Abstract
The distal portion of rotavirus (RV) VP4 spike protein (VP8*) is implicated in binding to cellular receptors, thereby facilitating viral attachment and entry. While VP8* of some animal RVs engage sialic acid, human RVs often attach to and enter cells in a sialic acid-independent manner. A recent study demonstrated that the major human RVs (P[4], P[6], and P[8]) recognize human histo-blood group antigens (HBGAs). In this study, we performed a phylogenetic analysis of RVs and showed further variations of RV interaction with HBGAs. On the basis of the VP8* sequences, RVs are grouped into five P genogroups (P[I] to P[V]), of which P[I], P[IV], and P[V] mainly infect animals, P[II] infects humans, and P[III] infects both animals and humans. The sialic acid-dependent RVs (P[1], P[2], P[3], and P[7]) form a subcluster within P[I], while all three major P genotypes of human RVs (P[4], P[6], and P[8]) are clustered in P[II]. We then characterized three human RVs (P[9], P[14], and P[25]) in P[III] and observed a new pattern of binding to the type A antigen which is distinct from that of the P[II] RVs. The binding was demonstrated by hemagglutination and saliva binding assay using recombinant VP8* and native RVs. Homology modeling and mutagenesis study showed that the locations of the carbohydrate binding interfaces are shared with the sialic acid-dependent RVs, although different amino acids are involved. The P[III] VP8* proteins also bind the A antigens of the porcine and bovine mucins, suggesting the A antigen as a possible factor for cross-species transmission of RVs. Our study suggests that HBGAs play an important role in RV infection and evolution.
INTRODUCTION
Group A rotaviruses (RVs) are major pathogens associated with acute gastroenteritis in young children and animals worldwide (37). RVs contain an 11-segment double-stranded RNA genome within a triple-layered protein capsid. The outermost layer consists of two major surface proteins, VP4 and VP7, which play an important role in initiating a viral infection via attachment and entry. VP7 is a glycoprotein forming a smooth shell of the virion. Through proteolysis, VP4 is cleaved into two fragments, named VP5* and VP8*. VP8*, the globular head of the spike, interacts with the host receptor, allowing virions to attach to host cells (see below), while VP5*, the stalk of the spike, may be responsible for penetration of the processed double-shell particles into hosts (10, 12, 56).
Early studies showed that some animal RVs recognize sialic acid as a host receptor for attachment (54). However, later studies using neuraminidase sensitivity assays on a large number of RVs showed that only a few P genotypes of animal RVs were sialic acid dependent, while human RVs and the majority of animal RVs were sialidase or neuraminidase insensitive (6, 7, 14, 28). Subsequent studies showed that the requirement of sialic acid is associated with the P genotypes but independent from the host origin of the strains (7), supporting the notion that VP4 is responsible for interaction with the host and viral attachment. The lack of a defined receptor for the sialidase-insensitive RVs prompted further studies that led to discoveries of a number of potential host receptors, including the heat shock protein (29, 30, 55), ganglioside (9, 18, 25), and integrins (8, 16, 20). While these studies provided valuable information, the roles of these proteins in the attachment or penetration of RVs and the involved viral proteins such as VP5* and/or VP8* remain elusive. A recent study, using nuclear magnetic resonance (NMR) spectroscopy, molecular modeling, and infectivity competition assays, demonstrated a direct interaction between a neuraminidase-insensitive human RV (Wa) and sialic acid, arguing against the widely accepted paradigm that sialic acids are irrelevant in host cell recognition by the neuraminidase-insensitive RVs (19). Thus, our current understanding of the interaction between RV and its receptor remains limited.
In a recent study, we demonstrated that the recombinant VP8* proteins of a number of human RVs interacted with the human histo-blood group antigens (HBGAs) (24). This interaction was found to be strain specific and appeared to be genetically related. Two closely related P genotypes, P[4] and P[8], were found to bind the Lewis b (Leb) and H-type 1 antigens, while the genetically more distant P[6] recognized H-type 1 only. The observed pattern of the binding was further supported by a binding assay using highly purified triple-layer RV particles. Thus, the sialic acid-independent human RVs may recognize alternative carbohydrates, the human HBGAs, as receptors.
These findings resemble the phenomena observed in caliciviruses (CVs). Different CVs recognize different carbohydrate receptors, in which the feline CVs and murine noroviruses (NoVs) recognize sialic acids (42, 50), while human NoVs recognize the human HBGAs (23, 32). Significant variation of HBGA binding patterns that related to all major ABO, secretor, and Lewis types have been described previously (22, 23, 46, 47). Structure and functional studies also showed that NoVs share an HBGA binding interface among strains within each genogroup, indicating that the host HBGAs play an important role in NoV evolution (45, 49). Corresponding to the polymorphism of human HBGAs, human NoVs have been found to be highly diverse in their interaction with different ABO, secretor, and Lewis HBGAs. The P genotypes of RVs are also highly diverse. Thus, questions have been raised of whether HBGAs also play a role in RV evolution and whether RVs have variation in HBGA binding patterns similar to those observed in NoVs.
A further issue is the host range and mechanism of cross-species transmission of RVs. It is known that most RVs have a host range limited to either humans or animals. For example, the three P genotypes P[4], P[8], and P[6], which are responsible for over 95% of human RV infections, are rarely found in animals (P[6] infects only sporadically swine). In contrast, some other P genotypes were only found in animals, such as P[13] in swine, P[21] in cattle, P[12] in horses, and P[22] in rabbits. However, a few other P genotypes can apparently be transmitted between species. For example, P[9] strains can infect humans, cats, and dogs (17, 40). The human P[14] strains might have originated from even-toed ungulates belonging to the mammalian order Artiodactyla (35). Thus, studies to determine the factor(s) that controls the host ranges and mechanism of cross-species transmission of RVs are necessary.
In this study, we performed a phylogenetic analysis on the VP8* sequences of RVs and classified RVs into five P genogroups (P[I] to P[V]) among 2,107 RV VP8* proteins representing 35 P genotypes in GenBank. We found a clear segregation of RVs among the five genogroups according to their infection in humans versus animals. In addition, we characterized the HBGA binding profiles of three RV strains representing the P[9], P[14], and P[25] genotypes in the P[III] genogroup. A new pattern of binding to the A antigen was observed, which is distinct from those of the P[II] RVs described previously. The potential roles of HBGAs in the host range, cross-species transmission between humans and animals, and evolution of RVs are discussed.
MATERIALS AND METHODS
RV VP8* sequences.
All available RV VP4 sequences in GenBank were downloaded. Sequences containing the intact protease-resistant VP8* core (amino acid [aa] 46 to 231) were selected for further analysis. A total of 2107 deduced amino acid sequences were collected and used to construct a local BLAST database by using BLAST+ executable software (ftp://ftp.ncbi.nlm.nih.gov/blast/executables/blast+/LATEST).
Sequence grouping and phylogenetic analysis.
Sequences of the prototype strains for each P genotype were used as query sequences to run BLAST searches against the GenBank database. Based on the alignment similarity scores (E-values) for query sequences with known P genotypes, 2,107 sequences in the database were assigned to one of the 35 P genotypes. All sequences in each P genotype were used to construct a local phylogenetic tree for the genotype, and then one or a few representative strains from each P genotype were selected to build a complete phylogenetic tree representing all 35 known P genotypes.
Phylogenetic and molecular evolutionary analyses were conducted using MEGA, version 4.1, software. Genetic distances were calculated using the Poisson correction parameters, and dendrograms were constructed using the neighbor-joining method (39). To determine the cutoff values for distance ranges of RVs within and between P genotypes and between P genogroups, the percentages of amino acid identities among all unique VP8* sequences were calculated using the pairwise distances program of the MEGA program (39).
Cloning, expression, and purification of VP8*.
cDNA of the VP8* sequences of a human P[9] strain (GenBank accession no., AB077766), a P[14] strain (GenBank accession no., GQ398013), and a P[25] strain (GenBank accession no., GU199495) were synthesized by GenScript (GenScript USA Inc., Piscataway, NJ). The VP8* core sequences were then subcloned into plasmid pGEX-4T-1 (GE Healthcare Life Sciences, Piscataway, NJ) between the BamHI and SalI sites. After confirmation of the inserts through DNA sequencing, glutathione S-transferase (GST)-VP8* core fusion proteins were expressed in Escherichia coli strain BL21 with an induction of isopropyl-β-d-thiogalactopyranoside (IPTG; 0.4 mM) at room temperature (∼22°C) overnight. The GST-VP8* fusion proteins were purified using glutathione Sepharose 4 Fast Flow (GE Healthcare Life Sciences) according to the manufacturer's protocol. Bacterial lysates were allowed to bind to the Sepharose beads for 2 h at room temperature, followed by washing with phosphate-buffered saline (PBS) 4 to 6 times, and the GST fusion proteins were eluted by using glutathione elution buffer (10 mM reduced glutathione, 50 mM Tris-Cl [pH 8.0]). Free VP8* was released from GST by thrombin digestion at room temperature overnight. The yields of the GST fusion proteins were around 5 to 10 mg/liter bacterial culture, and the purity of the proteins was checked by SDS-PAGE electrophoresis.
HBGA binding assay.
The binding of VP8* to HBGAs was measured by saliva- and synthetic oligosaccharide-based binding assays as described previously (23). A panel of previously well-defined A, B, O, secretor, and Lewis types of saliva samples were used as sources of HBGAs in the saliva-based binding assays. Boiled saliva samples were diluted 1:1,000 and used to coat 96-well microtiter plates (Dynex Immulon; Dynatech, Franklin, MA) at 4°C overnight. After blocking with 5% nonfat milk, 100 μl of VP8* proteins (10 μg/ml) was added and samples were incubated at 37°C for 1 h. The bound VP8* proteins were detected using a guinea pig serum anti-VP8* at 1:4,000 followed by horseradish peroxidase (HRP)-conjugated goat anti-guinea pig IgG (ICN, Aurora, OH). The signal intensities were displayed using a TMB (3,3′,5,5′-tetramethylbenzidine) kit (Kirkegaard and Perry Laboratories, Gaithersburg, MD). In each step, the plates were incubated for 1 h at 37°C and washed five times with PBS-Tween 20 using a plate washer.
For the oligosaccharide-based binding assays, microtiter plates were coated with recombinant VP8* (10 μg/ml) at 4°C overnight. After blocking with 5% nonfat milk, the synthetic oligosaccharide-polyacrylamide (PAA)-biotin conjugates (2 μg/ml) were added and incubated at 4°C overnight. Oligosaccharides used in the present study included conjugates of Lewis antigens (Lea, Leb, Lex, and Ley), H type1, H type 2, H type 3, type A and type B disaccharides, type A and type B trisaccharides, sialyl-Lea, and sialyl-Lex (GlycoTech, Inc., Gaithersburg, MD). Bound oligosaccharides were detected using HRP-conjugated-streptavidin (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) and displayed using the TMB kit mentioned above.
Binding of VP8* to bovine and porcine mucins.
Bovine and porcine mucins were obtained from Sigma-Aldrich Co. (St. Louis, MO) and were used as the source of HBGAs to bind the RV P[9], P[14], and P[25] VP8*. The same procedures as in the saliva-based binding assays were used except for coating the plates with the two animal mucins. The carbohydrate profiles of the two animal mucins were also determined by binding assays using monoclonal antibodies against human ABH and Lewis HBGAs described in our previous studies (23).
To further confirm the specificity of P[9] and P[14] VP8* protein binding to the A antigen of mucins from animals, porcine mucin was fractionated through a Superdex 200 column powered by an AKTA fast-protein liquid chromatography (FPLC) system (model 920; Amersham Biosciences, Piscataway, NJ). Each fraction was tested for the A antigen content and for binding to the P[9] and P[14] VP8* proteins. Moreover, the specificity of binding to the A antigen was further demonstrated by treating the mucins with a glycosidase specific to the N-acetylgalactosamine residue (ProZyme, Inc., Hayward, CA) prior to the binding assay.
Prediction of receptor binding interfaces of P[9] and P[14] VP8* by homology modeling and docking.
In order to map potential HBGA binding sites, we performed docking simulations using AutoDock, version 4.0 (http://autodock.scripps.edu/) (36, 39). A high-quality homology-based model of the P[9] VP8* protein was obtained from the ModBase database (http://modbase.compbio.ucsf.edu). This homology model (ModBase model Q86162) was built by using the crystal structure (Protein Data Bank identifier [PDB ID], 1KQR) (11) of a P[3] rhesus rotavirus VP8* (GenBank accession no. AY033150) as the template. The amino acid identity between the two capsid proteins was 51.9%, and the model was generated automatically by the ModBase server (http://modbase.compbio.ucsf.edu/). Multiple docking simulations were performed to assess the effects of sampling, validate the results, and perform sensitivity analysis. In particular, different simulation grids, effects of partial charges, and other parameter setups were assessed. The results were further analyzed using Polyview-MM in order to assess clustering of docking poses, residues in contact with the ligand, and overall convergence of the simulations.
Site-directed mutagenesis.
Mutant VP8* proteins with single amino acid substitutions were designed and constructed by site-directed mutagenesis using the wild-type VP8* constructs as templates. Site-directed mutagenesis was conducted using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and the corresponding primer pairs with mutations (the primer information is available upon request) as described previously (43, 44, 48, 49). Mutant VP8* proteins were expressed and purified using the same procedures as for the wild-type VP8*, described above. None of the designed single residue mutations in this study revealed any detectable reduction in reactivity to the hyperimmune serum against VP8* compared with that of the wild-type VP8*.
Preparation of RV TLPs by CsCl gradient centrifugation and their binding to saliva samples.
A cell culture-adapted RV P[9] strain, Arg 720 (5), was cultivated in MA104 cells. After three cycles of freeze-thawing, infected MA104 cultures were clarified by centrifugation at 4,500 × g for 30 min. The viruses in the supernatants were concentrated by pelleting at 141,000 × g for 3 h using an SW28 rotor (Beckman Coulter, Inc., Danvers, MA). Virus pellets were suspended in TNC buffer (50 mM Tris-Cl [pH 7.5], 150 mM NaCl, 10 mM CaCl2). CsCl was added and adjusted to a final density of refractive index 1.369 before the samples were centrifuged at 288,000 × g for 48 h using an SW41Ti rotor. Virus triple-layered particle (TLP) band was collected from the gradient by side puncture. The presence of TLPs in the band was further confirmed by the densities of the collected sample (1.36 g/cm3 in CsCl) and by electron microscopy (EM) examination with negative staining after the samples were dialyzed against the TNC buffer.
The saliva binding assay of TLP virions was performed using the same methods as for the binding of the VP8* protein described above. Briefly, boiled saliva samples at a dilution of 1:1,000 were used to coat 96-well microtiter plates at 4°C overnight. After blocking with 5% nonfat milk, the TLPs were added at 1:20 and incubated at 4°C overnight. The binding signals were then detected using the reagents included in the Rotaclone kit (Meridian Biosciences, Cincinnati, OH).
Hemagglutination (HA) of VP8* and TLPs of the P[9] RV.
Human red blood cells (RBCs) of A, B, and O types (Immucor, Inc., Norcross, GA) were washed twice in PBS before use. A total of 50 μl of 1% RBCs was added to 50-μl serial dilutions of either GST-VP8* fusion proteins or TLPs on 96-well V-bottom plates (Greiner Bio-One, Monroe, NC). Agglutination was determined after 3 h of incubation at 4°C.
RESULTS
Grouping of RVs into P genotypes based on VP8* sequences.
Of all VP4 sequences identified in GenBank using standard BLAST searches, 2,107 sequences contained an intact VP8* core (aa 46 to 231), and they were selected for the study. Due to incompleteness, many partial VP4 sequences in GenBank did not have an assigned P genotype. The P genotypes of these sequences were assigned by identifying the closest (lowest BLASTp E-value) match with assigned genotype. As a result, all 2,107 sequences were assigned to one of 35 distinct P genotypes.
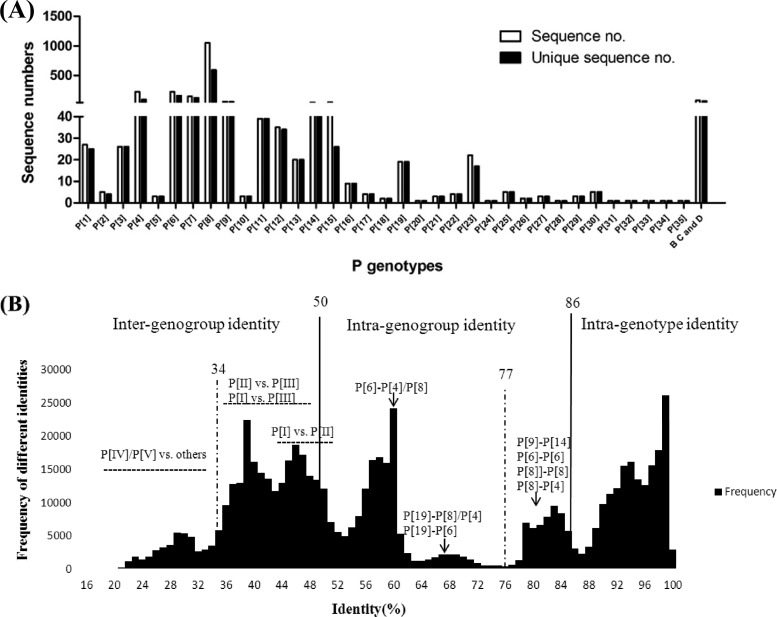
The numbers of sequences in each P genotype were not distributed evenly (Fig. 1A). For example, the three largest P genotypes were P[8] (1,052, 49.9%), P[6] (224, 10.6%), and P[4] (222, 10.5%), which counted for ∼71% of the total sequences studied. Low-abundance P genotypes had only a few sequences (P[5], P[10], P[18], P[21], P[26], and P[29]) or even one sequence (P[20], P[24], P[31], P[32], P[33], and P[34]). Besides group A RVs, 75 complete VP8* sequences from group B, C, and D RVs were identified. No VP4 or VP8* sequences of groups E, F, and G were found in GenBank.
Fig 1.
Sequence numbers for different P genotypes and the distribution of pairwise sequence identity of rotavirus VP8* proteins. (A) Sequence numbers for each of the genotypes. A total of 2,107 available VP8* sequences in GenBank were grouped into 35 genotypes of group A RVs and group B, C, and D RVs. The unique sequences were also included in this figure after excluding identical sequences concerning the region from aa 46 to 231. Identical sequences in the original VP8* sequence pool were excluded using BioEdit software (version 7.0.9.0). (B) The remaining 1,319 unique group A RV sequences were analyzed for pairwise sequence identities within or between genotypes. The graph was constructed by plotting all the calculated pairwise identities, with the percent identities in the abscissa (x axis) and the frequency of each calculated pairwise identities in the ordinate (y axis). To determine a cutoff value, we chose a ratio below and closest to 1 between inter- and intragenotype identities. Based on this principle, the proposed intra- and intergenotype and intra- and intergenogroup identity ranges for the potential cutoff value are shown. A cutoff value of 86% sequence identity for intergenotypes and a 50% cutoff value for intercluster (genogroup) distance were selected.
The host species of each P genotype also varied (Table 1). Many RV genotypes exhibit strict species specificity. For example, the major human genotypes P[8], P[4], and P[6] infect almost exclusively humans, with only 18 reported P[6] infections in swine. Genotypes P[10], P[28], and P[25] were also found in humans exclusively, although only a few sequences were reported for each genotype. Genotypes P[13], P[23], P[26], P[27], P[32], and P[34] appear to infect swine exclusively. Similarly, genotypes P[5], P[21], P[29], and P[33] were found in bovine, P[18] and P[12] in equine, P[16] and P[20] in murine, and P[17], P[30], P[31], and P[35] in avian species, respectively. Some P genotypes have apparently broader host ranges. For example, P[1], P[2], P[3], and P[7] RVs infect a wide variety of animal species, with a few cases reported for humans. Genotypes P[9] and P[14] cause disease in humans but have also been found in animal species in many countries (35, 38).
Table 1.
Species distribution of different P genotypes
| Genogroup | P genotype | Rank of species distribution |
|---|---|---|
| P[I] | P[1] | Bovine > human > porcine > simian > caprine > equine > camelid (guanaco) |
| P[2] | Simian > human | |
| P[3] | Canine > human > feline > simian > bovine > caprine (Capra hircus) | |
| P[5] | Bovine | |
| P[7] | Porcine > bovine > human | |
| P[10] | Human | |
| P[12] | Equine | |
| P[13] | Porcine | |
| P[15] | Ovine (lamb) > bovine | |
| P[16] | Murine | |
| P[18] | Equine | |
| P[20] | Murine | |
| P[21] | Bovine | |
| P[22] | Lupine | |
| P[23] | Porcine | |
| P[24] | Simian (rhesus) | |
| P[26] | Porcine | |
| P[27] | Porcine | |
| P[28] | Human | |
| P[29] | Bovine | |
| P[32] | Porcine | |
| P[33] | Bovine | |
| P[34] | Porcine | |
| P[II] | P[4] | Human |
| P[6] | Human > porcine | |
| P[8] | Human | |
| P[19] | Porcine > human | |
| P[III] | P[9] | Human > feline > canine |
| P[14] | Human > bovine > lupine > caprine > camelid (guanaco) > bovid (sable antelope) | |
| P[25] | Human | |
| P[IV] | P[11] | Bovine > human |
| P[V] | P[17] | Avian (chicken) > bovine |
| P[30] | Avian (chicken) | |
| P[31] | Avian (chicken) | |
| P[35] | Avian (turkey) |
Phylogenetic tree of RV VP8* sequences.
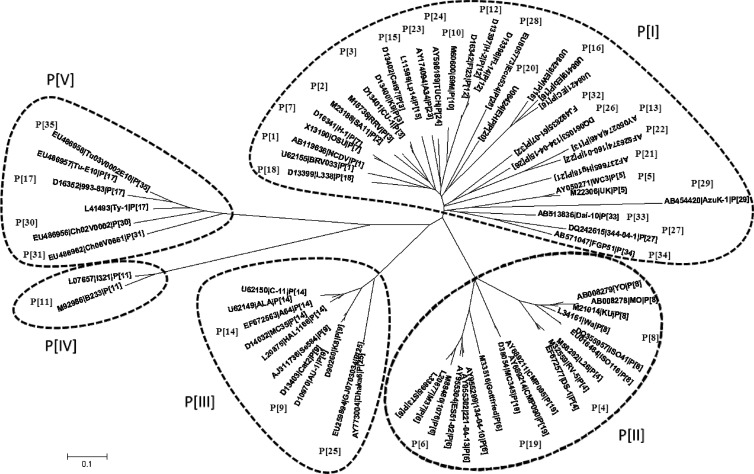
We first performed phylogenetic analyses for P genotypes containing a large number of sequences. The goal of this intermediate step was to facilitate the generation and assessment of the overall phylogenetic tree for all 35 P genotypes by selecting representative sequences for large clades (P genotypes). For genotypes P[8], P[4], and P[6] of human RVs, reference sequences representing major branches in the local phylogenetic trees of each P genotype were selected using the MEGA program, as described in Materials and Methods. For P genotypes with a limited number of sequences, one or two prototype sequences were selected. As a result, a total of 71 reference sequences representing all 35 P genotypes were selected to construct the integrated phylogenetic tree.
We also performed phylogenetic analysis among genetically closely related P genotypes to further validate the P genotype assignment and to assess the genetic relationship among closely related P genotypes (data not shown). In this analysis, sequences from most of the 35 P genotypes were segregated well according to their assigned P genotypes. However, overlaps were observed between P[2] and P[3] and between P[13] and P[22], indicating that strains in each of the two pairs may belong to the same genetic lineages as related to the biological function of VP8*. For the three largest genotypes, P[8], P[4], and P[6], of the human RVs, all sequences were clustered in well-defined clades, with two major branches in P[8] and P[6] but no major branches in P[4].
On the basis of the phylogenetic tree of the 71 reference sequences representing the 35 P genotypes (Fig. 2), RVs were grouped into five P genogroups (P[I] to P[V]) using 50% pairwise sequence identity between genogroups (Fig. 1B) as a cutoff value. Theoretically, if the numbers of sequences are evenly distributed among different genotypes, the cutoff value would fall within the minimum in the graph of pairwise identity versus frequency. However, in our study, the numbers of sequences varied significantly among different genotypes as shown in Fig. 1A, which resulted in bias with cutoff values falling around but not exactly in the minimum value. Genogroup P[I] contained the most P genotypes (n = 23), including the sialic acid-dependent genotypes P[1], P[2], P[3], and P[7] and many others that infect different animal species. P[II] included the most epidemic human genotypes P[8], P[4], and P[6] as well as the less prevalent P[19], which infects both humans and swine. P[III] included genotypes P[9], P[14], and P[25] (data not shown), while P[IV] contained only genotype P[11], which infects mainly bovines, with a few human cases. P[V] contained four P genotypes that infect avian species.
Fig 2.
Phylogenetic tree of rotavirus VP8*. The phylogenetic tree was generated based on the VP8*sequences (aa 46 to 231) using the neighbor-joining method. Seventy-one prototype and/or reference rotaviruses, representing all 35 known genotypes, were selected. Sequence information for the 71 selected strains is shown in the order of GenBank accession number, strain name, and P genotype.
The VP8* proteins of P[III] RVs recognized the type A HBGAs.
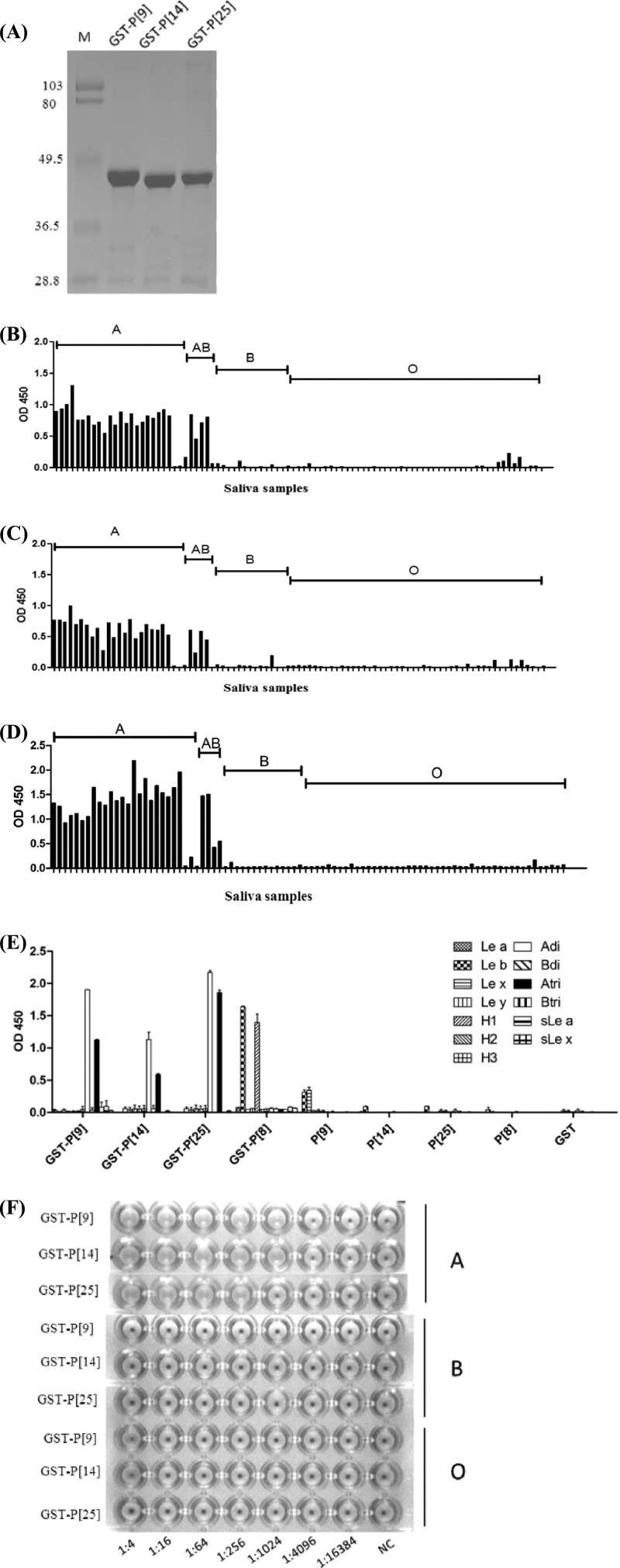
Three strains representing P[9], P[14], and P[25] genotypes of P[III] RVs were studied for their interaction with human HBGAs. The recombinant GST-VP8* fusion proteins of all three strains revealed strong binding to type A human saliva (Fig. 3B to D) as well as hemagglutination with human type A red blood cells (RBCs) (Fig. 3F). The specificity of reaction with the A antigen was further verified by oligosaccharide-based binding assay, in which the A disaccharide exhibited much higher binding affinity than that of the A trisaccharide (Fig. 3E). As a control, the recombinant VP8* protein from a P[8] strain characterized in our previous studies (24) bound to the H-type 1 and Leb oligosaccharides only. Also consistent with our previous observation (24), neither free VP8* proteins without a GST tag nor the purified GST alone show any binding signal (Fig. 3E).
Fig 3.
Hemagglutination and saliva- and synthetic oligosaccharide-based binding of P[9], P[14], and P[25] VP8* proteins. (A) SDS-PAGE analysis of the purified recombinant VP8* proteins of P[9], P[14], and P[25] rotaviruses. The GST-VP8* fusion protein is ∼45 kDa. “M” indicates a prestained protein marker (low range; Bio-Rad), with bands from the top to the bottom representing 103, 80, 49.5, 36.5, and 28.8 kDa, respectively. (B to D) Binding of P[9] (B), P[14] (C), and P[25] (D) GST-VP8* fusion proteins to a panel of well-characterized saliva samples. (E) Binding of the recombinant VP8*proteins to the synthetic oligosaccharides. Polyacrylamide (PAA)-biotin conjugates of Lewis antigens (Lea, Leb, Lex, and Ley), H type1 (H1), H type 2 (H2), H type 3(H3), type A and type B disaccharides (Adi and Bdi), type A and type B trisaccharides (Atri and Btri), sialyl-Lea (sLe a), and sialyl-Lex (sLe x) were used in the binding assay. The GST-VP8* fusion protein from a P[8] strain expressed previously in our lab was also used in the binding assay as a control to confirm the binding specificity observed in this study on the recombinant proteins of P[9], P[14], and P[25]. Recombinant proteins with or without the GST tag and the GST tag alone were tested. (F) Hemagglutination assay of the recombinant VP8* proteins. GST-VP8* proteins were serially diluted with PBS, and the PBS buffer without recombinant proteins was used as the negative control (NC). OD 450, optical density at 450 nm.
The VP8* proteins of P[III] RVs also bound bovine and porcine mucins.
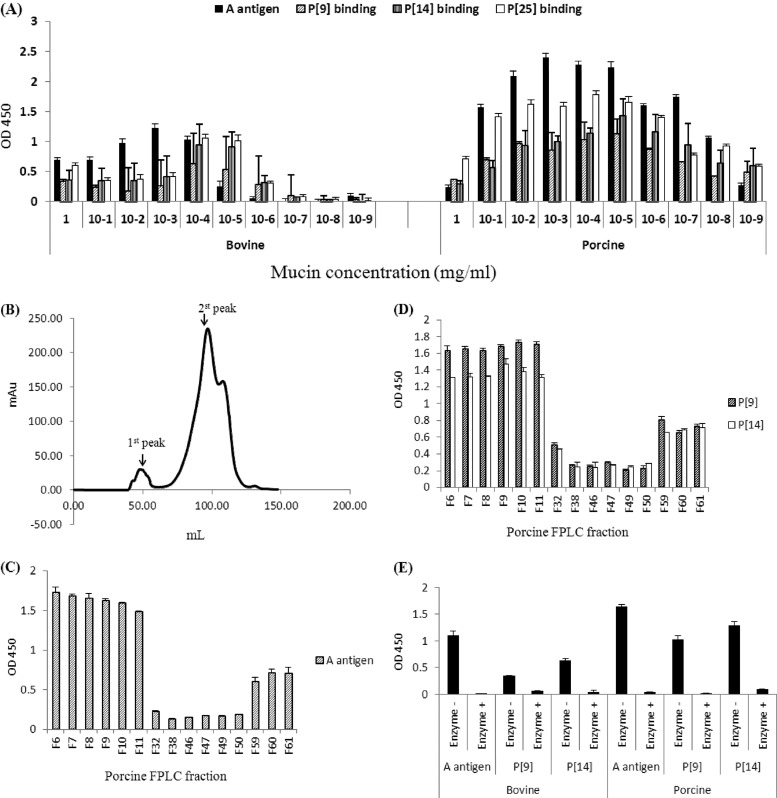
Since the type A antigens are expressed in many animal species, we tested the binding of the VP8* proteins of the three P[III] RVs with bovine and porcine mucins. Clear associations between the VP8* binding and the A antigen concentrations in the mucins of both species were observed (Fig. 4A). Further examination of the porcine mucin following a gel filtration fractionation indicated that the A antigens, mainly distributed in the high-molecular-weight fractions, correlated with the binding signals of the P[9] and P[14] VP8* proteins (Fig. 4B to D). To further confirm the results, the binding assay was performed after a treatment of the bovine and porcine mucins with the GalNAc glycosidase to remove the A epitope. Loss of binding following the enzyme treatment was observed for both P[9] and P[14] VP8* (Fig. 4E).
Fig 4.
Binding of recombinant rotavirus VP8* to porcine and bovine mucins. (A) GST-VP8* fusion proteins of P[9], P[14], and P[25] were tested for binding to serial dilutions of boiled porcine and bovine mucins. The A antigen signal in each dilution is shown. (B) Gel filtration profiles of the porcine mucins. Fractions from each major peak were collected. (C to D) The A antigen signals (C) and the binding of P[9] and P[14] VP8* (D) to these fractions were determined by enzyme-linked immunosorbent assay as shown (F6 to F11 were fractions from the 1st peak, F32 to F50 were fractions from the 2nd peak, and F59 to F61 were fractions after the 2nd peak). (E) Effect of enzymatic removal of the A epitope on the rotavirus VP8* binding to porcine and bovine mucins. F6 from the high-molecular-weight peaks (the 1st peaks) of the porcine and bovine mucins was treated with an N-acetylgalactosaminidase. The signals of the A antigen and the binding of the P[9] and P[14] VP8* to the mucins before and after the enzyme treatment were measured. Error bars show the standard deviations from two experiments. OD 450, optical density at 450 nm.
Mapping of the HBGA binding site on VP8* by docking simulations and mutagenesis analysis.
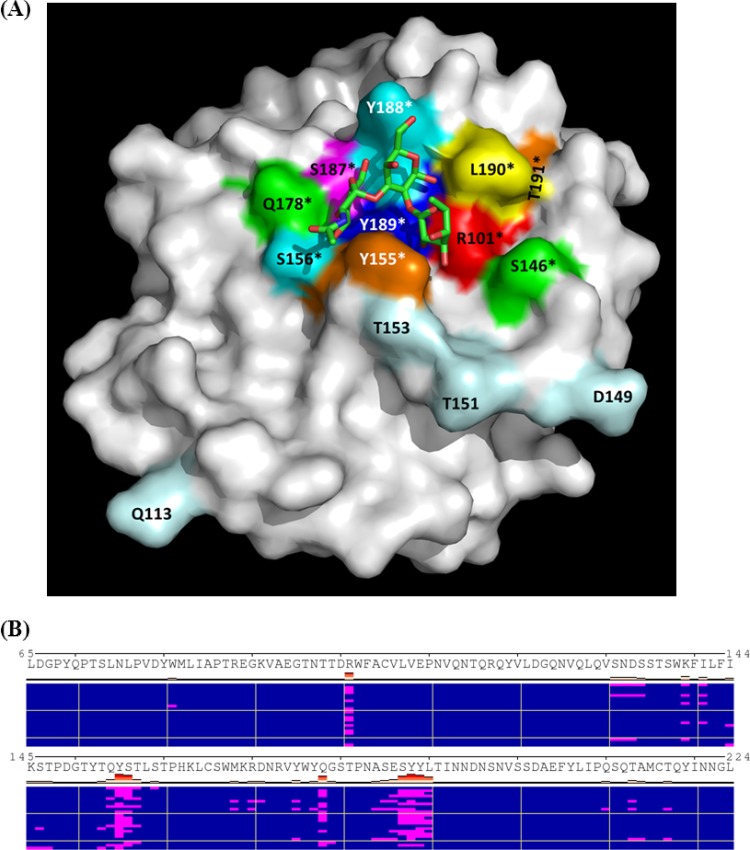
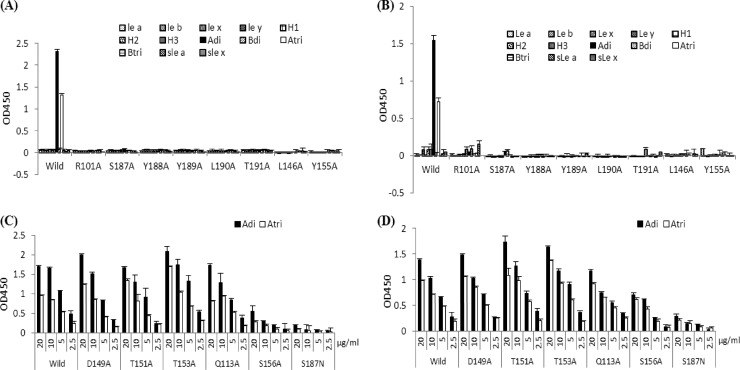
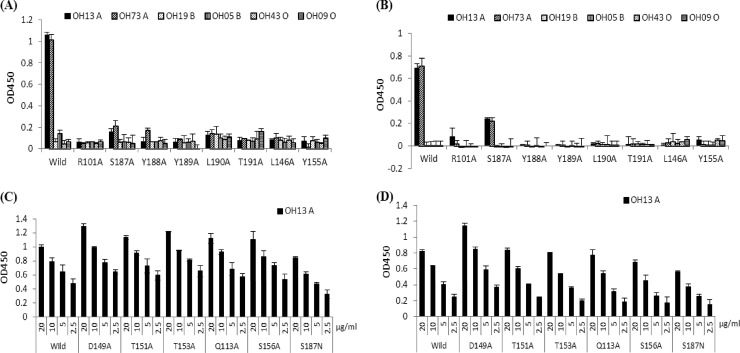
Docking simulations using AutoDock (version 4.0) and a homology model of the capsid protein of a P[9] strain (39) identified a shallow pocket around residue S187 as the top A antigen binding site. The predicted binding pocket corresponds to the carbohydrate binding site of the sialic acid-dependent RVs (Fig. 5) (11). Based on this model, we performed mutagenesis analysis for both P[9] and P[14] strains. Single amino acid mutations at eight residues—R101A, S146A, Y155A, S187A, Y188A, Y189A, L190A, and T191A—within the predicted binding pocket for both P[9] and P[14] strains have led to a complete loss of A antigen binding ability, shown by both the saliva- and oligosaccharide-based binding assays (Fig. 6A and B and Fig. 7A and B). Sequence alignment results showed a high degree of conservation of these amino acids of all three P genotypes (P[9], [14], and [25]) in P[III], except for a single amino acid change (S187N) in P[25] (data not shown). Interestingly, S187N mutation in P[25] seemingly did not affect the ability of the mutant to bind to type A saliva, although it did reduce the binding to type A oligosaccharides (Fig. 6C and D and 7C and D). Similarly, a mutation at S156, a residue at the edge of the binding pocket, resulted in reduced binding to the A oligosaccharides but not to A saliva. None of the single amino acid mutations away from the binding pocket (D149A, T151A, T153A, and Q113A) had an influence on the binding. Moreover, the binding results for these mutants were also confirmed by HA assay (data not shown). In conclusion, the P[III] human RVs and the sialic acid-dependent RVs share a carbohydrate binding site, although their amino acid compositions and modes of binding to their corresponding ligands may be different.
Fig 5.
Modeling of HBGA-binding interface of a P[9] VP8*. The homology model of a P[9] VP8* from ModBase was used for modeling the HBGA binding site by docking simulations. (A) P[9] VP8* is shown in surface representation, with indication of a predicted HBGA-binding pocket that is formed by residues R101, Y155, S156, Q178, S187, Y188, Y189, and L190. The best pose of the A trisaccharide in stick representations with carbon (green), oxygen (red), and nitrogen (blue) atoms of the trisaccharide is shown. The binding mode of the A trisaccharide in the binding pocket was predicted based on a calculated lowest energy. The amino acid residues involved in the HBGA interactions are labeled in color and with asterisks. Amino acid residues away from the binding pocket tested in the mutagenesis study are also labeled in light blue. (B) Clusters of docking poses of the A trisaccharide to VP8* by AutoDock. A total of 23 distinct clusters (of docking poses), each represented by a row, are shown. Residues in contact with the ligand in each pose (highlighted as red bars) are shown in magenta. Frequent hits of the protein by the A trisaccharide at residues around the binding pocket shown in panel A (R101, Y155, S156, Q178, S187, Y188, Y189, and L190) are apparent.
Fig 6.
Oligosaccharide binding of VP8* mutants with single amino acid changes in the binding pocket or away from the pocket. Most of the VP8* mutants with a single mutation in the binding pockets lost the binding activities completely for all tested HBGAs for both P[9] (A) and P[14] (B) rotavirus strains (protein concentrations of 10 μg/ml were used in the binding assay). Abbreviations of the oligosaccharide conjugates are the same as in Fig. 3. Mutants that retained binding to the A antigen were further tested for their binding to A di- and trisaccharides with serial dilutions of VP8* proteins in 20, 10, 5, and 2.5 μg/ml, as shown in panels C and D for P[9] and P[14], respectively. Error bars show the standard deviations from three experiments.
Fig 7.
Saliva-based binding results of various mutant VP8* proteins with single amino acid changes in or away from the binding interface. Saliva samples representing type A, B, and O secretors were used. Mutants (10 μg/ml) that dramatically lost the ability to bind the type A saliva are shown in panels A and B for P[9] and P[14], respectively. Mutants that retained the ability to bind to type A saliva are shown in panels C and D for P[9] and P[14], respectively, with variable concentrations of the VP8* proteins (20, 10, 5, and 2.5 μg/ml). Error bars show the standard deviations from three experiments.
RV virions bound HBGAs.
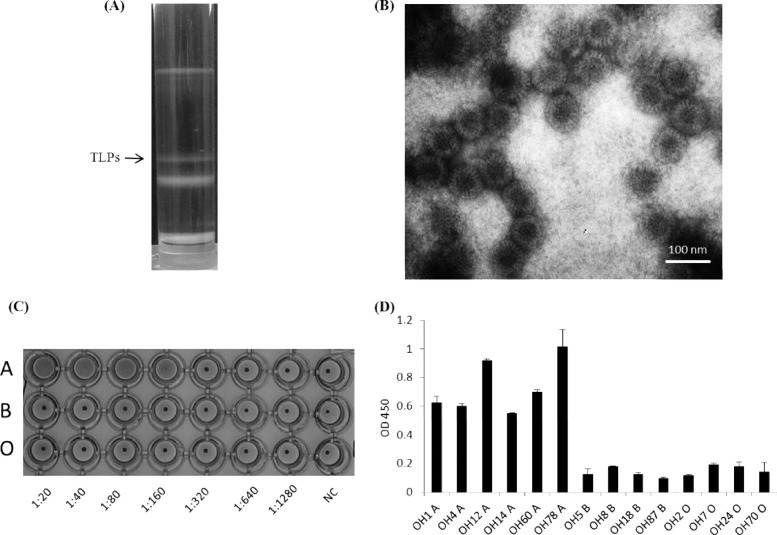
To further determine the biological relevance of our finding, binding assays on authentic RVs from a well-characterized P[9] RV (Arg 720) (5) were performed (Fig. 8). Triple-layered particles (TLPs) of Arg 720 purified by a CsCl gradient (Fig. 8A) were confirmed by the density of the band and electron microscopy (EM) (Fig. 8B). Strong binding of TLPs to the type A antigen was observed by both hemagglutination and saliva-based binding assays (Fig. 8C and D). These results suggested that the ability of RVs to bind HBGAs is an authentic nature of RVs, in which the spike protein VP8* is likely to be involved in the binding.
Fig 8.
Purification, hemagglutination (HA), and binding to saliva samples of the triple-layered particles (TLPs) of a P [9] rotavirus (Arg 720 strain). (A) CsCl gradient purification with indication of the TLP band. (B) The TLP band (density, 1.36 g/cm3 in CsCl) was confirmed by electron microscopy (EM) examination. (C) HA of the TLP virions. Twofold serial dilutions of TLPs were used in the HA assay, with PBS buffer without TLPs as the negative control (NC). The TLPs were found to specifically agglutinate the type A RBCs. (D) Specific binding of the TLPs to the type A saliva samples. The x axes show a panel of A-positive and -negative saliva samples, and the y axes show the optical density (OD) value of the binding signals. Error bars show the standard deviations from two experiments.
DISCUSSION
In this study, we performed an up-to-date phylogenetic analysis of RVs representing all 35 known P genotypes in GenBank. We focused on the VP8* sequences instead of the entire VP4, highlighting the most important portion of the spike protein in interaction with the host receptor, which is believed to be biologically relevant. In addition, since VP4 is large and many VP4 sequences in GenBank are incomplete, only limited numbers of sequences are available if full-length VP4 sequences are studied. Thus, our choice of the VP8* sequences is rational and informative.
Following construction of a phylogenetic tree and validation of genetic distances, five P genogroups (P[I] to P[V]) covering all 35 known P genotypes of RVs have been identified. While RVs have been found in many human and animal species, each of the five P genogroups seemed to have a limited host range or tropism. Three P genogroups (P[I], P[IV], and P[V]) were almost exclusively found in animals, one P genogroup (P[II]) infected only humans, and the remaining one (P[III]) infected both humans and animals. The sialic acid-dependent RVs formed a subcluster within P[I], supporting the previous observation that the requirement of sialic acid is related to the VP4 genotypes but not the host origins (7).
The genetic relatedness on recognition of a host receptor is also seen among human RVs. Our previous studies showed that multiple strains within each of the P[8], P[4], and P[6] genotypes in the P[II] genogroup showed the same binding specificity, with the H-related antigens (Leb and/or H type 1) as the common interaction epitope for all three genotypes. In this study, three strains from each of the three P genotypes (P[9], P[14], and P[25]) of P[III] RVs were studied, and all three strains revealed the same binding to the type A HBGAs. Furthermore, the recent study by Hu et al. (21) characterized another P[14] strain and revealed the same binding to the type A antigen as that observed by our study. Thus, we hypothesize that RVs within a genogroup may share a consensus HBGA profile based on their interaction with a common epitope, such as the H-related antigens for P[II] and the A antigen for P[III].
In this study, we estimated pairwise distances of inter- and intragenotypes as well as inter- and intragenogroups and derived putative P genotype and P genogroup classifications based on such a phylogenetic analysis (Fig. 1B). The 86% cutoff value for genotypes defined in this study was slightly lower than the 89% cutoff values described previously based on the full-length VP4 (15). This is because the VP8* regions used in this study are genetically more diverse than full-length VP4s, possibly due to an evolutionary selection from the hosts. While the majority of the P genotypes fell well within the 86% cutoff value, exceptions were observed. In P[8] and P[6], because clear branches of sequences have been observed within each of the two P genotypes, small numbers of sequences had apparently lower identities (77%) than the cutoff value. While being grouped within each P genotype, special attention should be paid to the biological difference between these branches in future studies.
A further issue is the genetic relatedness between P[8] and P[4]. Although the majority of strains in the two P genotypes were segregated by the 86% cutoff value, there were a number of sequences that fell within the cutoff value, indicating that these two P genotypes were closely related. These results are supported by the fact that both P[8] and P[4] RVs recognize the common H-related HBGAs (24). Epidemiologically, both P[8] and P[4] are highly predominant in many countries (1, 2, 4, 13, 26, 27, 33, 34, 41) and likely to have overlapping target populations (24). Thus, one could view the two most predominant P genotypes as one genetic lineage in assessment of disease burden and epidemic control.
Our results also raise a question about the potential role of the HBGAs in RV evolution. The facts that all three major human RV P genotypes (P[4], P[8], and P[6]) recognize the H-related antigens (Leb and H type 1) and that the vast majority of them infect humans suggest a strong evolutionary selection pressure from the human HBGAs. The variations in binding to different HBGAs (H related versus the A antigens) by different P genotypes within and between genogroups suggest a mechanism of selection by the polymorphic HBGAs of humans similar to that of human NoVs (45, 49). The shared carbohydrate binding sites between the sialic acid and A antigen binders, and possibly the H antigen binders, further suggest that the requirement of a carbohydrate receptor for host attachment is a common feature for RVs. Furthermore, the RV VP8* proteins are also very diverse in analogy to that of the P domain of NoVs (57). Thus, one can expect diverse interaction profiles with multiple HBGAs and/or non-HBGA carbohydrates being recognized by different RV genotypes and/or genogroups, particularly for those sialic acid-independent animal RVs in P[I], P[IV], and P[V].
It is known that RV transmission is restricted within certain species, which is supported by our phylogenic analysis. We hypothesize that the absence of necessary carbohydrates in certain species that is required for RV infection could be barriers against RV transmission to these species. For example, one of the major forms of sialic acid recognized by the sialic acid-dependent RVs is the Neu5Gc (N-glycolylneuraminic acid) (9) that was found in many primate and other vertebrate species but not in humans (3). This might explain why many sialic acid-dependent RVs infect animals but not humans. In addition, the polymorphism of the secretor (FUT 2) and Lewis (FUT 3) genes that are responsible for synthesis of the major Lewis antigens (Lea, Leb, Lex, and Ley) has been found in humans but not in other mammals (31), which may explain why the P[4], P[8], and P[6] RVs exclusively infect humans. On the other hand, species with common carbohydrates may facilitate cross-species transmission of certain RVs. For example, P[9] and P[14] of P[III] recognize the type A antigens of humans. Similar A antigens are also expressed in many animal species (51–53), which could be the reason that P[9] and P[14] RVs infect both humans and animals. Likewise, the sialic acid-dependent strains in P[1], P[2], P[3], and P[7] might share binding to the Neu5Gc antigens in those species that are susceptible to these P types, while the Neu5Gc binding is missing in many other species that are sialic acid independent. Alternatively, those animals may express forms of sialic acids differing from Neu5Gc. Future study to explore these possibilities is necessary.
The predicted relationship between P genogroups and host range or tropisms of RVs based on their carbohydrate receptor specificities may be important for understanding the epidemiology and development of strategies to control and prevent RV disease. For example, the predominance of the P[II] RVs that is responsible for more than 95% of RV epidemics in humans is likely associated with their broad spectrum of host ranges via recognition of the H-related antigens in the general population. This feature is similar to that of the predominant GII (mainly GII.4) of NoVs that also recognize the H-related HBGAs. Thus, a strategy such as vaccine targeting to these P genotypes would be important for disease control and prevention. On the other hand, the relative lower prevalence of P[9] and P[14] could be due to their relatively narrow target populations that have type A antigen (∼30% of the general population in the North American and European countries). While these P genotypes may not be responsible for the major outbreaks of epidemics, strategies to prevent cross-species transmission are necessary.
Further proof of the A antigen as a receptor of RVs came from a separate study (21) on another P[14] RV, supporting our hypothesis that P[14] and maybe all members of P[III] recognize the type A antigens. This study elucidated the crystal structure of the A antigen-binding site on VP8* and demonstrated an abrogation of the RV infectivity by anti-A-type antibodies. It also confirmed the A antigen-binding site predicted by our docking simulation experiments, with 6 (R101, S187, Y188, Y189, L190, and T191) of 9 amino acids predicted by our study having direct contacts with the A antigen according to the crystallography study (21). Our mutagenesis studies on two of the other three amino acids (S146, Y155, and S156) (Fig. 5) resulted in loss of the A antigen binding in the case of the S146A and Y155A mutants (Fig. 6 and 7). These data suggested that both mutations caused a change of the local conformation, influencing indirectly the structural integrity and function of the binding site. Another possibility is that the binding mode of native A antigens somewhat differs from those observed in the crystallography study using a trisaccharide.
In summary, our study provides strong evidence for the genetic relatedness of VP8* in RV recognition of HBGAs. Two major genogroups (P[II] and P[III]) of RVs cause acute gastroenteritis in humans, and both genogroups recognize the human HBGAs, indicating that the human HBGAs play an important role in the infection and therefore evolution of RVs. The genotype- and genogroup-specific variations of RVs in interaction with different HBGAs suggest a functional selection of RVs by the polymorphic human HBGAs. These variations may also play a role in the host tropism and cross-species transmission of RVs among human and many animal species. Further studies to elucidate these relationships, including exploring variations among animal RVs, are warranted.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health through the National Institute of Allergy and Infectious Diseases (R01 AI37093 and R01 AI055649) and National Institute of Child Health and Human Development (P01 HD13021) to X.J. and R21AI092434-01A1 to M.T. Computational resources were provided by the Cincinnati Children's Hospital Medical Center.
We thank Jon R. Gentsch for sharing materials, Dongsheng Zhang for technical support, and Ming Xia for discussion and suggestions regarding this study.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
Footnotes
Published ahead of print 3 July 2012
REFERENCES
- 1. Beards G, Graham C. 1995. Temporal distribution of rotavirus G-serotypes in the West Midlands region of the United Kingdom, 1983–1994. J. Diarrhoeal Dis. Res. 13:235–237 [PubMed] [Google Scholar]
- 2. Beards GM, Desselberger U, Flewett TH. 1989. Temporal and geographical distributions of human rotavirus serotypes, 1983 to 1988. J. Clin. Microbiol. 27:2827–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bishop JR, Gagneux P. 2007. Evolution of carbohydrate antigens—microbial forces shaping host glycomes? Glycobiology 17:23R–34R [DOI] [PubMed] [Google Scholar]
- 4. Bishop RF, Unicomb LE, Barnes GL. 1991. Epidemiology of rotavirus serotypes in Melbourne, Australia, from 1973 to 1989. J. Clin. Microbiol. 29:862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castello AA, et al. 2009. Characterization of genotype P[9]G12 rotavirus strains from Argentina: high similarity with Japanese and Korean G12 strains J. Med. Virol. 81:371–381 [DOI] [PubMed] [Google Scholar]
- 6. Ciarlet M, Estes MK. 1999. Human and most animal rotavirus strains do not require the presence of sialic acid on the cell surface for efficient infectivity J. Gen. Virol. 80(Part 4):943–948 [DOI] [PubMed] [Google Scholar]
- 7. Ciarlet M, et al. 2002. Initial interaction of rotavirus strains with N-acetylneuraminic (sialic) acid residues on the cell surface correlates with VP4 genotype, not species of origin. J. Virol. 76:4087–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coulson BS, Londrigan SL, Lee DJ. 1997. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc. Natl. Acad. Sci. U. S. A. 94:5389–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delorme C, et al. 2001. Glycosphingolipid binding specificities of rotavirus: identification of a sialic acid-binding epitope. J. Virol. 75:2276–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denisova E, et al. 1999. Rotavirus capsid protein VP5* permeabilizes membranes. J. Virol. 73:3147–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dormitzer PR, Sun ZY, Wagner G, Harrison SC. 2002. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 21:885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dowling W, Denisova E, LaMonica R, Mackow ER. 2000. Selective membrane permeabilization by the rotavirus VP5* protein is abrogated by mutations in an internal hydrophobic domain. J. Virol. 74:6368–6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esteban LE, et al. 2010. Molecular epidemiology of group A rotavirus in Buenos Aires, Argentina 2004–2007: reemergence of G2P[4] and emergence of G9P[8] strains J. Med. Virol. 82:1083–1093 [DOI] [PubMed] [Google Scholar]
- 14. Fukudome K, Yoshie O, Konno T. 1989. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology 172:196–205 [DOI] [PubMed] [Google Scholar]
- 15. Gorziglia M, Larralde G, Kapikian AZ, Chanock RM. 1990. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc. Natl. Acad. Sci. U. S. A. 87:7155–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graham KL, et al. 2003. Integrin-using rotaviruses bind alpha2beta1 integrin alpha2 I domain via VP4 DGE sequence and recognize alphaXbeta2 and alphaVbeta3 by using VP7 during cell entry. J. Virol. 77:9969–9978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffin DD, et al. 2002. Characterization of nontypeable rotavirus strains from the United States: identification of a new rotavirus reassortant (P2A[6],G12) and rare P3[9] strains related to bovine rotaviruses. Virology 294:256–269 [DOI] [PubMed] [Google Scholar]
- 18. Guo CT, et al. 1999. Ganglioside GM(1a) on the cell surface is involved in the infection by human rotavirus KUN and MO strains. J. Biochem. 126:683–688 [DOI] [PubMed] [Google Scholar]
- 19. Haselhorst T, et al. 2009. Sialic acid dependence in rotavirus host cell invasion. Nat. Chem. Biol. 5:91–93 [DOI] [PubMed] [Google Scholar]
- 20. Hewish MJ, Takada Y, Coulson BS. 2000. Integrins alpha2beta1 and alpha4beta1 can mediate SA11 rotavirus attachment and entry into cells. J. Virol. 74:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu L, et al. 2012. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature 485:256–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang P, et al. 2003. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J. Infect. Dis. 188:19–31 [DOI] [PubMed] [Google Scholar]
- 23. Huang P, et al. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 79:6714–6722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang P, et al. 2012. Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner. J. Virol. 86:4833–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Isa P, Realpe M, Romero P, Lopez S, Arias CF. 2004. Rotavirus RRV associates with lipid membrane microdomains during cell entry. Virology 322:370–381 [DOI] [PubMed] [Google Scholar]
- 26. Khamrin P, et al. 2007. Changing pattern of rotavirus G genotype distribution in Chiang Mai, Thailand from 2002 to 2004: decline of G9 and reemergence of G1 and G2. J. Med. Virol. 79:1775–1782 [DOI] [PubMed] [Google Scholar]
- 27. Kirkwood C, et al. 2002. Report of the Australian Rotavirus Surveillance Program, 2001/2002. Commun. Dis. Intell. 26:537–540 [PubMed] [Google Scholar]
- 28. Kuhlenschmidt TB, Hanafin WP, Gelberg HB, Kuhlenschmidt MS. 1999. Sialic acid dependence and independence of group A rotaviruses. Adv. Exp. Med. Biol. 473:309–317 [DOI] [PubMed] [Google Scholar]
- 29. Lopez S, Arias CF. 2006. Early steps in rotavirus cell entry. Curr. Top. Microbiol. Immunol. 309:39–66 [DOI] [PubMed] [Google Scholar]
- 30. Lopez S, Arias CF. 2004. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol. 12:271–278 [DOI] [PubMed] [Google Scholar]
- 31. Marionneau S, et al. 2001. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 83:565–573 [DOI] [PubMed] [Google Scholar]
- 32. Marionneau S, et al. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masendycz P, Bogdanovic-Sakran N, Kirkwood C, Bishop R, Barnes G. 2001. Report of the Australian Rotavirus Surveillance Program, 2000/2001. Commun. Dis. Intell. 25:143–146 [PubMed] [Google Scholar]
- 34. Masendycz P, Bogdanovic-Sakran N, Palombo E, Bishop R, Barnes G. 2000. Annual report of the Rotavirus Surveillance Programme, 1999/2000. Commun. Dis. Intell. 24:195–198 [PubMed] [Google Scholar]
- 35. Matthijnssens J, et al. 2009. Are human P[14] rotavirus strains the result of interspecies transmissions from sheep or other ungulates that belong to the mammalian order Artiodactyla? J. Virol. 83:2917–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morris GM, et al. 2009. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30:2785–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parashar UD, Gibson CJ, Bresse JS, Glass RI. 2006. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 12:304–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phan TG, et al. 2007. Genetic characterization of group A rotavirus strains circulating among children with acute gastroenteritis in Japan in 2004–2005. Infect. Genet. Evol. 7:247–253 [DOI] [PubMed] [Google Scholar]
- 39. Pieper U, et al. 2011. ModBase, a database of annotated comparative protein structure models, and associated resources. Nucleic Acids Res. 39:D465–D474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Racz ML, et al. 2000. Molecular characterization of porcine rotaviruses from the southern region of Brazil: characterization of an atypical genotype G[9] strain. J. Clin. Microbiol. 38:2443–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Santos N, Hoshino Y. 2005. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 15:29–56 [DOI] [PubMed] [Google Scholar]
- 42. Stuart AD, Brown TD. 2007. Alpha2,6-linked sialic acid acts as a receptor for Feline calicivirus. J. Gen. Virol. 88:177–186 [DOI] [PubMed] [Google Scholar]
- 43. Tan M, Hegde RS, Jiang X. 2004. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J. Virol. 78:6233–6242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan M, et al. 2003. Mutations within the P2 domain of norovirus capsid affect binding to human histo-blood group antigens: evidence for a binding pocket. J. Virol. 77:12562–12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tan M, Jiang X. 2011. Norovirus-host interaction: multi-selections by human histo-blood group antigens. Trends Microbiol. 19:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tan M, Jiang X. 2005. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 13:285–293 [DOI] [PubMed] [Google Scholar]
- 47. Tan M, Jiang X. 2010. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 6:e1000983 doi:10.1371/journal.ppat.1000983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan M, et al. 2008. Elucidation of strain-specific interaction of a GII-4 norovirus with HBGA receptors by site-directed mutagenesis study. Virology 379:324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tan M, et al. 2009. Conservation of carbohydrate binding interfaces: evidence of human HBGA selection in norovirus evolution. PLoS One 4:e5058 doi:10.1371/journal.pone.0005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taube S, et al. 2009. Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J. Virol. 83:4092–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tomova E, Sarafian V, Ishev V. 1999. Expression of human blood group antigens A and B in stomach cells of C. carpio, C. auratus, R. ridibunda and H. sapiens. Folia Med. 41:12–17 [PubMed] [Google Scholar]
- 52. Tomova ES, Popov AA, Sarafian VS. 2001. Expression of human blood group antigens A and B in kidney and lung of some vertebrates. Folia Biol. 49:251–257 [PubMed] [Google Scholar]
- 53. Tomova ES, Sarafian VS. 2010. Expression of histo-blood group antigens in vertebrate gonads. Acta Biol. Hung. 61:64–72 [DOI] [PubMed] [Google Scholar]
- 54. Yolken RH, Willoughby R, Wee SB, Miskuff R, Vonderfecht S. 1987. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J. Clin. Invest. 79:148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zarate S, et al. 2003. Interaction of rotaviruses with Hsc70 during cell entry is mediated by VP5. J. Virol. 77:7254–7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zarate S, et al. 2000. The VP5 domain of VP4 can mediate attachment of rotaviruses to cells. J. Virol. 74:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zheng DP, et al. 2006. Norovirus classification and proposed strain nomenclature. Virology 346:312–323 [DOI] [PubMed] [Google Scholar]