 34 SIVgor-infected gorillas, characterize additional SIVgor strains, resample SIV-positive and -negative individuals over time, reconstitute gorilla groups by using noninvasive methods among nonhabituated animals, and obtain evidence for SIVgor transmissions within and between groups. Finally, we could document the genetic diversity of SIVgor in different gorilla groups and found viral adaptation in the envelope of SIVgor over time in its natural host.
During the study period, we identified SIV antibodies in 16% of the gorilla samples, corresponding to at least 34 SIVgor-positive gorillas living in an area of approximately 200 km2 in the center of the Campo Ma'an Park. Similar to the case for SIVcpzPtt and SIVcpzPts, SIVgor prevalences were also unevenly distributed, but the SIVgor prevalence at a site did not reach the 30% to 40% rates observed in certain central and eastern chimpanzee populations (22, 55; Y. Li et al, presented at the Cold Spring Harbor Laboratory Meeting on Retroviruses, Cold Spring Harbor, NY, 2012; F. Liegeois et al., presented at The Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 2012). For other nonhuman primate species, prevalences can also vary, from 0% to >50%, according to species or geographic localities (reviewed in reference 27). Importantly, 11 of the 34 gorillas were resampled at different time intervals, allowing us to monitor infected nonhabituated gorillas noninvasively. We were also able to identify four social groups and three putative social groups with SIVgor-infected animals by combining microsatellite analysis and field data. Thus, we confirmed, in a 7.5-year survey, the previously reported data from Arandjelovic et al. which showed the effectiveness of noninvasive monitoring of wild gorilla groups during a 2-year period with frequent missions (1). During our 7.5-year study, SIVgor-positive animals and social groups with SIVgor-infected gorillas were detected on up to 17 occasions (for group A), during the same or independent missions. Between 2010 and 2011, follow-up missions were conducted at shorter intervals, and as a consequence, resampling of individuals and groups increased accordingly. The identified social groups had overlapping home ranges and varied in size from 2 to 15 individuals, with different male/female sex ratios, which is typical of western lowland gorillas (1, 15, 43). We determined the SIV infection rates (13 to 29%) in four identified nonhabituated social groups. We were also able to identify a single adult male (ID38) infected with SIV, illustrating that migrating individuals infected with SIVgor can be responsible for the spread of viruses in the area by fighting for a dominant position or by other routes while transferring to a new gorilla social group (2, 29, 43). Despite previous studies reporting that the use of RNAlater is not optimal for DNA analyses (36), we obtained a microsatellite amplification success rate comparable to those in previous studies on noninvasive samples, showing that storage of fecal samples in RNAlater allows for combined studies on the host and its pathogens.
By combining the analyses of viral genetic diversity, the information on the social groups, and the genotypes of the individuals, we could infer potential routes of transmission. In a previous study, we reported that SIVgor strains infecting individuals from two groups identified at nesting sites were highly similar (34). In this study, we confirmed this pattern for two additional groups. However, in this new survey, divergent strains were also found to circulate within groups (three examples were found). In fact, there are no limiting natural geographic barriers in the studied area, and we found that different groups circulate with overlapping ranges. These characteristics made possible both male and female transfers (11), and thus SIV strain circulation, among various groups of gorillas.
The ratio of infected females to total females in a social group was generally higher than the ratio of infected males to total males. In gorilla groups where several females and only one male are sexually active (24, 47), this observation could support the hypothesis that SIVgor transmission between individuals in a group occurs mostly via the horizontal route, by sexual contact. Furthermore, we identified two pairs in which sexual transmission was highly suspected, since the implicated males and females were not genetically linked but were infected with highly similar viruses. We can thus hypothesize that SIVgor transmission by sexual contact also drives the epidemic in lowland gorillas. Rudicell and colleagues showed that the SIVcpzPts transmission probability is approximately 0.001 transmission event per coital act for eastern chimpanzees, which is comparable to the HIV-1 transmission probability for humans by the same route (44). Nevertheless, horizontal transmission could also occur via blood contact during grooming or aggressive behavior.
Besides horizontal transmission, we reported cases of suspected vertical transmission of SIVs in gorillas. Mother-to-infant viral transmission (MTIT) could occur in utero or by breastfeeding, since gorillas are weaned at approximately 4 years of age and therefore can be infected after birth (21, 64). Sexual transmission between related individuals (at first degree) is unlikely, as females and males leave the natal group at sexual maturity to avoid inbreeding (12, 24, 47). By deducting microsatellite matches and highly related viruses, we identified five putative vertical transmissions, but because we had no data on the ages of the apes, it was impossible to ascertain whether we were looking at father-daughter pairs or mother-son pairs or to discriminate mothers or daughters among linked female pairs. Interestingly, in a few cases, the presumed mother-infant pairs involved individuals from different groups, e.g., ID1 (group E) and ID33 (group G), which suggested that the infected infants did not die early of SIV infection and were able to emigrate from their respective natal groups. This behavior is common in healthy weaned female and male western lowland gorillas and has been reported in several studies (24, 43, 47). MTIT of nonpathogenic SIV infection, such as SIVsm infection of sooty mangabeys or SIVagm infection of African green monkeys, is rarely seen (reviewed in reference 7). In contrast, for SIVcpz, which is pathogenic in its natural host, vertical transmissions are observed, and infants born to SIVcpzPts-positive mothers have a significantly higher mortality rate than those born to uninfected mothers (22). In addition, many naturally SIVcpzPtt-infected chimpanzees in captivity have died as infants upon arrival in sanctuaries (reviewed in reference 13). Further studies are thus needed to confirm the putative vertical transmission of SIVgor strains and the survival rate of infected infants, as this characteristic is highly informative regarding the impact of SIVgor on the structure and survival of gorilla populations. More generally, it would be interesting to better understand if there is a link between SIV/HIV pathogenicity and MTIT.
Since we identified two possible cases of seroconversion during the time of survey, we show here that there are ongoing SIVgor transmissions in wild-living gorilla populations. Nevertheless, only a few samples were available to confirm these seroconversions, and even with the use of PCR amplification controls, we cannot exclude the possibility of false-negative results, mainly because we detected SIVgor antibodies in fecal samples by cross-reactivity with the Inno-Lia HIV-1/2 assay. In fact, the sensitivity of this assay has not been determined for SIVgor infection, since there is no captive SIV-positive gorilla for comparison. Furthermore, the test was performed on fecal samples, which result in lower sensitivities and may also have had antibody shedding over time.
SIVgor evolution within its natural host has never been characterized, since no naturally SIV-infected gorilla has been documented in captivity. In this study, we characterized the virus infecting the positive nonhabituated gorilla ID1 in April 2004 and December 2008. The clones obtained from the V1V4 gp120 region (∼1,000 bp) were very similar at a given time, showing that the low viral loads in fecal samples can be a drawback to noninvasive genetic diversity studies. However, we could compare the strains infecting the animal 4.7 years apart. We showed that the V1V4 region was significantly longer in 2008 than in 2004 (+24 aa), mainly due to a longer V1 loop. Furthermore, we observed a significantly increased number of PNGS in the V1V2 region over time. Like that of SIVcpz in naturally infected chimpanzees (13, 37) or SIVsmm in naturally infected sooty mangabeys (10), SIVgor V1V2 appears to be highly glycosylated. An increasing number of PNGS in the gp120 hypervariable loops of HIV-1 has been associated with the escape of viral mutants (31, 59). Also, longer HIV-1 V1V2 loops (particularly in V1) with increased numbers of PNGS in Env have been correlated with escape from HIV-1-specific neutralizing antibodies at both the individual and population levels (6, 54), possibly by the shielding of envelope epitopes from antibody recognition (8, 28, 41, 46). Furthermore, these V1V2 characteristics were recently associated with disease progression in humans (9). Whether they are also associated with an AIDS-like disease progression in gorillas has still to be determined. In addition, a switch from a GPL to a GPM motif (found in HIV-1 group O) in the V3 crown was observed. To our knowledge, such a switch or its implication has not been reported in the literature yet, apart from a follow-up study of a SIVcpzPtt infection in a naturally infected captive chimpanzee over 5 years (GPAM-to-GPGM switch) (13). The role of such a mutation in the V3 crown and the biological properties of the corresponding viruses need to be studied further.
In conclusion, our study demonstrates the feasibility of monitoring SIV infection in wild-living nonhabituated gorillas over a long period in a region of Cameroon where SIVgor prevalence is relatively high. We not only identified new SIVgor strains during our survey, showing ongoing SIVgor transmissions in the wild, but also obtained follow-up samples from infected gorillas and their putative social groups, from which we could retrieve important information on the SIVgor infection rate, routes of transmission, and viral evolution within the natural host. The possibility that SIVgor is pathogenic for its natural host is a major concern given the highly endangered status of western lowland gorillas, and it can be studied only by noninvasive surveys implemented over a sufficiently long period to allow for the documentation of both new SIVgor infections and deaths of infected gorillas. It will also be important to compare SIVgor infection in gorillas with SIVcpz infection in chimpanzee populations with different social structures, which could have different consequences on the modes and efficiency of viral transmission, viral evolution at the population level, pathogenicity, and survival of the populations in general. Many different pathogens can be detected in fecal samples, as reported in previous studies (18, 25, 26, 30); coupling long-term monitoring of SIVgor infection with investigations of the presence of other viruses or parasites will provide us with a better picture of the role that SIV plays in the health and survival of wild gorillas at both the individual and population levels. Finally, noninvasive monitoring can also be applied more generally to the study of other pathogens in wildlife.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff and the SIV team from PRESICA for logistical support in Cameroon and the Cameroonian Ministries of Health, Environment and Forestry, and Research for permission to collect samples in Cameroon.
This study was supported by grants from the National Institutes of Health (R01 AI50529), the Agence Nationale de Recherches sur le SIDA, France (ANRS 12125, ANRS 12182, and ANRS 12255), and the Institut de Recherche pour le Développement (IRD). Lucie Etienne is supported by a Ph.D. grant from Sidaction and Fonds de Dotation Pierre Bergé.
Footnotes
Published ahead of print 27 June 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Arandjelovic M, et al. 2010. Effective non-invasive genetic monitoring of multiple wild western gorilla groups. Biol. Conserv. 143:1780–1791 [Google Scholar]
- 2. Bermejo M. 2004. Home-range use and intergroup encounters in western gorillas (Gorilla g. gorilla) at Lossi Forest, North Congo. Am. J. Primatol. 64:223–232 [DOI] [PubMed] [Google Scholar]
- 3. Bonin A, et al. 2004. How to track and assess genotyping errors in population genetics studies. Mol. Ecol. 13:3261–3273 [DOI] [PubMed] [Google Scholar]
- 4. Boom R, et al. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Botstein D, White RL, Skolnick M, Davis RW. 1980. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 32:314–331 [PMC free article] [PubMed] [Google Scholar]
- 6. Bunnik EM, et al. 2010. Adaptation of HIV-1 envelope gp120 to humoral immunity at a population level. Nat. Med. 16:995–997 [DOI] [PubMed] [Google Scholar]
- 7. Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. 2012. Natural SIV hosts: showing AIDS the door. Science 335:1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ching L, Stamatatos L. 2010. Alterations in the immunogenic properties of soluble trimeric human immunodeficiency virus type 1 envelope proteins induced by deletion or heterologous substitutions of the V1 loop. J. Virol. 84:9932–9946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curlin ME, et al. 2010. HIV-1 envelope subregion length variation during disease progression. PLoS Pathog. 6:e1001228 doi:10.1371/journal.ppat.1001228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demma LJ, Vanderford TH, Logsdon JM, Jr, Feinberg MB, Staprans SI. 2006. Evolution of the uniquely adaptable lentiviral envelope in a natural reservoir host. Retrovirology 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doran-Sheehy DM, Greer D, Mongo P, Schwindt D. 2004. Impact of ecological and social factors on ranging in western gorillas. Am. J. Primatol. 64:207–222 [DOI] [PubMed] [Google Scholar]
- 12. Douadi MI, et al. 2007. Sex-biased dispersal in western lowland gorillas (Gorilla gorilla gorilla). Mol. Ecol. 16:2247–2259 [DOI] [PubMed] [Google Scholar]
- 13. Etienne L, et al. 2011. Characterization of a new simian immunodeficiency virus strain in a naturally infected Pan troglodytes troglodytes chimpanzee with AIDS related symptoms. Retrovirology 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evett IW, Weir BS. 1998. Interpreting DNA evidence: statistical genetics for forensic scientists. Sinauer & Associates, Inc, Sunderland, MA [Google Scholar]
- 15. Gatti S, Levrero F, Menard N, Gautier-Hion A. 2004. Population and group structure of western lowland gorillas (Gorilla gorilla gorilla) at Lokoue, Republic of Congo. Am. J. Primatol. 63:111–123 [DOI] [PubMed] [Google Scholar]
- 16. Guindon S, et al. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59:307–321 [DOI] [PubMed] [Google Scholar]
- 17. Guschanski K, et al. 2009. Counting elusive animals: comparing field and genetic census of the entire mountain gorilla population of Bwindi Impenetrable National Park, Uganda. Biol. Conserv. 142:290–300 [Google Scholar]
- 18. Harvala H, et al. 2011. Detection and genetic characterization of enteroviruses circulating among wild populations of chimpanzees in Cameroon: relationship with human and simian enteroviruses. J. Virol. 85:4480–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hearne CM, Ghosh S, Todd JA. 1992. Microsatellites for linkage analysis of genetic traits. Trends Genet. 8:288–294 [DOI] [PubMed] [Google Scholar]
- 20. Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16:1099–1106 [DOI] [PubMed] [Google Scholar]
- 21. Keele BF, et al. 2009. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460:515–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keele BF, et al. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313:523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korber B. 2000. HIV signature and sequence variation analysis, p 55–72 In Rodrigo AG, Learn GH., Jr (ed), Computational analysis of HIV molecular sequences. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 24. Levrero F, et al. 2006. Living in nonbreeding groups: an alternative strategy for maturing gorillas. Am. J. Primatol. 68:275–291 [DOI] [PubMed] [Google Scholar]
- 25. Liu W, et al. 2010. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature 467:420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu W, et al. 2008. Molecular ecology and natural history of simian foamy virus infection in wild-living chimpanzees. PLoS Pathog. 4:e1000097 doi:10.1371/journal.ppat.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Locatelli S, Peeters M. 2012. Cross-species transmission of simian retroviruses: how and why they could lead to the emergence of new diseases in the human population. AIDS 26:659–673 [DOI] [PubMed] [Google Scholar]
- 28. Ly A, Stamatatos L. 2000. V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 74:6769–6776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magliocca F, Gautier-Hion A. 2004. Inter-group encounters in western lowland gorillas at a forest clearing. Folia Primatol. 75:379–382 [DOI] [PubMed] [Google Scholar]
- 30. Makuwa M, et al. 2005. Identification of hepatitis B virus genome in faecal sample from wild living chimpanzee (Pan troglodytes troglodytes) in Gabon. J. Clin. Virol. 34(Suppl 1):S83–S88 [DOI] [PubMed] [Google Scholar]
- 31. Mascola JR, Montefiori DC. 2003. HIV-1: nature's master of disguise. Nat. Med. 9:393–394 [DOI] [PubMed] [Google Scholar]
- 32. Milne I, et al. 2009. TOPALi v2: a rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics 25:126–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navidi W, Arnheim N, Waterman MS. 1992. A multiple-tubes approach for accurate genotyping of very small DNA samples by using PCR: statistical considerations. Am. J. Hum. Genet. 50:347–359 [PMC free article] [PubMed] [Google Scholar]
- 34. Neel C, et al. 2010. Molecular epidemiology of simian immunodeficiency virus infection in wild-living gorillas. J. Virol. 84:1464–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nei M. 1987. Molecular evolutionary genetics. Columbia University Press, New York, NY [Google Scholar]
- 36. Nsubuga AM, et al. 2004. Factors affecting the amount of genomic DNA extracted from ape faeces and the identification of an improved sample storage method. Mol. Ecol. 13:2089–2094 [DOI] [PubMed] [Google Scholar]
- 37. Ondoa P, et al. 2001. Genetic variability of the V1 and V2 env domains of SIVcpz-ant and neutralization pattern of plasma viruses in a chimpanzee infected naturally. J. Med. Virol. 65:765–776 [DOI] [PubMed] [Google Scholar]
- 38. Paetkau D, Strobeck C. 1994. Microsatellite analysis of genetic variation in black bear populations. Mol. Ecol. 3:489–495 [DOI] [PubMed] [Google Scholar]
- 39. Peeters M, et al. 1997. Geographical distribution of HIV-1 group O viruses in Africa. AIDS 11:493–498 [DOI] [PubMed] [Google Scholar]
- 40. Peeters M, et al. 1989. Isolation and partial characterization of an HIV-related virus occurring naturally in chimpanzees in Gabon. AIDS 3:625–630 [DOI] [PubMed] [Google Scholar]
- 41. Pinter A, et al. 2004. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 78:5205–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Plantier JC, et al. 2009. A new human immunodeficiency virus derived from gorillas. Nat. Med. 15:871–872 [DOI] [PubMed] [Google Scholar]
- 43. Robbins MM, et al. 2004. Social structure and life-history patterns in western gorillas (Gorilla gorilla gorilla). Am. J. Primatol. 64:145–159 [DOI] [PubMed] [Google Scholar]
- 44. Rudicell RS, et al. 2010. Impact of simian immunodeficiency virus infection on chimpanzee population dynamics. PLoS Pathog. 6:e1001116 doi:10.1371/journal.ppat.1001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rudicell RS, et al. 2011. High prevalence of simian immunodeficiency virus infection in a community of savanna chimpanzees. J. Virol. 85:9918–9928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sagar M, Wu X, Lee S, Overbaugh J. 2006. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J. Virol. 80:9586–9598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stokes EJ, Parnell RJ, Olejniczak C. 2003. Female dispersal and reproductive success in wild western lowland gorillas (Gorilla gorilla gorilla). Behav. Ecol. Sociobiol. 54:329–339 [Google Scholar]
- 48. Sullivan KM, Mannucci A, Kimpton CP, Gill P. 1993. A rapid and quantitative DNA sex test: fluorescence-based PCR analysis of X-Y homologous gene amelogenin. Biotechniques 15:636–638, 640–641 [PubMed] [Google Scholar]
- 49. Taberlet P, et al. 1996. Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Res. 24:3189–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takehisa J, et al. 2009. Origin and biology of simian immunodeficiency virus in wild-living western gorillas. J. Virol. 83:1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vallari A, et al. 2011. Confirmation of putative HIV-1 group P in Cameroon. J. Virol. 85:1403–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van der Kuyl AC, Kuiken CL, Dekker JT, Goudsmit J. 1995. Phylogeny of African monkeys based upon mitochondrial 12S rRNA sequences. J. Mol. Evol. 40:173–180 [DOI] [PubMed] [Google Scholar]
- 54. van Gils MJ, et al. 2011. Longer V1V2 region with increased number of potential N-linked glycosylation sites in the HIV-1 envelope glycoprotein protects against HIV-specific neutralizing antibodies. J. Virol. 85:6986–6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Van Heuverswyn F, et al. 2007. Genetic diversity and phylogeographic clustering of SIVcpzPtt in wild chimpanzees in Cameroon. Virology 368:155–171 [DOI] [PubMed] [Google Scholar]
- 56. Van Heuverswyn F, et al. 2006. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature 444:164. [DOI] [PubMed] [Google Scholar]
- 57. Vergne L, et al. 2003. Biological and genetic characteristics of HIV infections in Cameroon reveals dual group M and O infections and a correlation between SI-inducing phenotype of the predominant CRF02_AG variant and disease stage. Virology 310:254–266 [DOI] [PubMed] [Google Scholar]
- 58. Waits LP, Luikart G, Taberlet P. 2001. Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol. Ecol. 10:249–256 [DOI] [PubMed] [Google Scholar]
- 59. Wei X, et al. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312 [DOI] [PubMed] [Google Scholar]
- 60. Wilberg MJ, Dreher BP. 2004. GENECAP: a program for analysis of multilocus genotype data for non-invasive sampling and capture-recapture population estimation. Mol. Ecol. Notes 4:783–785 [Google Scholar]
- 61. Woods JG, et al. 1999. Genetic tagging of free-ranging black and brown bears. Wildl. Soc. Bull. 27:616–627 [Google Scholar]
- 62. WWF 22 June 2012, accession date Campo-Ma'an National Park, Cameroon: http://wwf.panda.org/what_we_do/where_we_work/project/projects/index.cfm?uProjectID=CM0858&source=ge [Google Scholar]
- 63. WWF 22 June 2012, accession date Western lowland gorillas. http://wwf.panda.org/what_we_do/endangered_species/great_apes/gorillas/western_lowland_gorilla/
- 64. Yamagiwa J, Kahekwa J, Basabose AK. 2003. Intra-specific variation in social organization of gorillas: implications for their social evolution. Primates 44:359–369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.