Abstract
Canine distemper virus (CDV) uses signaling lymphocyte activation molecule (SLAM), expressed on immune cells, as a receptor. However, epithelial and neural cells are also affected by CDV in vivo. Wild-type CDV strains showed efficient replication with syncytia in Vero cells expressing dog nectin4, and the infection was blocked by an anti-nectin4 antibody. In dogs with distemper, CDV antigen was preferentially detected in nectin4-positive neurons and epithelial cells, suggesting that nectin4 is an epithelial cell receptor for CDV and also involved in its neurovirulence.
TEXT
Distemper is a severe infectious disease that mainly affects dogs and other canids (5). The causative agent is canine distemper virus (CDV), which is closely related to measles virus (MV) (23). CDV belongs to the genus Morbillivirus in the family Paramyxoviridae and possesses a single-stranded negative-sense RNA genome encoding six structural and two nonstructural proteins (23). Two surface glycoproteins, H and F, play key roles in virus entry. The H protein is responsible for the receptor binding, and the F protein mediates membrane fusion. Signaling lymphocyte activation molecule (SLAM) expressed on cells of the immune system is a receptor for CDV (16). SLAM serves as a common receptor for morbilliviruses (1). Using SLAM as a receptor, CDV primarily replicates in lymphocytes and macrophages in the respiratory tract and then disseminates throughout the body (22). However, SLAM-negative cells in epithelia and the central nervous system (CNS) are also affected by CDV in vivo (2, 21). Recently, nectin4 was identified as an epithelial cell receptor for MV (13, 14). In humans, nectin4 is expressed mainly in the placenta and, to lesser extents, in the tonsil, oral mucosa, trachea, esophagus, nasopharynx, prostate, lung, and stomach (13, 14, 20). Although MV also exhibits neurovirulence and causes a persistent infection of the CNS, subacute sclerosing panencephalitis (SSPE), neither SLAM nor nectin4 was detected in neural cells of the human CNS (13, 14, 20). The frequency of SSPE is 1/5,000 to 1/100,000 in reported cases of acute measles (3, 19). In contrast, acute infection of animals with CDV is often accompanied by severe neurological manifestations, which are rarely seen in patients with acute measles (2, 21). The aim of the present study was to elucidate the roles for nectin4 in CDV pathogenesis, including its neurovirulence.
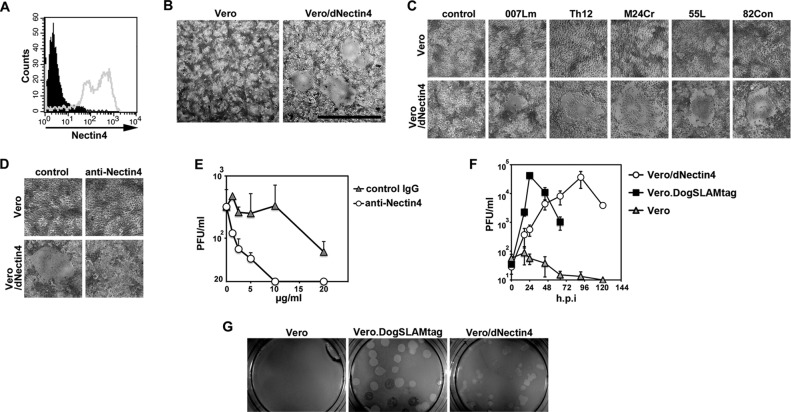
Six wild-type CDV strains (Ac96I, 007Lm, Th12, M24Cr, 55L, and 82Con) isolated from dogs with distemper by using Vero.DogSLAMtag cells were employed in the present study. Some of these strains were reported previously (7, 8, 10). Within 2 days after infection, they all induced syncytia in Vero cells constitutively expressing dog nectin4 (Vero/dNectin4), but not in the parental Vero cells (Fig. 1A, B, and C). The formation of syncytia was completely blocked by 20 μg/ml of a goat anti-human nectin4 polyclonal antibody (R&D Systems) and clearly reduced by 10 μg/ml of the antibody (Fig. 1D). Production of infectious virus particles was inhibited by the anti-nectin4 antibody in a dose-dependent manner (Fig. 1E). Although CDV replicated poorly in Vero cells, it replicated efficiently in Vero/dNectin4 cells (Fig. 1F), as observed in Vero.DogSLAMtag cells. CDV produced plaques in Vero.DogSLAMtag and Vero/dNectin4 cells, but not in the parental Vero cells, although PFU were reduced by ∼3-fold in Vero/dNectin4 cells compared to Vero.DogSLAMtag cells (Fig. 1G). The size of plaques was also smaller in Vero/dNectin4 cells than in Vero.DogSLAMtag cells (Fig. 1G). These findings indicate that dog nectin4 functions as a CDV receptor, similar to the case with MV (13, 14).
Fig 1.
Infection of Vero/dNectin4 cells with CDV. (A) Vero/dNectin4 (gray empty profile) and parental Vero (filled black profile) cells were stained with a goat anti-human nectin4 polyclonal antibody (R&D Systems), followed by staining with Alexa Fluor 488-conjugated anti-goat IgG. (B) Vero/dNectin4 (right panel) and parental Vero (left panel) cells were infected with the Ac96I strain. At 48 h postinfection, the cells were observed under a phase-contrast microscope. Bar, 1 mm. (C) Vero/dNectin4 (lower panels) and parental Vero (upper panels) cells were infected with wild-type strains of CDV (007Lm, Th12, M24Cr, 55L, or 82Con) or left uninfected (control). At 48 h postinfection, the cells were observed under a phase-contrast microscope. (D) Vero/dNectin4 (lower panels) and parental Vero (upper panels) cells were infected with the wild-type Ac96I CDV strain in the presence (anti-Nectin4) or absence (control) of the goat anti-human nectin4 polyclonal antibody. At 48 h postinfection, the cells were observed under a phase-contrast microscope. (E) Vero/dNectin4 cells pretreated with increasing concentrations of anti-Nectin4 antibody or control IgG were infected with 1,000 PFU of strain Ac96I and cultured with the same concentrations of the antibody or control IgG. At 48 h postinfection, the virus titers of the supernatants were determined in plaque assays. (F) Vero/dNectin4, Vero.DogSLAMtag, and parental Vero cells were infected with the wild-type Ac96I CDV strain at a multiplicity of infection of 0.05. At various time intervals, the virus titers were determined in plaque assays. (F) Vero/dNectin4 (right panel), Vero.DogSLAMtag (middle panel), and the parental Vero (left panel) cells in 12 well-cluster plates were infected with the wild-type Th12 strain and overlaid with medium containing 1% agarose. At 7 days postinfection, the plaques were observed under a stereoscope.
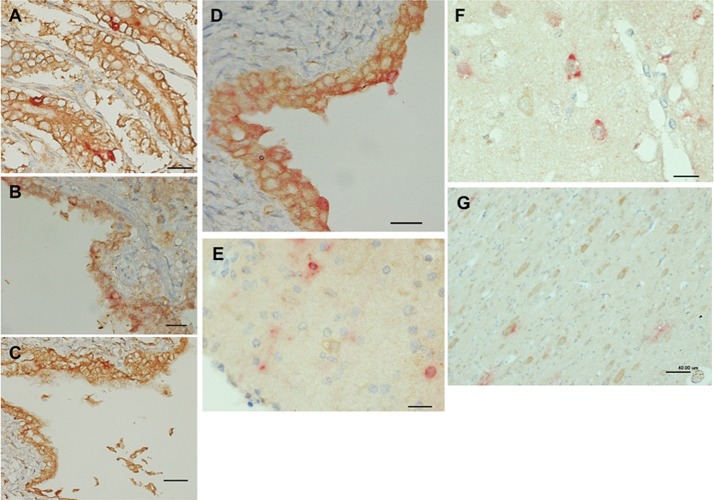
Seven dogs with distemper were necropsied, and tissues were subjected to histopathological analyses. Hematoxylin and eosin staining of the tissue samples revealed pathognomonic changes with CDV infection, including lymphoid depletion, catarrhal enteritis, bronchointerstitial pneumonia, and nonsuppurative encephalitis (data not shown) (9). Eosinophilic intracytoplasmic and intranuclear inclusion bodies were observed in the brain, lymphoid organs, and lung (data not shown). Immunohistochemical double staining for CDV antigen and nectin4 was conducted in two ways. In the first method, CDV antigen was stained pink by Fast red II and nectin4 was stained brown by diaminobenzidine (Fig. 2). In the second method, CDV antigen and nectin4 were labeled with the red fluorescent probe Alexa Fluor 594 and green fluorescent probe Alexa Fluor 488 (Fig. 3). Nectin4 was expressed in all epithelia of the lung, kidney, intestine, and urinary bladder (Fig. 2A to D and 3A to C and data not shown). CDV antigen was detected in accordance with some of the nectin4-positive epithelial cells (Fig. 2A to D and 3A to C and data not shown). Importantly, and in contrast to the reports for humans, nectin4 was detected in the brain of dogs, and CDV antigen was preferentially detected in the nectin4-positive neurons (Fig. 2E to G and 3D). These findings suggest that nectin4 may contribute to infection of the CNS with CDV.
Fig 2.
Immunohistochemical double staining for CDV antigen and nectin4. Tissue sections of 2-μm thickness were deparaffinized, rehydrated, and subjected to heat-induced antigen retrieval before incubation with antibodies. Subsequently, blocking of endogenous peroxidase was performed. The tissue sections were then incubated with a mouse monoclonal anti-CDV antibody (Adtec, Japan) and a goat anti-nectin4 polyclonal antibody (R&D Systems). Using an EnVision kit (Dako) and 3′,3-diaminobenzidine (Sigma), CDV antigen and nectin4 were visualized (pink and brown staining, respectively), according to the manufacturer's protocol. (A) Intestine; (B) lung; (C) renal pelvis; (D) urinary bladder; (E) cerebellum; (F) cerebrum; (G) midbrain. Bars, 20 μm (A, B, D, E, and F) or 40 μm (C and G).
Fig 3.
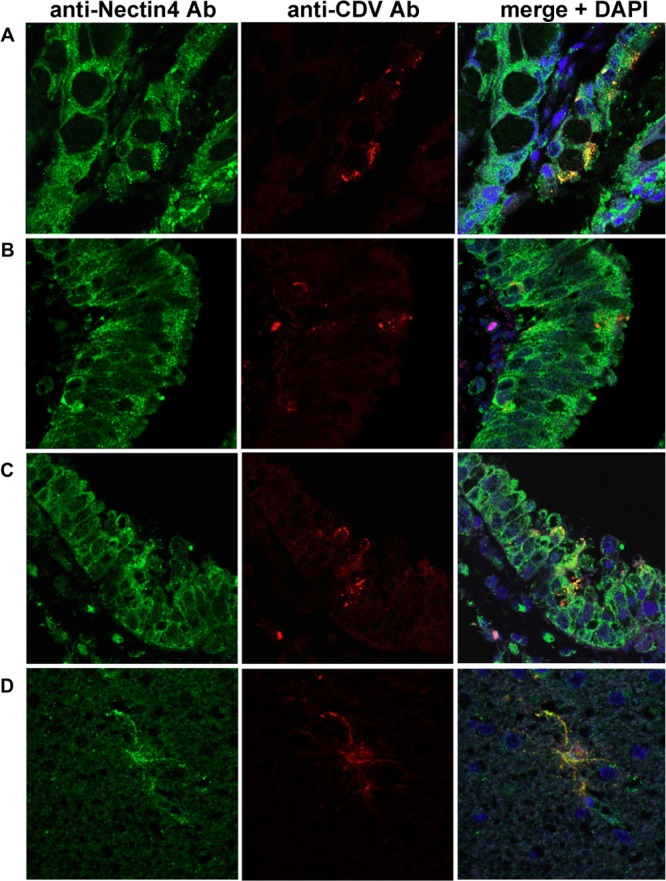
Immunofluorescence double staining for CDV antigen and nectin4. Tissue sections of 2-μm thickness were deparaffinized, rehydrated, and subjected to heat-induced antigen retrieval before incubation with antibodies. CDV antigen was stained with a mouse anti-CDV monoclonal antibody (Adtec, Japan) and Alexa Fluor 594-conjugated secondary (red) antibody. Nectin4 was stained with a goat anti-nectin4 polyclonal antibody (R&D Systems) and Alexa Fluor 488-conjugated secondary antibody (green). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). (A) Intestine; (B and C) lung; (D) brain.
Although SLAM is not expressed in epithelial and neural cells, CDV antigen is often detected at high levels in the epithelia of various organs and the CNS of dogs with distemper (21). Several candidate molecules that support SLAM-independent CDV infection have previously been reported. Fujita et al. (6) reported that a heparin-like molecule supports the entry of CDV into cells of the human embryonic kidney 293 cell line. CD9 has also been described to support cell-to-cell fusion by CDV (12, 15, 17). A recent study further showed that chicken embryo fibroblasts and monkey kidney Vero cells express unidentified receptor molecules for CDV with molecular masses of 57 and 42 kDa, respectively (4). However, that study did not provide a solid conclusion for the in vivo epithelial and neural receptors of CDV (4, 6, 12, 15, 17). For MV, a breakthrough came with the identification of nectin4 as an epithelial receptor for the virus, implying an efficient mechanism for MV transmission, although the mechanisms for MV infection of neural cells remained unclear (13, 14). In the present study, we investigated CDV as a model of morbillivirus infection of the CNS, since the virus often causes severe infection of the CNS in infected animals (21). Our data demonstrated that dog canine nectin4 functions as a CDV receptor and is expressed in neural cells of the brain as well as the epithelia of various organs. In the brain, nectin4-positive neurons were preferentially targeted by CDV. Therefore, CDV infection of the CNS could be partly explained by the knowledge that dog canine nectin4 acts as a receptor for CDV. Like SLAM, nectin4 seems to be a common receptor for morbilliviruses, because critical residues of the H protein required for nectin4 binding are highly conserved among morbilliviruses (11, 18). Thus, the findings in the present study contribute to our understanding of the pathogenesis of CDV, and possibly other morbilliviruses.
ACKNOWLEDGMENTS
We thank Y. Yanagi for providing the Vero.DogSLAMtag cells.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labor and Welfare of Japan, and the Takeda Science Foundation. W. Pratakpiriya was supported by a granted Chulalongkorn University tuition fee scholarship.
Footnotes
Published ahead of print 3 July 2012
REFERENCES
- 1. Adombi CM, et al. 2011. Monkey CV1 cell line expressing the sheep-goat SLAM protein: a highly sensitive cell line for the isolation of peste des petits ruminants virus from pathological specimens. J. Virol. Methods 173:306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appel MJ, Summers BA. 1995. Pathogenicity of morbilliviruses for terrestrial carnivores. Vet. Microbiol. 44:187–191 [DOI] [PubMed] [Google Scholar]
- 3. Bellini WJ, et al. 2005. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J. Infect. Dis. 192:1686–1693 [DOI] [PubMed] [Google Scholar]
- 4. Chen J, Liang X, Chen PF. 2011. Canine distemper virus utilizes different receptors to infect chicken embryo fibroblasts and Vero cells. Virol. Sin. 26:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deem SL, Spelman LH, Yates RA, Montali RJ. 2000. Canine distemper in terrestrial carnivores: a review. J. Zoo Wildl. Med. 31:441–451 [DOI] [PubMed] [Google Scholar]
- 6. Fujita K, et al. 2007. Host range and receptor utilization of canine distemper virus analyzed by recombinant viruses: involvement of heparin-like molecule in CDV infection. Virology 359:324–335 [DOI] [PubMed] [Google Scholar]
- 7. Lan NT, Yamaguchi R, Hirai T, Kai K, Morishita K. 2009. Relationship between growth behavior in Vero cells and the molecular characteristics of recent isolated classified in the Asia 1 and 2 groups of canine distemper virus. J. Vet. Med. Sci. 71:457–461 [DOI] [PubMed] [Google Scholar]
- 8. Lan NT, et al. 2006. Comparative analyses of canine distemper viral isolates from clinical cases of canine distemper in vaccinated dogs. Vet. Microbiol. 115:32–42 [DOI] [PubMed] [Google Scholar]
- 9. Lan NT, et al. 2009. First isolation and characterization of canine distemper virus in Vietnam with the immunohistochemical examination of the dog. J. Vet. Med. Sci. 71:155–162 [DOI] [PubMed] [Google Scholar]
- 10. Lan NT, Yamaguchi R, Uchida K, Sugano S, Tateyama S. 2005. Growth profiles of recent canine distemper isolates on Vero cells expressing canine signalling lymphocyte activation molecule (SLAM). J. Comp. Pathol. 133:77–81 [DOI] [PubMed] [Google Scholar]
- 11. Leonard VH, et al. 2008. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 118:2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loffler S, et al. 1997. CD9, a tetraspan transmembrane protein, renders cells susceptible to canine distemper virus. J. Virol. 71:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muhlebach MD, et al. 2011. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480:530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noyce RS, et al. 2011. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 7:e1002240 doi:10.1371/journal.ppat.1002240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmid E, et al. 2000. Antibodies to CD9, a tetraspan transmembrane protein, inhibit canine distemper virus-induced cell-cell fusion but not virus-cell fusion. J. Virol. 74:7554–7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seki F, Ono N, Yamaguchi R, Yanagi Y. 2003. Efficient isolation of wild strains of canine distemper virus in Vero cells expressing canine SLAM (CD150) and their adaptability to marmoset B95a cells. J. Virol. 77:9943–9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singethan K, et al. 2006. CD9-dependent regulation of canine distemper virus-induced cell-cell fusion segregates with the extracellular domain of the haemagglutinin. J. Gen. Virol. 87:1635–1642 [DOI] [PubMed] [Google Scholar]
- 18. Tahara M, et al. 2008. Measles virus infects both polarized epithelial and immune cells by using distinctive receptor-binding sites on its hemagglutinin. J. Virol. 82:4630–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takasu T, et al. 2003. A continuing high incidence of subacute sclerosing panencephalitis (SSPE) in the Eastern Highlands of Papua New Guinea. Epidemiol. Infect. 131:887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takeda M, Tahara M, Nagata N, Seki F. 2011. Wild-type measles virus is intrinsically dual-tropic. Front. Microbiol. 2:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Moll P, Alldinger S, Baumgartner W, Adami M. 1995. Distemper in wild carnivores: an epidemiological, histological and immunocytochemical study. Vet. Microbiol. 44:193–199 [DOI] [PubMed] [Google Scholar]
- 22. von Messling V, Milosevic D, Cattaneo R. 2004. Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl. Acad. Sci. U. S. A. 101:14216–14221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L-F, et al. 2012. Family Paramyxoviridae, p 672–685 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy, 9th ed Elsevier Academic Press, London, United Kingdom [Google Scholar]