Abstract
Cationic β-cyclodextrin derivatives were recently introduced as highly effective, potentially universal blockers of three binary bacterial toxins: anthrax toxin of Bacillus anthracis, C2 toxin of Clostridium botulinum, and iota toxin of Clostridium perfringens. The binary toxins are made of two separate components: the enzymatic A component, which acts on certain intracellular targets, and the binding/translocation B component, which forms oligomeric channels in the target cell membrane. Here we studied the voltage and salt dependence of the rate constants of binding and dissociation reactions of two structurally different β-cyclodextrins (AmPrβCD and AMBnTβCD) in the PA63, C2IIa, and Ib channels (B components of anthrax, C2, and iota toxins, respectively). With all three channels, the blocker carrying extra hydrophobic aromatic groups on the thio-alkyl linkers of positively charged amino groups, AMBnTβCD, demonstrated significantly stronger binding compared with AmPrβCD. This effect is seen as an increased residence time of the blocker in the channels, whereas the time between blockages characterizing the binding reaction on-rate stays practically unchanged. Surprisingly, the voltage sensitivity, expressed as a slope of the logarithm of the blocker residence time as a function of voltage, turned out to be practically the same for all six cases studied, suggesting structural similarities among the three channels. Also, the more-effective AMBnTβCD blocker shows weaker salt dependence of the binding and dissociation rate constants compared with AmPrβCD. By estimating the relative contributions of the applied transmembrane field, long-range Coulomb, and salt-concentration-independent, short-range forces, we found that the latter represent the leading interaction, which accounts for the high efficiency of blockage. In a search for the putative groups in the channel lumen that are responsible for the short-range forces, we performed measurements with the F427A mutant of PA63, which lacks the functionally important phenylalanine clamp. We found that the on-rates of the blockage were virtually conserved, but the residence times and, correspondingly, the binding constants dropped by more than an order of magnitude, which also reduced the difference between the efficiencies of the two blockers.
Introduction
Several pathogenic species of Bacillus and Clostridium employ a unique and well-designed means of intoxicating eukaryotic cells—they secrete binary exotoxins that consist of two individual nonlinked proteins: an active/enzymatic (A) component and a binding/translocation (B) component (1–4). To gain access for their A components into the cytosol, the binary toxins rely on a similar cellular uptake mechanism. The B component of these toxins binds to a receptor on the surface of the target cells, self-assembles to form a ring-shaped oligomeric prepore that is able to bind the A components, and, after receptor-mediated endocytosis, is converted into an ion-conductive pore that mediates A component translocation from acidified endosomal vesicles into the cytosol.
With the exception of binary anthrax toxin, no extensive studies searching for effective therapies against the binary toxins have been reported. Remarkably, the binding/translocation B components are structurally conserved between the Bacillus and Clostridium families. They share a high level of amino acid homology and numerous functional similarities (2), whereas the enzymatic A components of these toxins are quite distinct and inhibit different normal cell functions. The similarities between the channel-forming B components suggest that these channels can be a specific universal target in the search for new, effective, broad-spectrum antitoxins against the Bacillus and Clostridium pathogenic species.
In this study we focus on three binary toxins: anthrax toxin of Bacillus anthracis, C2 toxin of Clostridium botulinum, and iota toxin of C. perfringens. After proteolytic activation, the truncated B components of these toxins (PA63, C2IIa, and Ib) assemble into ring-shaped oligomers, the so-called prepores on the surface of eukaryotic cells or in solution. C2IIa and Ib prepores are heptamers (5), and PA63 was reported to form heptamers (6) as well as octamers (7). After the binding of A components, the cell-bound complexes are internalized by receptor-mediated endocytosis (8–16) and reach endosomal vesicles where the A components translocate across the endosomal membranes into the cytosol using the PA63, C2IIa, and Ib pores as translocation corridors (5,16–21). In mildly acidic conditions, their B components form cation-selective oligomeric channels in planar lipid membranes. The channel cationic selectivity decreases in the sequence PA63 > C2IIa > Ib (17,18,22,23). The mushroom-like (125-Å-diameter and 70-Å-long cap, and 100-Å-long stem) membrane-spanning (PA63)7 pores were observed by negative-stain electron microscopy (24). With both the PA63 and C2IIa channels, the phenylalanine clamps (φ-clamps) F427 and F428, respectively, were found to catalyze the unfolding and translocation of the A component moieties across the membrane (25–29). The Phe at the corresponding position is also conserved in Ib (29), but the importance of the φ-clamp for the iota toxin transport is not clear as of yet.
One of the new promising inhibitory approaches involves obstructing the membrane pores with molecules having the same structural symmetry as the oligomeric pores (30–34). It was demonstrated that the tailor-made β-cyclodextrin derivatives carrying seven positively charged groups, 7+β-CD, efficiently blocked the PA63 pores in subnanomolar concentrations in vitro, and protected cultured macrophage-like cells and Fischer F344 rats against intoxication with lethal toxin (30,31) and DBA/2 mice against B. anthracis infection (35). Of most importance, we recently showed that AMBnTβCD efficiently protects cultured epithelial cells against intoxication with two clostridial binary toxins (C2 and iota) and blocks the ion current through C2IIa and Ib channels in planar lipid membranes (34).
The nature of the physical forces involved in 7+β-CD’s interactions with the PA63, C2IIa, and Ib channels remains unclear. On the basis of experiments with PA63, we previously reported an order of magnitude increase in the inhibitory activity of seven positively charged aromatic hepta-6-alkylarylamine β-CD derivatives compared with their hepta-6-aminoalkyl analogs (31). Here, to study contributions of different forces to 7+β-CDs binding, we examine the basic features of PA63, C2IIa, and Ib pore blockage by two structurally different derivatives: per-6-S-(3-amino)propylthio-β-cyclodextrin (AmPrβCD), and per-6-S-(3-aminomethyl)benzylthio-β-cyclodextrin (AMBnTβCD) (Fig. 1). AMBnTβCD differs from AmPrβCD by the aromatic rings inserted into alkyl chains carrying positively charged amino groups. To address the importance of the φ-clamp in the blockage, we also studied the F427A mutant of PA63 (25).
Figure 1.
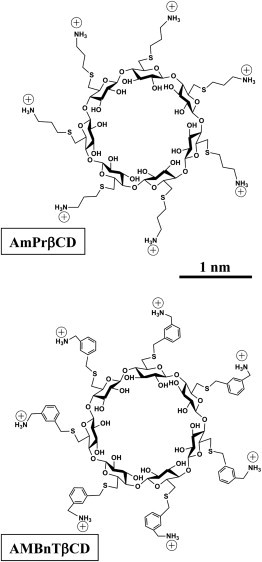
Two sevenfold symmetrical synthetic molecules, AmPrβCD (top panel) and AMBnTβCD (bottom panel) were used as blockers of the PA63, C2IIa, and Ib pores.
We start with a description of the static (average currents) and dynamic (noise power spectra) features of the single PA63, C2IIa, Ib, and PA63 F427A channels reconstituted into planar lipid bilayers in blocker-free solutions, and proceed with the characterization of AmPrβCD and AMBnTβCD binding to these channels as a function of the applied transmembrane voltage and different KCl concentrations. In particular, we show that binding of both derivatives to the PA63 F427A mutant, quantified by the equilibrium binding constant, decreases by more than an order of magnitude, thus confirming the importance of the φ-clamp for channel interaction with the blockers. Our main conclusion is that although both long-range Coulomb forces and interactions of the blocker charge with the transmembrane field are able to increase the residence time of the blocker in the channel by orders of magnitude, the leading interactions are related to salt-concentration-independent, short-range forces.
Materials and Methods
Reagents
PA63 was purchased from List Biological Laboratories (Campbell, CA). The recombinant C2 toxin’s C2IIa component and iota toxin’s Ib component were prepared and activated as described previously (5,36,37). The F427A mutant of PA63 was created by Quick-Change mutagenesis of the pYS5 expression plasmid, and the protein was purified by procedures equivalent to those previously described (38). We used the following chemical reagents: KCl, MES, KOH, and HCl (Sigma-Aldrich, St. Louis, MO); purum hexadecane (Fluka, Buchs, Switzerland); diphytanoyl phosphatidylcholine (DPPC; Avanti Polar Lipids, Alabaster, AL); pentane (Burdick and Jackson, Muskegon, MI), and agarose (Bethesda Research Laboratory, Gaithersburg, MD). Milli-Q water was used to prepare solutions. AMBnTβCD and AmPrβCD were custom synthesized at CycloLab (Budapest, Hungary) as described previously (31).
Channel reconstitution into planar lipid bilayers
To form solvent-free planar lipid bilayers using the lipid monolayer opposition technique (39), we employed a 5 mg/ml stock solution of DPPC in pentane. Bilayer lipid membranes were formed on a 60-80-μm diameter aperture in a 15-μm-thick Teflon film that separated the two compartments as described in detail elsewhere (33). The 0.1–2 M aqueous solutions of KCl were buffered at pH 6 (MES) at room temperature (23 ± 0.5)°C. Single channels were formed by adding 0.5 to 1 μl of 20 μg ml−1 solution of PA63 (wild-type (WT) and F427A mutant), (0.2–0.5) μl of 48 ng ml−1 solution of C2IIa, or (0.2–1) μl of 2.5 μg ml−1 solution of Ib to the 1.5 ml aqueous phase on the cis half of the chamber. For multichannel experiments, we applied ∼1–2 μl of 0.2 mg ml−1 stock PA63. Under this protocol, the channel insertions were always directional. The applied potential was defined as positive if it was higher on the side of protein addition.
Results
PA63, C2IIa, and Ib channels, but not the PA63 F427A channel, share 1/f current noise characteristics
Typical ion currents through the three single channels of PA63, C2IIa, and Ib, reconstituted into planar lipid membranes in blocker-free 1 M KCl solutions, are presented in Fig. 2 A. The currents are shown at 1-ms resolution to illustrate the vigorous flickering of the channels between open and completely closed states. This type of fast flickering was reported previously (17,22,40,41) and recently discussed in more detail (33). The current through the channel formed by the F427A mutant of PA63 is shown in Fig. 2 B. The removal of the φ-clamp leads to a significant current increase, in agreement with earlier studies (25), and changes the channel dynamics. First, the F427A channel conductance does not flicker to a completely closed state. Second, the time hierarchy of the flickering is altogether different. As the current power spectra plotted in Fig. 3 against frequency f demonstrate, for all the WT PA63, C2IIa, and Ib channels, the flickering is described by the complex non-Markov kinetics. The data in Fig. 3 were normalized by dividing the current power spectral density by the square of the mean current, and thus represent the relative fluctuation level to facilitate comparison of the dynamic properties of channels with different conductance. In similarity to our previous finding with PA63 (33), the power spectral density of these relative current fluctuations was voltage independent (data not shown), suggesting that fluctuations in current i reflect equilibrium fluctuations in conductance g, (where quantities in brackets are averages). The 1/f-type shapes of the spectra for the WT channels, , imply that the dynamics of their conformational transitions are characterized by a broad distribution of characteristic times that span many orders of magnitude. Interestingly, the current noise of the F427A mutant of PA63, which lacks the functionally important φ-clamp, is mostly devoid of this complex hierarchy of times even in the noisiest, partially closed states (Figs. 2 B and 3, marked with a star). The significance of these changed dynamics for the A component translocation remains to be clarified.
Figure 2.
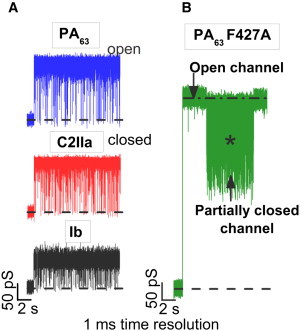
(A) In the absence of blockers, conductance of the PA63, C2IIa, and Ib single channels reconstituted into planar lipid membranes demonstrate fast flickering between the open and closed states. (B) The F427A mutant of PA63 forms channels with much higher conductance and modified dynamics compared with those of the WT. Measurements were taken in 1 M KCl solutions at pH 6 buffered by 5 mM MES. The applied voltage for the data in panels A and B was 50 mV. The currents are given at 1-ms time resolution.
Figure 3.
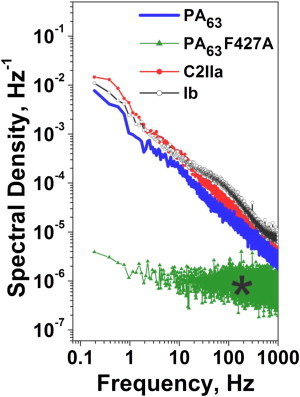
Power spectral densities of the normalized currents demonstrate a basic difference in the dynamic properties of the WT and the PA63 F427A mutant pores. The WT PA63, C2IIa, and Ib single channels display an expressed 1/f behavior, whereas the mutant does not. The experimental conditions are the same as for Fig. 2.
The spectra measured here for currents through WT single channels agree well with the results obtained earlier from multichannel membranes (40,42), thus demonstrating that the origin of the 1/f noise in the present systems is not related to channel-channel interactions. Rather, it is an inherent property of individual channels (43). In addition, PA63, C2IIa, and Ib exhibited strong voltage-dependent gating (not shown) of an unknown origin that is observed with many β-barrel channels. This type of gating is highly asymmetrical (being more pronounced at cis-negative voltages) and decreases in the order PA63 > C2IIa ≫ Ib.
AmPrβCD- and AMBnTβCD-pore interactions
Fig. 4 A illustrates the effects of AmPrβCD and AMBnTβCD on the currents through the three WT channels. To show the effect more clearly, the time resolution of the current records in the figure was limited to 10 ms. Under this resolution, the 1/f fast flickering is filtered out to a significant extent. It can be seen that both blockers induce extra current interruptions in all three channels, with the lifetime in the blocked state (or blocker residence time) depending on the type of blocker molecule. Particularly, AMBnTβCD provides much longer current interruptions than its close analog AmPrβCD, which is missing aromatic rings on the linkers carrying positive charges (Fig. 1). The average times between successive current interruptions decrease with blocker concentration (30,33,34). Fig. 4 B shows that both blockers are significantly less potent with the F427A mutant of PA63. The dwell times of the blockers in this channel are much shorter than in the WT PA63. Please note the order-of-magnitude finer timescale in the figure and higher blocker concentration.
Figure 4.
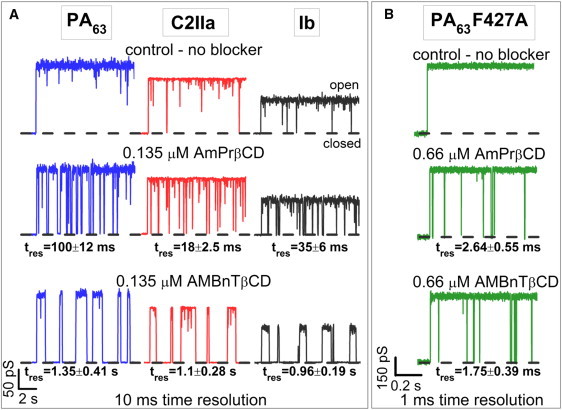
(A) Conductance of single PA63, C2IIa, and Ib channels in the absence (top) and presence of 0.135 μM AmPrβCD (middle row) and AMBnTβCD (bottom row) blockers in the cis side of the chamber. Recordings are shown at 10-ms time resolution. AMBnTβCD displays a significantly longer binding lifetime with all channels compared with AmPrβCD, whereas the time between the blockage events, characterizing the on-rate of the binding reaction, seems to be practically unchanged. (B) The PA63 F427A mutant shows much shorter blockages for both AmPrβCD (middle track) and AMBnTβCD (bottom track) blockers. Recordings are shown at 1-ms time resolution. Other conditions for A and B are the same as for Fig. 2.
The blocker residence times are also strong functions of the applied voltage. The results presented in Fig. 5 were determined either by direct measurements and statistical analysis of the time distributions, which were single exponential (30,33,34), or by power spectral analysis of current fluctuations using the fitting by single Lorentzian spectra (33). Therefore, the PA63, C2IIa, and Ib current fluctuations induced by AmPrβCD and AMBnTβCD could be described by a two-state Markov process. Fig. 5 demonstrates that for both blockers and all three WT channels, the blockage times are practically exponential in the voltage applied to the membrane. Although they differ consistently by the absolute value for the two blockers and for the three channels in the case of AmPrβCD, surprisingly, the slopes of the logarithm of the lifetimes versus voltage dependence are very close to each other, though somewhat lower for the less-efficient AmPrβCD ( = (13.9 ± 0.3) × 10−3 (mV)−1) than for AMBnTβCD ( = (16.5 ± 0.5) × 10−3 (mV)−1). In any case, all six systems can be characterized by the effective gating charge or apparent valence (44) of 1.1 ± 0.1 elementary charges.
Figure 5.
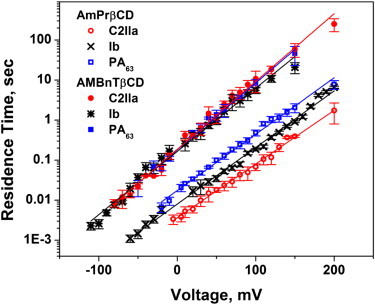
Residence times of blocker binding to the channels plotted as functions of transmembrane voltage reveal exponential voltage dependence. Membranes were bathed by 1 M KCl solution at pH 6. Blocker concentrations ranged from nanomolar to micromolar to ensure reliable statistics. Residence times were calculated from probability versus time histograms of the blockage events or by spectral analysis of current fluctuations as described previously (33). The solid lines through the data show single-exponential regression.
The on- and off-rates of the blockage reaction were studied as functions of salt concentration. Fig. 6 shows that for two different blockers with three different WT channels, a decrease in salt concentration leads to a substantial increase in the lifetimes, suggesting the involvement of long-range Coulomb interactions. However, the relative magnitude of the increase depends on the channel and on the blocker. Interestingly, with the more-efficient AMBnTβCD, which carries extra hydrophobic aromatic groups (Fig. 1), the salt-dependent increase is much smaller than for AmPrβCD, especially in the case of the PA63 and C2IIa pores.
Figure 6.
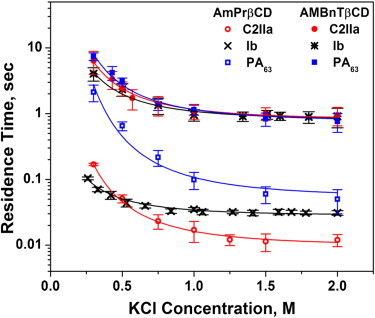
Residence times of blocker binding to the channels as functions of bulk salt concentration show different degrees of salt dependence for the three channels and two blockers. The salt dependence is most pronounced for AmPrβCD interacting with the PA63 pore. For the more-efficient AMBnTβCD, the dependence is weaker. The solid lines through the data are drawn according to Eqs. 3 and 4 with the fitting parameters given in the text. The applied voltage was +50 mV.
The on-rates of the blockage for AmPrβCD and AMBnTβCD are given in Table 1 for three voltages. It can be seen that the on-rates are quite comparable for all of the blocker/channel combinations studied. We also observed the on-rate decrease at increasing salt concentrations with AmPrβCD (data not shown, but see Nestorovich et al. (33)). The on-rate constant was calculated from the times between the successful blockages, , and blocker concentration in the bulk as . The data show a significant spread because we faced several challenges when determining the values. First, at the low blocker concentrations, there were indications of blocker sorption on the hydrophobic surfaces of the vials, syringes, pipette tips, and Teflon walls of the bilayer chamber. Second, under certain experimental conditions (e.g., at low voltages), the main difficulty with the direct determination was the need to separate the blockages from the fast flickering. The equal amplitudes of the current fluctuations for these two processes complicated the problem (see related discussions in Nestorovich et al. (33) and Blaustein et al. (41)). Third, the strong voltage gating of these channels at high transmembrane voltages, which in turn increases in the presence of the blocker (33), made the values at high voltages (>100 mV) statistically less reliable.
Table 1.
Rate constants of binding reaction, kon, for the two blockers and PA63 (WT and F427A mutant), C2IIa, and Ib channels measured at three different transmembrane voltages
|
kon, (Ms)−1 |
|||
|---|---|---|---|
| 50 mV |
100 mV |
150 mV |
|
| AmPrβCD | |||
| PA63 | (1.7 ± 0.7) × 107 | (2.4 ± 0.9) × 107 | (2.5 ± 0.6) × 107 |
| PA63 F427A | (2.0 ± 0.5) × 107 | (3.7 ± 0.8) × 107 | (5.6 ± 0.4) × 107 |
| C2IIa | (3.1 ± 1.2) × 107 | (3.9 ± 1.5) × 107 | (5.3 ± 1.6) × 107 |
| Ib | (1.9 ± 0.9) × 107 |
(2.1 ± 0.7) × 107 |
(3.0 ± 1.0) × 107 |
| AMBnTβCD |
|||
| PA63 | (2.9 ± 0.7) × 107 | (4.1 ± 1.7) × 107 | (4.4 ± 1.1) × 107 |
| PA63 F427A | (2.1 ± 0.5) × 107 | (6.3 ± 0.3) × 107 | (1.2 ± 0.5) × 108 |
| C2IIa | (2.2 ± 0.4) × 107 | (2.7 ± 0.9) × 107 | (3.4 ± 1.7) × 107 |
| Ib | (1.0 ± 0.6) × 107 | (1.2 ± 0.5) × 107 | (1.3 ± 0.3) × 107 |
Data were obtained in 1 M KCl at pH 6 buffered by 5 mM MES.
Table 2 compares the off-rates for the two blockers and all channels studied, including the F427A mutant of PA63 at 0.3 M KCl. It can be seen that removal of the φ-clamp increases the off-rates by more than an order of magnitude, which at the approximately conserved on-rates leads to a decreased efficiency of the blockers. This result correlates nicely with the multichannel measurements in Fig. 7, which show that the IC50 values characterizing PA63 F427A multichannel conductance inhibition by the two blockers are significantly higher and very close. Compared with its value for the WT PA63, the AmPrβCD’s IC50 increases 20 times (from 0.65 nM to 15 nM), whereas for the more effective AMBnTβCD the increase is more significant (∼200-fold, from 0.14 nM to 25 nM).
Table 2.
Rate constants of dissociation reaction, koff, for the two blockers and PA63 (WT and F427A mutant), C2IIa, and Ib channels measured in 0.3 M KCl, at pH 6 at 50 mV applied voltage
|
koff, (s)−1 |
||
|---|---|---|
| AmPrβCD | AMBnTβCD | |
| PA63 | 0.47 ± 0.13 | 0.13 ± 0.02 |
| PA63 F427A | 10.8 ± 2.3 | 12.5 ± 2.3 |
| C2IIa | 5.9 ± 0.2 | 0.16 ± 0.06 |
| Ib | 11.9 ± 2.8 | 0.24 ± 0.06 |
Figure 7.
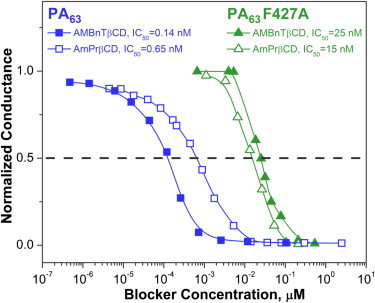
Deletion of the φ-clamp significantly decreases the efficacy of the blockers. The multichannel conductances of WT PA63 (two curves on the left) and the F427A mutant of PA63 (two curves on the right) are inhibited by AmPrβCD (open symbols) and AMBnTβCD (filled symbols) blockers, with the IC50 values differing by orders of magnitude. Membranes were bathed by 0.1 M KCl solution at pH 6, and the applied voltage was 20 mV.
Discussion
One surprising observation of our study is related to the voltage dependence of the lifetimes in the blocked states (Fig. 5). Indeed, the slope of this dependence, which gives an apparent electrical distance (41,44–46) and is usually interpreted as the depth of blocker penetration in the channel, turns out to be practically the same for all six cases. They include two different blockers and three different channels that belong not only to three different species but also to two different families, Bacillus and Clostridium. This finding strongly suggests that the structural features responsible for blocker binding are remarkably similar among all three channels.
Whatever the reason for the similarity in the blockage voltage sensitivity, the expressed voltage dependence itself points toward the blocker charge interaction with the applied transmembrane field as an important component of blockage regulation. In addition, the data in Fig. 6 suggest that there is another long-range electrostatic component, , which is most pronounced at small salt concentrations . To dissect the blocker-channel interaction into different quantifiable components, we will use the framework of a simple model of solute dynamics in the channel (47). We will assume that the total interaction can be characterized by a rectangular potential well of depth occupying the entire blocker-accessible cylindrical part of the pore:
| (1) |
where ze is the blocker charge, δ is the dimensionless apparent electrical distance, and V is the applied voltage. Here the second term represents the blocker interaction with the transmembrane field and is the salt-concentration-independent, short-range component of interaction manifested by the saturating parts of the dependencies in Fig. 6. In this analysis, we will ignore the weak dependence of the on-rate on salt concentration and voltage, and consider it constant. Then, for a deep potential well, the residence time of the blocker molecule in the channel as a function of the applied voltage and salt concentration can be written as
| (2) |
where is the Boltzmann constant, T is the absolute temperature, and 1 / v is a prefactor determined by a number of parameters, such as the difference in the radii of the blocker and the blocker-accessible part of the pore, the length of the pore, and the diffusion coefficient of the blocker (48).
From Eq. 2, for a constant applied voltage we have
| (3) |
where is the salt-concentration-independent residence time. To crudely evaluate the salt-concentration-dependent contribution, as a possible candidate we consider Coulomb interaction between the fixed charges on the walls of the pores and the seven elementary charges on the blocker molecule. In bulk water, an estimate for the energy of this interaction can be written as a function of salt concentration and distance r between the charges as
| (4) |
where is the dielectric constant of vacuum, ε is a ratio of the dielectric constant of water to that of vacuum, and is the concentration-dependent Debye length.
The solid lines in Fig. 6 give the best fits of Eq. 4 with z = 7 to the experimental data obtained for the two blockers and three channels. The effective charge of the channel pore was used as a fitting parameter for each of the six systems, and distance r was chosen as 1.2 nm for all of the systems. The results in the case of the AmPrβCD blocker are as follows: = 8.2 e for the PA63 pore, = 5.9 e for the C2IIa pore, and = 2.2 e for the Ib pore, showing the decreased pore charge in the sequence. This finding qualitatively agrees with the predictions based on the data for the conductance salt dependence and selectivity of the three channels (17,22,41,42). For the more-effective blocker, AMBnTβCD, the salt-independent interactions are more pronounced. The Coulomb component is now described by the set of the following effective charges: = 4.9 e for the PA63 pore, = 4.2 e for the C2IIa pore, and = 3.4 e for the Ib pore, showing the same sequence. Thus, the strongest Coulomb component, accounting for ∼4 per molecule when salt concentration in the bulk is reduced from 2 M to 0.3 M KCl, is seen for the PA63 pore blockage with AmPrβCD.
Note that Eq. 4 gives only an extremely crude description of the salt-concentration-dependent component of the blocker-channel interaction. There are several main reasons for this. First, these equations hold true for a point charge interacting with another point charge, and not for the complex constellation of charges on the blocker molecule and on the channel protein. Second, they describe Coulomb interactions in water, and not in the highly inhomogeneous water/protein media of the channel lumen. Third, calculations of the total electrostatic energy should necessarily include solvation energies and energies associated with possible conformational changes (49). Nevertheless, even this simple description allows one to obtain a reasonable set of charge and distance parameters, and an acceptable dependence of the interaction on salt concentration, thus suggesting significant involvement of long-range electrostatics, especially at physiological salt conditions. The important role of long-range electrostatics was also demonstrated in a number of recent studies devoted to PA63 channel interactions with its substrates (26,50–57).
Estimation of the salt-concentration-independent part, , is most difficult because it requires knowledge of the prefactor 1 / v in Eq. 2. Because the calculation of this prefactor requires detailed information about the structure and potential profile of the blocker-channel interaction (48), we will use an alternative consideration, namely, the partition coefficient. The partition coefficient, which is defined as a ratio of blocker concentration in the channel, , to its concentration in the bulk, , can be written as
| (5) |
Blocker concentration in the channel can be estimated based on the results of our previous study of AmPrβCD interaction with the anthrax pore (33) and structural predictions for the pore. The values of τon and taken from Fig. 3, b and c, of Nestorovich et al. (33) for 1 M KCl and = 3 × 10−8 M allow us to calculate the pore occupancy at this blocker concentration as 0.084. From the structure of the blocker (Fig. 1) and the structural predictions of Katayama et al. (24), the channel volume available for the center of the blocker molecule can be evaluated as 12 nm3. This leads to the following estimate for blocker concentration in the channel: 0.7 × 1025 m−3 or 1.2 × 10−2 M. Then, using Eq. 5, we obtain 13 kBT or ∼8 kcal/mol. Indeed, this is a lower estimate for the depth of the potential well because the effective binding volume (58) of the blocker molecule can be much smaller than the volume evaluated from the structural prediction used here.
Although recent research demonstrated that interactions between cyclodextrins and protein nanopores are remarkably sophisticated (59), our experiments with the F427A mutant of PA63 strongly suggest that a significant part of the salt-independent interactions described by is coming from the blockers’ interaction with the φ-clamp. Removal of the clamp reduces the blocker-channel interaction by ∼2 kcal/mol for the AmPrβCD and by ∼3 kcal/mol for the AMBnTβCD blocker. Note that mutations in the ϕ-clamp were previously shown to significantly affect the binding affinity of a number of compounds with both PA63 (25) and C2IIa channels (29), preferring aromatic moieties by 0.7 kcal/mol per aromatic ring (25).
The on-rates of the blockage given in Table 1 do not show any clear dependence on the applied voltage. The AMBnTβCD on-rate also did not exhibit dependence on salt concentration (data not shown). As we reported earlier (33), the AmPrβCD on-rate decreases by ∼4-fold as salt concentration increases from 0.3 M KCl to 2.0 M KCl. When the on-rate data from Fig. 6 B of Nestorovich et al. (33) are replotted as a function of salt activity, they demonstrate a good linear regression that can be characterized by the osmotic sensitivity of −29 water molecules (60), suggesting that the mechanism of this salt dependence is the preferential solvation of AmPrβCD in the bulk by salt ions. More importantly, the data in Table 1 given for 1 M KCl solutions do not reveal any statistically significant difference between the on-rate constants for the two blockers. Averaging the on-rates measured for the three channels and three voltages, we obtain the following values: kon = (2.9 ± 1.1) × 107 (M·s)−1 for AmPrβCD, and kon = (2.6 ± 1.2) × 107 (M·s)−1 for the much more efficient AMBnTβCD. This allows us to conclude that the most important interactions determining blocker efficiency are interactions between the blocker molecule and the channel lumen analyzed above.
Thus, the blockage efficiency of the translocation pores of the three binary toxins depends on a number of parameters. In this study, we used rather high salt concentrations of ≥0.3 M because, in similarity to our findings with PA63 and AmPrβCD (33), at the decreasing KCl concentrations, all three channels started to display a supplementary mode of blocker action. In addition to the reversible blockage obeying a two-state Markov process, the presence of blockers enhanced the voltage gating of these channels, making their closed states more favorable. Together with the increasing contribution of Coulomb interactions, this supplementary mode further improved the efficiency of the blockers but made the quantitative analysis of the two process acting in parallel difficult due to problems with their separation. Among the studied channels, the blocker-enhanced gating was minimal for Ib and maximal for PA63. According to our measurements on multichannel membranes, which do not discriminate between the two modes of action, for AMBnTβCD this resulted in IC50 = (0.13 ± 0.1) nM for PA63, IC50 = (1.5 ± 0.5) nM for C2IIa, and IC50 = (23 ± 10) nM for Ib channels at “physiological” 0.1 M KCl.
Concluding Remarks
A better understanding of the physical forces that are responsible for the pore-blocker interaction is imperative for the directed chemical synthesis of effective blockers with broad-spectrum activity against pore-forming bacterial toxins. Analysis of the dependence of the interaction strength and underlying kinetics on the salt concentration, the applied potential, and, in the case of the PA63 pore, the presence of the φ-clamp, allows one to identify at least three components:
-
1.
The residence time at high salt concentrations interpolated to zero voltage allows an estimation of salt-concentration-independent, short-range interactions, which predominate in all six cases studied. In the case of AMBnTβCD, its binding to the PA63, C2IIa, and Ib pores is further enhanced by the presence of aromatic groups most probably interacting with the φ-clamp (25) in the lumen of these channels. This conclusion is supported by the F427A mutation of PA63, which not only reduces the binding constants by more than an order of magnitude but makes the blockage by AmPrβCD and AMBnTβCD indistinguishable, thus abolishing the effect of addition of the aromatic groups.
-
2.
The dependence of the blockers’ residence times on salt concentration reveals the contribution of long-range Coulomb interactions at moderate and low salt concentrations. At physiological salt concentrations, Coulomb interactions increase the binding efficiency by orders of magnitude. At salt concentrations < 0.5 M, the AmPrβCD residence time increases in the order Ib < C2IIa < PA63, and so does the cationic selectivity of the channels. This pattern is Ib ≈ C2IIa < PA63 for the AMBnTβCD binding. The difference in the affinity of positively charged blockers toward the PA63, C2IIa, and Ib channels was previously attributed to the different number of negatively charged amino acids on the lumen of these pores (22,29,42).
-
3.
The increase in the blockers’ residence times with the applied voltage demonstrates the existence of an additional electrostatic component of the interaction. Usually, the residence time dependence on the applied voltage allows for an estimation of the depth of the blocker penetration into the pore, but this may not be the case for the mesoscopic channels studied here. Nevertheless, the interaction of the charges with the transmembrane electric field is an additional factor in regulating the blocker efficacy.
Acknowledgments
We thank Tanisha Robinson, Rasem Fattah, and Clinton E. Leysath for excellent technical assistance. E.M.N. thanks Dr. Philip Gurnev (Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health) for his help in setting up her laboratory.
This study was supported by the Intramural Research Programs of the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases, and by startup funds from The Catholic University of America. V.A.K. is an employee and shareholder of Innovative Biologics.
Contributor Information
Sergey M. Bezrukov, Email: bezrukos@mail.nih.gov.
Ekaterina M. Nestorovich, Email: nestorovich@cua.edu.
References
- 1.Barth H., Aktories K., Stiles B.G. Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus proteins. Microbiol. Mol. Biol. Rev. 2004;68:373–402. doi: 10.1128/MMBR.68.3.373-402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth H., Stiles B.G. Binary actin-ADP-ribosylating toxins and their use as molecular Trojan horses for drug delivery into eukaryotic cells. Curr. Med. Chem. 2008;15:459–469. doi: 10.2174/092986708783503195. [DOI] [PubMed] [Google Scholar]
- 3.Collier R.J. Membrane translocation by anthrax toxin. Mol. Aspects Med. 2009;30:413–422. doi: 10.1016/j.mam.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai J., Nagahama M., Kobayashi K. Clostridium perfringens iota-toxin: structure and function. Toxins (Basel) 2009;1:208–228. doi: 10.3390/toxins1020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth H., Blocker D., Aktories K. Cellular uptake of Clostridium botulinum C2 toxin requires oligomerization and acidification. J. Biol. Chem. 2000;275:18704–18711. doi: 10.1074/jbc.M000596200. [DOI] [PubMed] [Google Scholar]
- 6.Petosa C., Collier R.J., Liddington R.C. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 7.Kintzer A.F., Thoren K.L., Krantz B.A. The protective antigen component of anthrax toxin forms functional octameric complexes. J. Mol. Biol. 2009;392:614–629. doi: 10.1016/j.jmb.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh Y., Klimpel K.R., Leppla S.H. The chymotrypsin-sensitive site, FFD315, in anthrax toxin protective antigen is required for translocation of lethal factor. J. Biol. Chem. 1994;269:29039–29046. [PubMed] [Google Scholar]
- 9.Cunningham K., Lacy D.B., Collier R.J. Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc. Natl. Acad. Sci. USA. 2002;99:7049–7053. doi: 10.1073/pnas.062160399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milne J.C., Furlong D., Collier R.J. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 1994;269:20607–20612. [PubMed] [Google Scholar]
- 11.Mogridge J., Mourez M., Collier R.J. Involvement of domain 3 in oligomerization by the protective antigen moiety of anthrax toxin. J. Bacteriol. 2001;183:2111–2116. doi: 10.1128/JB.183.6.2111-2116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrami L., Liu S., van der Goot F.G. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 2003;160:321–328. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagahama M., Hagiyama T., Sakurai J. Binding and internalization of Clostridium botulinum C2 toxin. Infect. Immun. 2009;77:5139–5148. doi: 10.1128/IAI.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pust S., Barth H., Sandvig K. Clostridium botulinum C2 toxin is internalized by clathrin- and Rho-dependent mechanisms. Cell. Microbiol. 2010;12:1809–1820. doi: 10.1111/j.1462-5822.2010.01512.x. [DOI] [PubMed] [Google Scholar]
- 15.Blöcker D., Behlke J., Barth H. Cellular uptake of the Clostridium perfringens binary iota-toxin. Infect. Immun. 2001;69:2980–2987. doi: 10.1128/IAI.69.5.2980-2987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiles B.G., Hale M.L., Popoff M.R. Clostridium perfringens iota toxin: characterization of the cell-associated iota b complex. Biochem. J. 2002;367:801–808. doi: 10.1042/BJ20020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmid A., Benz R., Aktories K. Interaction of Clostridium botulinum C2 toxin with lipid bilayer membranes. Formation of cation-selective channels and inhibition of channel function by chloroquine. J. Biol. Chem. 1994;269:16706–16711. [PubMed] [Google Scholar]
- 18.Bachmeyer C., Benz R., Popoff M.R. Interaction of Clostridium botulinum C2 toxin with lipid bilayer membranes and Vero cells: inhibition of channel function by chloroquine and related compounds in vitro and intoxification in vivo. FASEB J. 2001;15:1658–1660. doi: 10.1096/fj.00-0671fje. [DOI] [PubMed] [Google Scholar]
- 19.Blöcker D., Pohlmann K., Barth H. Clostridium botulinum C2 toxin: low pH-induced pore formation is required for translocation of the enzyme component C2I into the cytosol of host cells. J. Biol. Chem. 2003;278:37360–37367. doi: 10.1074/jbc.M305849200. [DOI] [PubMed] [Google Scholar]
- 20.Blöcker D., Bachmeyer C., Barth H. Channel formation by the binding component of Clostridium botulinum C2 toxin: glutamate 307 of C2II affects channel properties in vitro and pH-dependent C2I translocation in vivo. Biochemistry. 2003;42:5368–5377. doi: 10.1021/bi034199e. [DOI] [PubMed] [Google Scholar]
- 21.Gibert M., Marvaud J.C., Popoff M.R. Differential requirement for the translocation of clostridial binary toxins: iota toxin requires a membrane potential gradient. FEBS Lett. 2007;581:1287–1296. doi: 10.1016/j.febslet.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Knapp O., Benz R., Popoff M.R. Interaction of Clostridium perfringens iota-toxin with lipid bilayer membranes. Demonstration of channel formation by the activated binding component Ib and channel block by the enzyme component Ia. J. Biol. Chem. 2002;277:6143–6152. doi: 10.1074/jbc.M103939200. [DOI] [PubMed] [Google Scholar]
- 23.Blaustein R.O., Koehler T.M., Finkelstein A. Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid bilayers. Proc. Natl. Acad. Sci. USA. 1989;86:2209–2213. doi: 10.1073/pnas.86.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katayama H., Janowiak B.E., Fisher M.T. GroEL as a molecular scaffold for structural analysis of the anthrax toxin pore. Nat. Struct. Mol. Biol. 2008;15:754–760. doi: 10.1038/nsmb.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krantz B.A., Melnyk R.A., Collier R.J. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science. 2005;309:777–781. doi: 10.1126/science.1113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krantz B.A., Finkelstein A., Collier R.J. Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J. Mol. Biol. 2006;355:968–979. doi: 10.1016/j.jmb.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Melnyk R.A., Collier R.J. A loop network within the anthrax toxin pore positions the phenylalanine clamp in an active conformation. Proc. Natl. Acad. Sci. USA. 2006;103:9802–9807. doi: 10.1073/pnas.0604000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang A.E., Neumeyer T., Aktories K. Amino acid residues involved in membrane insertion and pore formation of Clostridium botulinum C2 toxin. Biochemistry. 2008;47:8406–8413. doi: 10.1021/bi800615g. [DOI] [PubMed] [Google Scholar]
- 29.Neumeyer T., Schiffler B., Benz R. Clostridium botulinum C2 toxin. Identification of the binding site for chloroquine and related compounds and influence of the binding site on properties of the C2II channel. J. Biol. Chem. 2008;283:3904–3914. doi: 10.1074/jbc.M709807200. [DOI] [PubMed] [Google Scholar]
- 30.Karginov V.A., Nestorovich E.M., Bezrukov S.M. Blocking anthrax lethal toxin at the protective antigen channel by using structure-inspired drug design. Proc. Natl. Acad. Sci. USA. 2005;102:15075–15080. doi: 10.1073/pnas.0507488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karginov V.A., Nestorovich E.M., Bezrukov S.M. Search for cyclodextrin-based inhibitors of anthrax toxins: synthesis, structural features, and relative activities. Antimicrob. Agents Chemother. 2006;50:3740–3753. doi: 10.1128/AAC.00693-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karginov V.A., Nestorovich E.M., Hecht S.M. Inhibition of S. aureus α-hemolysin and B. anthracis lethal toxin by β-cyclodextrin derivatives. Bioorg. Med. Chem. 2007;15:5424–5431. doi: 10.1016/j.bmc.2007.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nestorovich E.M., Karginov V.A., Bezrukov S.M. Blockage of anthrax PA63 pore by a multicharged high-affinity toxin inhibitor. Biophys. J. 2010;99:134–143. doi: 10.1016/j.bpj.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nestorovich E.M., Karginov V.A., Barth H. Tailored β-cyclodextrin blocks the translocation pores of binary exotoxins from C. botulinum and C. perfringens and protects cells from intoxication. PLoS ONE. 2011;6:e23927. doi: 10.1371/journal.pone.0023927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moayeri M., Robinson T.M., Karginov V.A. In vivo efficacy of β-cyclodextrin derivatives against anthrax lethal toxin. Antimicrob. Agents Chemother. 2008;52:2239–2241. doi: 10.1128/AAC.00009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barth H., Preiss J.C., Aktories K. Characterization of the catalytic site of the ADP-ribosyltransferase Clostridium botulinum C2 toxin by site-directed mutagenesis. J. Biol. Chem. 1998;273:29506–29511. doi: 10.1074/jbc.273.45.29506. [DOI] [PubMed] [Google Scholar]
- 37.Perelle S., Domenighini M., Popoff M.R. Evidence that Arg-295, Glu-378, and Glu-380 are active-site residues of the ADP-ribosyltransferase activity of iota toxin. FEBS Lett. 1996;395:191–194. doi: 10.1016/0014-5793(96)01035-6. [DOI] [PubMed] [Google Scholar]
- 38.Pomerantsev A.P., Pomerantseva O.M., Leppla S.H. A Bacillus anthracis strain deleted for six proteases serves as an effective host for production of recombinant proteins. Protein Expr. Purif. 2011;80:80–90. doi: 10.1016/j.pep.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachmeyer C., Orlik F., Benz R. Mechanism of C2-toxin inhibition by fluphenazine and related compounds: investigation of their binding kinetics to the C2II-channel using the current noise analysis. J. Mol. Biol. 2003;333:527–540. doi: 10.1016/j.jmb.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 41.Blaustein R.O., Lea E.J., Finkelstein A. Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions. Single-channel analysis. J. Gen. Physiol. 1990;96:921–942. doi: 10.1085/jgp.96.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orlik F., Schiffler B., Benz R. Anthrax toxin protective antigen: inhibition of channel function by chloroquine and related compounds and study of binding kinetics using the current noise analysis. Biophys. J. 2005;88:1715–1724. doi: 10.1529/biophysj.104.050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bezrukov S.M., Winterhalter M. Examining noise sources at the single-molecule level: 1/f noise of an open maltoporin channel. Phys. Rev. Lett. 2000;85:202–205. doi: 10.1103/PhysRevLett.85.202. [DOI] [PubMed] [Google Scholar]
- 44.French R.J., Shoukimas J.J. An ion’s view of the potassium channel. The structure of the permeation pathway as sensed by a variety of blocking ions. J. Gen. Physiol. 1985;85:669–698. doi: 10.1085/jgp.85.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodhull A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strichartz G.R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J. Gen. Physiol. 1973;62:37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bezrukov S.M., Berezhkovskii A.M., Szabo A. Diffusion model of solute dynamics in a membrane channel: mapping onto the two-site model and optimizing the flux. J. Chem. Phys. 2007;127:115101. doi: 10.1063/1.2766720. [DOI] [PubMed] [Google Scholar]
- 48.Berezhkovskii A.M., Pustovoit M.A., Bezrukov S.M. Channel-facilitated membrane transport: average lifetimes in the channel. J. Chem. Phys. 2003;119:3943–3951. [Google Scholar]
- 49.Gilson M.K., Honig B. Calculation of the total electrostatic energy of a macromolecular system: solvation energies, binding energies, and conformational analysis. Proteins. 1988;4:7–18. doi: 10.1002/prot.340040104. [DOI] [PubMed] [Google Scholar]
- 50.Pentelute B.L., Barker A.P., Collier R.J. A semisynthesis platform for investigating structure-function relationships in the N-terminal domain of the anthrax Lethal Factor. ACS Chem. Biol. 2010;5:359–364. doi: 10.1021/cb100003r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pentelute B.L., Sharma O., Collier R.J. Chemical dissection of protein translocation through the anthrax toxin pore. Angew. Chem. Int. Ed. Engl. 2011;50:2294–2296. doi: 10.1002/anie.201006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basilio D., Juris S.J., Finkelstein A. Evidence for a proton-protein symport mechanism in the anthrax toxin channel. J. Gen. Physiol. 2009;133:307–314. doi: 10.1085/jgp.200810170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basilio D., Kienker P.K., Finkelstein A. A kinetic analysis of protein transport through the anthrax toxin channel. J. Gen. Physiol. 2011;137:521–531. doi: 10.1085/jgp.201110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basilio D., Jennings-Antipov L.D., Finkelstein A. Trapping a translocating protein within the anthrax toxin channel: implications for the secondary structure of permeating proteins. J. Gen. Physiol. 2011;137:343–356. doi: 10.1085/jgp.201010578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thoren K.L., Worden E.J., Krantz B.A. Lethal factor unfolding is the most force-dependent step of anthrax toxin translocation. Proc. Natl. Acad. Sci. USA. 2009;106:21555–21560. doi: 10.1073/pnas.0905880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown M.J., Thoren K.L., Krantz B.A. Charge requirements for proton gradient-driven translocation of anthrax toxin. J. Biol. Chem. 2011;286:23189–23199. doi: 10.1074/jbc.M111.231167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janowiak B.E., Fischer A., Collier R.J. Effects of introducing a single charged residue into the phenylalanine clamp of multimeric anthrax protective antigen. J. Biol. Chem. 2010;285:8130–8137. doi: 10.1074/jbc.M109.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilson M.K., Given J.A., McCammon J.A. The statistical-thermodynamic basis for computation of binding affinities: a critical review. Biophys. J. 1997;72:1047–1069. doi: 10.1016/S0006-3495(97)78756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banerjee A., Mikhailova E., Bayley H. Molecular bases of cyclodextrin adapter interactions with engineered protein nanopores. Proc. Natl. Acad. Sci. USA. 2010;107:8165–8170. doi: 10.1073/pnas.0914229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gurnev P.A., Harries D., Bezrukov S.M. Osmotic stress regulates the strength and kinetics of sugar binding to the maltoporin channel. J. Phys. Condens. Matter. 2010;22:454110. doi: 10.1088/0953-8984/22/45/454110. [DOI] [PMC free article] [PubMed] [Google Scholar]


