tRNA precursors, which are transcribed by RNA polymerase III, undergo end-maturation, splicing, and base modifications. Hypomodified tRNAs, such as tRNAVal(AAC), lacking 7-methylguanosine and 5-methylcytidine modifications, are subject to degradation by a rapid tRNA decay pathway. Here the authors searched for genes which, when overexpressed, restored stability of tRNAVal(AAC) molecules in a modification-deficient trm4Δtrm8Δ mutant. They identified TEF1 and VAS1, encoding elongation factor eEF1A and valyl-tRNA synthetase respectively, which likely protect hypomodified tRNAVal(AAC) by direct interactions. They also identified MAF1 whose product is a general negative regulator of RNA polymerase III. Expression of a Maf1-7A mutant that constitutively repressed RNA polymerase III transcription resulted in increased stability of hypomodified tRNAVal(AAC). These results support a model whereby inhibition of tRNA transcription leads to stabilization of hypomodified tRNAVal(AAC) due to more efficient protection by tRNA-interacting proteins.
Keywords: rapid tRNA decay, tRNA, RNA polymerase III, tRNA transcription, Maf1, translation elongation factor
Abstract
tRNA precursors, which are transcribed by RNA polymerase III, undergo end-maturation, splicing, and base modifications. Hypomodified tRNAs, such as tRNAVal(AAC), lacking 7-methylguanosine and 5-methylcytidine modifications, are subject to degradation by a rapid tRNA decay pathway. Here we searched for genes which, when overexpressed, restored stability of tRNAVal(AAC) molecules in a modification-deficient trm4Δtrm8Δ mutant. We identified TEF1 and VAS1, encoding elongation factor eEF1A and valyl-tRNA synthetase respectively, which likely protect hypomodified tRNAVal(AAC) by direct interactions. We also identified MAF1 whose product is a general negative regulator of RNA polymerase III. Expression of a Maf1-7A mutant that constitutively repressed RNA polymerase III transcription resulted in increased stability of hypomodified tRNAVal(AAC). Strikingly, inhibition of tRNA transcription in a Maf1-independent manner, either by point mutation in RNA polymerase III subunit Rpc128 or decreased expression of Rpc17 subunit, also suppressed the turnover of the hypomodified tRNAVal(AAC). These results support a model where inhibition of tRNA transcription leads to stabilization of hypomodified tRNAVal(AAC) due to more efficient protection by tRNA-interacting proteins.
INTRODUCTION
Regulation of the tRNA transcription machinery and the post-transcriptional steps that generate functional tRNAs are of fundamental importance for protein biosynthesis and cell survival. tRNAs precursors are transcribed by RNA polymerase III (Pol III), a large enzyme consisting of 17 subunits and requiring several auxiliary factors. tRNA transcription needs to be controlled in response to nutrient availability and other environmental circumstances; up-regulation of tRNA transcription can promote cell proliferation and transformation as well as tumorigenesis in mice (Marshall et al. 2008). Mammalian Pol III is controlled by the tumor suppressors p53 and Rb, and Maf1 protein, which is conserved also in all eukaryotes (Pluta et al. 2001; White 2004). In yeast, Maf1 is the only known general negative Pol III regulator which ensures coupling of tRNA biosynthesis to cell growth and metabolism (Boguta and Graczyk 2011).
Primary tRNA transcripts are extended at the 5′ and 3′ termini and may contain introns. Processing of the 5′ leader and trimming of the 3′ trailer occur in the nucleus and are followed by CCA addition at the newly formed 3′ termini. In yeast, the end-processed tRNAs are transported to the cytoplasm where introns are removed (for review, see Phizicky and Hopper 2010). The nuclear export of yeast tRNA is regulated by environmental conditions in coordination with Maf1-mediated transcription control, thereby coupling tRNA synthesis and maturation (Karkusiewicz et al. 2011).
In addition to the processing at the termini and intron removal, tRNAs undergo nucleotides modification, both in the nucleus and in the cytoplasm. Modifications in or around the anticodon loop affect translation and cell growth, while some body tRNA modifications contribute to its folding and stability (Chernyakov et al. 2008a). Inappropriately modified or folded tRNAs can be the target of at least two turnover mechanisms. Pre-tRNAiMet lacking m1A is destroyed by the nuclear surveillance pathway in which the pre-tRNA is first polyadenylated by the TRAMP complex, and then degraded from the 3′ end by the nuclear exosome (Anderson and Parker 1998; Kadaba et al. 2004; Kuai et al. 2004; Wyers et al. 2005). Another pathway, called rapid tRNA decay (RTD), causes partial loss of the hypomodified tRNA in mutant lacking specific modifications (Alexandrov et al. 2006; Kotelawala et al. 2008). One substrate for the RTD pathway is tRNAVal(AAC), lacking the m7G46 and m5C49 modifications. This tRNA is degraded at an elevated temperature by 5′→3′ exonucleases, Xrn1 and Rat1 (Chernyakov et al. 2008b). A more recent study has demonstrated that lack of specific modifications leads to instability of the tRNA acceptor stem and exposes the 5′ end of the tRNA molecule to degradation by Xrn1 nuclease (Whipple et al. 2011). Instability of acceptor stem causes tRNA degradation mediated by CCACCA addition at the 3′ end (Wilusz et al. 2011).
Here we show that degradation of hypomodified tRNA is prevented when tRNA transcription is decreased. Inhibition of Pol III activity in trm4Δtrm8Δ cells lacking the m7G46 and m5C49 modifications in their tRNAs resulted in lower degradation of tRNAVal(AAC). A similar effect, i.e., stabilization of hypomodified tRNAVal(AAC), could be achieved in the trm4Δtrm8Δ mutant by overexpression of genes encoding translation elongation factor eEF1A or valyl-tRNA synthetase, two tRNA-interacting proteins. According to our model, in the context of a globally lower tRNA level, tRNAVal(AAC) competes more efficiently for tRNA-interacting proteins which help it achieve proper conformation and/or protect from nucleases.
RESULTS
Identification of library plasmids that overcome growth defect related to degradation of tRNAVal(AAC)
Our rationale was that degradation of hypomodified tRNAVal(AAC) could be limited in vivo by some factors or as yet undescribed activities affecting the RTD pathway. In an attempt to identify such putative factors increasing stability of hypomodified tRNA, we used a genetic strategy employing the growth phenotype of the trm4Δtrm8Δ mutant, which is not viable at 37°C due to degradation of the tRNAVal(AAC) lacking the m7G46 and m5C49 modifications (Chernyakov et al. 2008b). We therefore looked for multicopy suppressors of the trm4Δtrm8Δ growth deficiency at an elevated temperature.
The trm4Δtrm8Δ strain was transformed with a yeast genomic library based on the pFL44L multicopy vector (Stettler et al. 1993). Sixty-five transformants growing at an elevated temperature (37°C) were selected from ∼5 × 103 transformed cells. For each of them we determined whether their temperature resistance required the presence of the library plasmid. Twenty-nine candidates showed the dependence of the suppression level upon the presence of the plasmid. Sequencing of the plasmids from those cells identified 16 inserts containing various tRNAVal(AAC) genes and 13 other inserts. Selection of genomic inserts containing tRNAVal(AAC) genes was expected in our screen since complementation of the thermosensitive (ts) phenotype of trm4Δtrm8Δ mutant by an overdose of the tRNAVal(AAC) gene had been reported previously (Alexandrov et al. 2006). All the other plasmids were retransformed into trm4Δtrm8Δ and assayed for growth at an elevated temperature. Sequencing and subsequent subcloning of the suppressor plasmids led to the identification of five genes: TEF1, VAS1, MAF1, RVB2, and POP5. One plasmid contained a 5′-terminal part of the RPC160 gene encoding the largest subunit of RNA polymerase III.
TEF1 encodes the translation elongation factor eEF1A. This protein is expressed from two genes in the yeast genome, TEF1 and TEF2, which contain identical ORFs. eEF1A is the eukaryotic homolog of Escherichia coli EF-Tu and binds in a GTP-dependent manner to aminoacyl tRNAs to deliver them to the ribosome (Mateyak and Kinzy 2010). We found that not only TEF1 but also TEF2 suppressed the trm4Δtrm8Δ defect when overexpressed (TW Turowski, unpubl.). Thus, the phenotypic effect of the tRNAVal(AAC) instability could be compensated by an overdosage of eEF1A for augmenting the eEF1A–tRNA interaction.
Interestingly, eEF1A was not the only tRNA-binding protein among the identified suppressors (Fig. 1). VAS1 encodes valyl-tRNA synthetase, which interacts with tRNAVal directly. This suggested that the temperature resistance of trm4Δtrm8Δ brought about by high level of VAS1 expression could likewise be due to stabilization of the hypomodified tRNAVal(AAC) by the interacting protein. Such an interaction could help the tRNAVal(AAC) achieve a correct conformation despite the lack of proper modifications or could simply mechanistically protect it from the degradation machinery.
FIGURE 1.
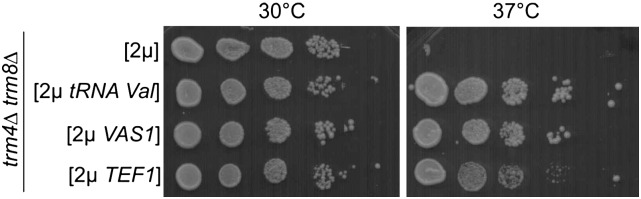
Multicopy suppressors of trm4Δtrm8Δ defect encoding known tRNA-interacting proteins. trm4Δtrm8Δ cells were transformed with multicopy plasmid pFL44L carrying VAS1 or TEF1. tRNAVal(AAC) gene as a positive control and empty plasmid as a negative control. Transformants were grown overnight at 30°C in SD-ura liquid media, diluted to OD600 = 1.0 and serially 10-fold diluted, spotted on YPD plates, and incubated at 30°C or 37°C for 2 d.
To address specificity of RTD suppression by tRNA-interacting proteins, we introduced TEF1 and VAS1 plasmids to tan1Δtrm44Δ mutant. The lack of Tan1 and Trm44 results in growth defect at 37°C due to degradation of tRNASer(CGA) and tRNASer(UGA) missing Um44 and ac4C12 modifications (Kotelawala et al. 2008). The temperature-sensitive phenotype of tan1Δtrm44Δ was suppressed by TEF1 but not by VAS1 overdose (Supplemental Fig. S1). We thus confirmed that VAS1 suppresses RTD-related phenotype of trm4Δtrm8Δ by specifically affecting the tRNAVal(AAC) levels.
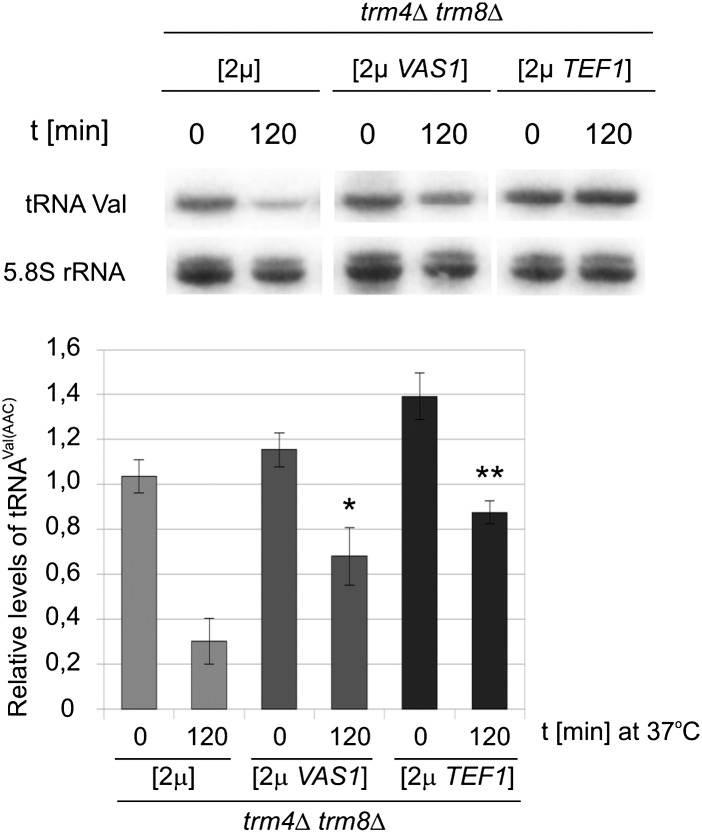
Next we examined the effects of TEF1 or VAS1 overdose on the stability of the hypomodified tRNAVal(AAC). Using Northern blotting we monitored the degradation of tRNAVal(AAC) following a shift of trm4Δtrm8Δ cells to an elevated temperature (Fig. 2). The level of Pol I-synthesized 5.8S rRNA served as an internal control. As published before (Chernyakov et al. 2008b), tRNAVal(AAC) lacking the m7G46 and m5C49 modifications is rapidly degraded at 37°C (Fig. 2). Importantly, overexpression of TEF1 or VAS1 suppressed the tRNAVal(AAC) loss. Thus, an overdose of eEF1A or valyl-tRNA synthetase suppresses phenotype of trm4Δtrm8Δ mutant by preventing degradation of hypomodified tRNAVal(AAC) at an elevated temperature.
FIGURE 2.
Multicopy suppressors of trm4Δtrm8Δ stabilize hypomodified tRNAVal(AAC). trm4Δtrm8Δ cells transformed with multicopy plasmid carrying either VAS1 [2μ VAS1] or TEF1 [2μ TEF1] or empty plasmid [2μ] were grown at 23°C and shifted to 37°C for 2 h. RNA was isolated and analyzed by Northern hybridization with tRNAVal(AAC) probe and 5.8S rRNA probe (loading control). For quantification of tRNAVal(AAC) levels were normalized to 5.8S rRNA. Bars represent magnitude of tRNAVal(AAC) change calculated relative to expression in trm4Δtrm8Δ cells bearing empty plasmid at 0 time point. Standard deviation (SD) was calculated on the basis of three independent experiments. P-values—P < 0.01 for the probe indicated with a single asterisk (*) and P < 0.005 for the probe indicated with two asterisks (**)—were calculated relative to control strain transformed with empty plasmid [2μ] at 120 time point.
Whether the hypomodified tRNAVal(AAC) also interacts with proteins encoded by the other suppressors of the trm4Δtrm8Δ phenotype is only speculative. POP5 encodes a subunit of RNAse P which cleaves tRNA precursors to generate mature 5′ ends. Rvb2, a component of Rvb1/Rvb2 reptin complex, is essential in the assembly of several macromolecular complexes in transcription regulation and chromatin remodeling (Jha and Dutta 2009). The suppression by POP5 and RVB2 may be therefore indirect. Here, we have focused on two of the suppressors identified, TEF1 and VAS1, as their encoded proteins were known tRNA interactors. The remaining suppressors are of interest as well and will be studied later.
Hypomodified tRNAVal(AAC) is stabilized by Maf1 overproduction
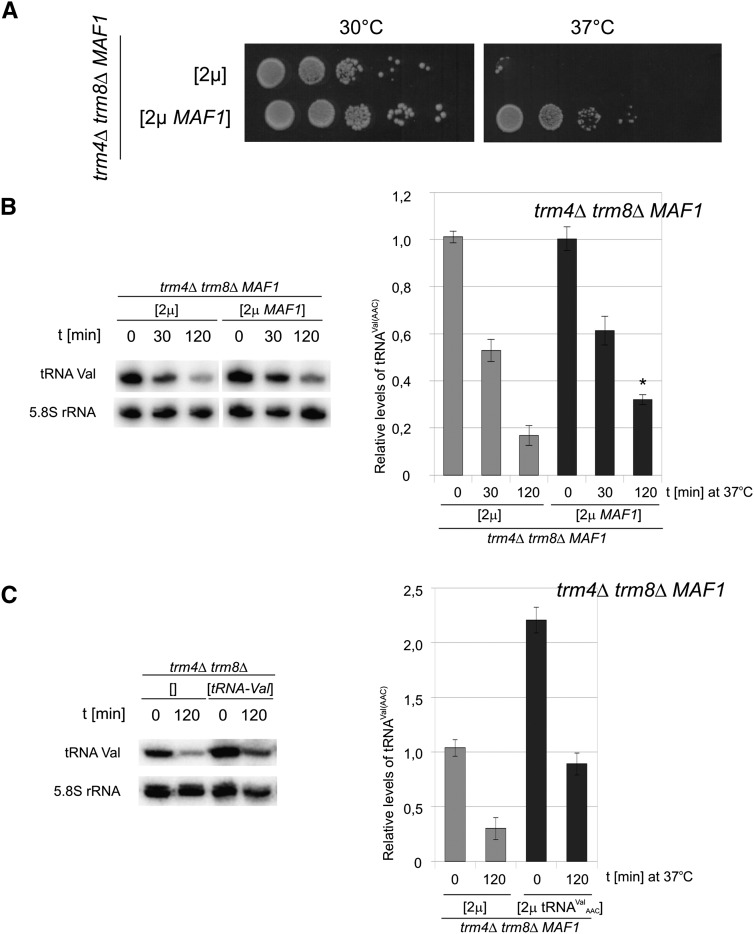
Identification of the gene encoding Maf1 as a high copy suppressor of the trm4Δtrm8Δ phenotype was surprising (Fig. 3A). Maf1 is a global negative regulator of Pol III transcription which, in an active state, decreases pre-tRNA synthesis (Pluta et al. 2001; Upadhya et al. 2002). Simple overexpression of MAF1 gene has little effect on the Pol III transcription (Desai et al. 2005) since activation of Maf1 requires its dephosphorylation (Oficjalska-Pham et al. 2006). By employing Northern blotting we confirmed that the steady state level of hypomodified tRNAVal(AAC) was actually not changed by increased expression of Maf1-encoding gene (Fig. 3B). The molecular mechanism of trm4Δtrm8Δ suppression by overexpression of MAF1 is therefore not related to a compensatory synthesis of tRNAVal(AAC). In contrast, an overdose of the tRNAVal(AAC) gene more than doubled the steady state level of this tRNA at the permissive temperature (Fig. 3C).
FIGURE 3.
Overproduction of Maf1 suppresses thermosensitive phenotype and stabilizes hypomodified tRNAVal(AAC) in trm4Δtrm8Δ cells. (A) trm4Δtrm8Δ cells were transformed with multicopy pFL44L plasmid with MAF1 [2μ MAF1] or empty plasmid [2μ]. Transformants were grown overnight at 30°C in SD-ura liquid media, diluted to OD600 = 1.0 and serially 10-fold diluted, spotted on YPD plates, and incubated at 30°C or 37°C for 2 d. (B) trm4Δtrm8Δ cells harboring control empty plasmid [2μ] or plasmid with MAF1 [2μ MAF1] were grown in SC-ura medium at 30°C to OD = 1.0, shifted to 37°C, and incubated for the indicated time. RNA was isolated and analyzed by Northern hybridization with tRNAVal(AAC) probe and 5.8S rRNA probe (loading control). For quantification of tRNAVal(AAC) levels were normalized to 5.8S rRNA. Bars represent magnitude of tRNAVal(AAC) change calculated relative to expression in trm4Δtrm8Δ cells bearing empty plasmid at 0 time point. SD was calculated on the basis of three independent experiments. P-value < 0.01 for the indicated probe (*) was calculated relative to control strain transformed with empty plasmid [2μ] at 120 time point. (C) trm4Δtrm8Δ transformants with multicopy plasmid carrying gene coding tRNAVal(AAC) [2μ tRNAVal(AAC)] or empty plasmid [2μ] were grown at 23°C and shifted to 37°C for 2 h, RNA was isolated and analyzed by Northern hybridization as in B. Relative levels of tRNAVal(AAC) were quantified (note that the scale is different than in B).
To evaluate the effect of Maf1 on the RTD pathway we monitored the degradation of tRNAVal(AAC) following a shift of trm4Δtrm8Δ cells to an elevated temperature (Fig. 3B). Importantly, MAF1 overexpression partially suppressed the tRNAVal(AAC) loss. Quantification of the Northern blots revealed that after 2 h at the elevated temperature tRNAVal(AAC) is present at only 20% of the level before temperature shift in the trm4Δtrm8Δ strain transformed with a control plasmid. In contrast, in the trm4Δtrm8Δ strain transformed with a Maf1-encoding multicopy plasmid, the tRNAVal(AAC) level was 40% of the level before the temperature shift (twofold more than without overexpression of Maf1). An overnight incubation at 37°C results in a complete loss of tRNAVal(AAC) and death of trm4Δtrm8Δ cells, both suppressed by MAF1 overdose (Fig. 3A; Supplemental Fig. S2).
In summary, overproduction of Maf1, a Pol III repressor, affords limited stabilization of hypomodified tRNAVal(AAC).
Maf1-7A mutant, a constitutively active Pol III repressor, stabilizes hypomodified tRNAVal(AAC)
Identification of Maf1-encoding gene in the screen for proteins involved in stabilization of hypomodified tRNAVal(AAC) raised the question of the mode of action of Maf1. Maf1 shuttles between the nucleus and the cytoplasm (Towpik et al. 2008). Its function in the cytoplasm is unknown, but one possibility is that cytoplasmic Maf1 could be directly involved in tRNA stabilization. However, our attempts to identify a direct Maf1–tRNAVal(AAC) interaction failed (I Karkusiewicz and TW Turowski, unpubl.). We therefore explored the possibility of an indirect role of Maf1 in tRNA stabilization deriving from the known negative effect of Maf1 on tRNA transcription. We expected that an enhancement of the Maf1-mediated repression by its dephosphorylation would promote stabilization of hypomodified tRNAVal(AAC). We therefore used the constitutively dephosphorylated Maf1-7A mutant (Huber et al. 2009). Cells expressing Maf1-7A had reduced basal tRNA levels due to an increased capacity of Maf1-7A to bind and inhibit Pol III (Huber et al. 2009). First we verified the functionality of Maf1-7A encoded by a centromeric plasmid by genetic complementation (Supplemental Fig. S3A). Then the dephosporylated state of Maf1-7A was verified under our experimental conditions. Crude extracts were prepared from trm4Δtrm8Δmaf1Δ cells expressing Maf1-7A from a centromeric plasmid. Differentially phosphorylated forms of Maf1 were resolved by SDS-PAGE and identified by immunoblotting at various times after culture transfer to 37°C. As reported previously, Maf1-7A is constitutively dephosphorylated whereas wild-type Maf1 remains phosphorylated upon this transition (Supplemental Fig. S3B). Moreover, total tRNA is reduced in Maf1-7A mutant to the same extend as previously identified using other experimental conditions (Supplemental Fig. S3C; Huber et al. 2009).
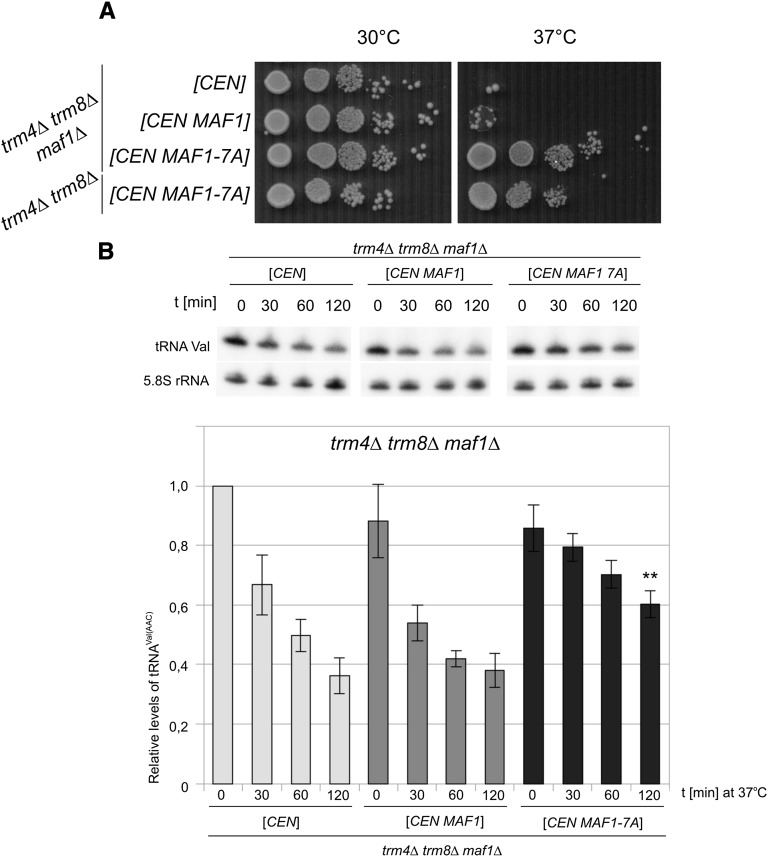
Having established the expected properties of Maf1-7A we studied its effect on the growth of trm4Δtrm8Δ mutant at elevated temperature. Notably, expression of Maf1-7A in both trm4Δtrm8Δmaf1Δ and trm4Δtrm8Δ cells resulted in suppression of their temperature-sensitive phenotype (Fig. 4A). Additionally plasmid encoding Maf1-7A suppressed the temperature-sensitive phenotype of tan1Δtrm44Δ mutant related to increased turnover of tRNASer(CGA) and tRNASer(UGA) (Supplemental Fig. S4).
FIGURE 4.
Maf1-7A mutant suppresses thermosensitive phenotype and stabilizes tRNAVal(AAC) in trm4Δtrm8Δ cells. trm4Δtrm8Δmaf1Δ strain, generated as described in Materials and Methods, was transformed with empty plasmid pRS415 [CEN], pAH095 encoding wild-type Maf1 [CEN MAF1], or pAH247 encoding Maf1-7A mutant [CEN MAF1-7A]. (A) Transformants were grown overnight at 30°C in SD-ura liquid media, cultures were diluted to OD600 = 1.0 and serially 10-fold diluted, spotted on YPD plate, and incubated at 30°C or 37°C for 2 d. (B) Cells were grown in SC-Leu at 23°C, transferred to 37°C, and harvested as indicated. RNA was isolated and analyzed by Northern hybridization with tRNAVal(AAC) probe and 5.8S rRNA probe (loading control). For quantification of tRNAVal(AAC) levels were normalized to 5.8S rRNA. Bars represent magnitude of tRNAVal(AAC) change calculated relative to expression in trm4Δtrm8Δ cells bearing empty plasmid at 0 time point. SD was calculated on the basis of three independent experiments. P-value < 0.005 for the indicated probe (**) was calculated relative to control strain transformed with empty plasmid [CEN] at 120 time point.
To further characterize effect of Maf1-7A, we investigated whether suppression was linked to stabilization of hypomodified tRNA. By Northern blotting we compared levels of tRNAVal(AAC) in trm4Δtrm8Δmaf1Δ cells harboring control vector and centromeric plasmids encoding wild-type Maf1 or Maf1-7A (Fig. 4B). Before the temperature shift level of tRNAVal(AAC) is slightly higher in control cells lacking Maf1. Following 2-h incubation of cells at 37°C, ∼60% of tRNAVal(AAC) was, however, degraded and the extent of degradation was similar in the presence of wild-type Maf1 expressed at basal level. Indeed tRNAVal(AAC) was significantly more stable in the presence of Maf1-7A since only 40% was degraded.
These results show that the variant of Maf1 repressor, previously described in the literature as dephosphorylated and interacting with Pol III even under unstressed conditions (Huber et al. 2009), efficiently promotes stabilization of hypomodified tRNAVal(AAC).
Decreased Pol III transcription stabilizes hypomodified tRNAVal(AAC)
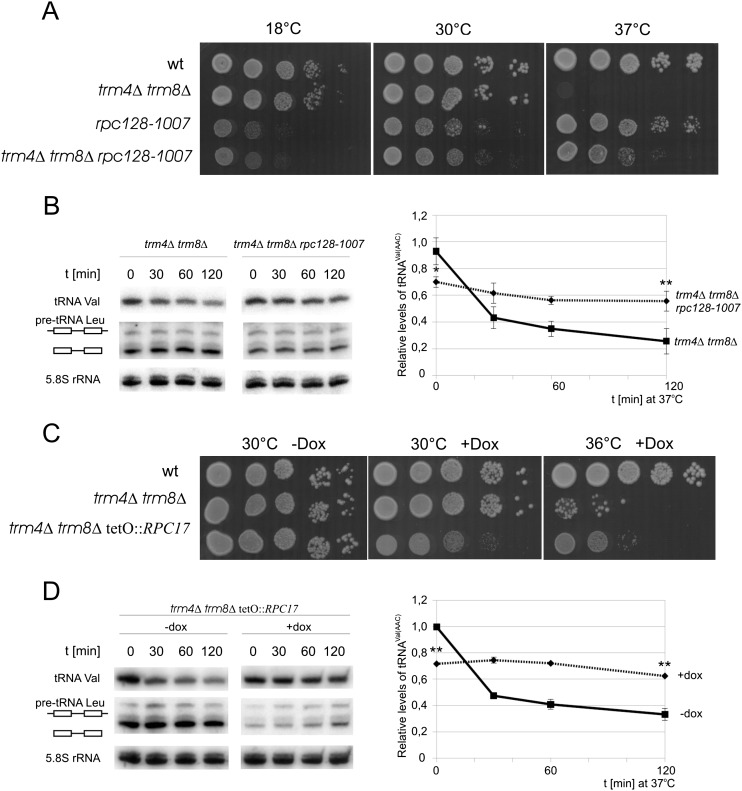
Next we asked whether repression of Pol III transcription by non-Maf1-mediated mechanisms would also stabilize tRNAVal(AAC) in trm4Δtrm8Δ cells. First we employed rpc128-1007 mutation in the Rpc128 Pol III subunit, which halves the overall tRNA transcription (Ciesla et al. 2007). The cold-sensitive (cs) rpc128-1007 mutant was crossed with the trm4Δtrm8Δ strain and the meiotic progeny was analyzed (see Materials and Methods). The observed phenotypic segregation indicated that rpc128-1007 suppressed the ts growth defect of trm4Δtrm8Δ (Fig. 5A). In accordance with the suppressed growth defect, Northern blotting revealed that the hypomodified tRNAVal(AAC) was more stable in the presence of rpc128-1007 mutation, although de novo transcription is decreased, resulting in reduced level of total tRNA (Fig. 5B; Supplemental Fig. S5A). Quantification of the blots employing an intron probe showed that the rpc128-1007 mutation decreased de novo synthesis of pre-tRNALeu in the trm4Δtrm8Δ strain by 40%. The steady state level of tRNAVal(AAC) at the permissive temperature was also significantly lower in triple trm4Δtrm8Δ rpc128-1007 mutant (∼75% of that in double trm4Δtrm8Δ mutant). However, following the temperature shift, tRNAVal(AAC) level was reduced only by 20% in trm4Δtrm8Δ rpc128-1007 in contrast to the reduction exceeding 70% in trm4Δtrm8Δ.
FIGURE 5.
Partial inactivation of Pol III subunit results in suppression of trm4Δtrm8Δ ts phenotype and tRNAVal(AAC) stabilization. (A) Cold-sensitive rpc128-1007 mutation in Pol III subunit Rpc128 was introduced to trm4Δtrm8Δ strain by genetic cross, resulting in triple trm4Δtrm8Δrpc128-1007 mutant. Serial 10-fold dilutions of BY4741 (wt), rpc128-1007, trm4Δtrm8Δ, and trm4Δtrm8Δrpc128-1007 cells were spotted on YPD plates and incubated at indicated temperatures. (B) Cells were grown in YPD to OD600 = 1.0 at 30°C, transferred to 37°C, and harvested as indicated. RNA was isolated and examined by Northern hybridization with probe specific for tRNAVal(AAC). Plots represent levels of tRNAVal(AAC) normalized to loading control (5.8S rRNA). The magnitude of change was calculated relative to expression in trm4Δtrm8Δ strain at 0 time point. SD was calculated on the basis of three independent experiments. P-values—P < 0.01 for the probe indicated with a single asterisk (*) and P < 0.005 for the probe indicated with two asterisks (**)—were calculated relative to control strain at each time point. (C) Gene encoding Rpc17 subunit of Pol III was cloned in trm4Δtrm8Δ strain under the regulated tetO7 promoter to allow down-regulation of RPC17 expression by doxycycline. Serial 10-fold dilutions of trm4Δtrm8Δ tetO7∷RPC17 and control cells were spotted to YPD plate containing 5 μg/mL of doxycycline and incubated for 2 d at indicated temperatures. Phenotypic suppression of the ts phenotype trm4Δtrm8Δ was most efficient at 36°C. (D) trm4Δtrm8Δ tetO7∷RPC17 cells were grown in YPD with or without 2 μg/mL of doxycycline to OD600 = 1.0 at 23°C, transferred to 37°C, and harvested as indicated. RNA was isolated and examined by Northern hybridization with probe specific for tRNAVal(AAC). Plots represent levels of RNAVal(AAC) normalized to loading control (5.8S rRNA). The magnitude of change was calculated relative to expression in culture without doxycycline at zero (0) time point. SD was calculated on the basis of three independent experiments. P-values—P < 0.005 for the probes indicated with two asterisks (**)—were calculated relative to control strain at each time point.
As a yet another approach we decreased Pol III transcription by partial inactivation of its Rpc17 subunit through expression of RPC17 gene cloned under the regulated tetO7 promoter. Addition of doxycycline to the growth medium decreased the level of Rpc17 protein and reduced tRNA transcription whereas in the absence of the antibiotic Pol III was unaffected (Ferri et al. 2000). To monitor the effect of Rpc17 level on the stability of hypomodified tRNAVal(AAC) we created a trm4Δtrm8Δ strain expressing RPC17 from the tetO7 promoter. In the presence of doxycycline, Northern blot with an intron probe of pre-tRNALeu indicated that repression of Rpc17 expression impaired Pol III transcription and reduced tRNA levels (Fig. 5D; Supplemental Fig. S5B). Northern blotting revealed also that tRNAVal(AAC) was mostly degraded when the Rpc17 level was high in the absence of doxycycline but was more than twice stable when Rpc17 expression and Pol III activity were decreased in the presence of doxycycline. In keeping with the tRNAVal(AAC) stabilization the ts phenotype of the trm4Δtrm8Δ strain expressing RPC17 from the tetO7 promoter was suppressed in the presence of doxycycline (Fig. 5C).
Altogether these results clearly indicate an inverse relationship between the stability of hypomodified tRNAVal(AAC) and the activity of Pol III-directed transcription.
DISCUSSION
Our interest in the global regulation of tRNA levels, involving transcription, maturation and decay, led us to screen for gene products that control the stability of hypomodified tRNAVal(AAC). We anticipated that we would uncover genes compensating for the lowered steady state level and specific modifications missing in tRNAVal(AAC), in addition to those involved in indirect mechanisms affecting tRNA decay. As anticipated, most of the insert cloned contained one of the thirteen copies of genes encoding tRNAVal(AAC). Surprisingly, no genes encoding tRNA-methyltransferases were uncovered. Actually, two other categories of gene products were identified: those affecting global tRNA transcription—Maf1 and truncated Pol III subunit Rpc160—and those directly interacting with tRNAVal(AAC)—elongation factor eEF1A and valyl-tRNA synthetase.
In yeast, Maf1 is the only known general and global negative regulator of Pol III which mediates several signaling pathways (Upadhya et al. 2002). Maf1 inhibits tRNA transcription via a mechanism that depends on the dephosphorylation and nuclear accumulation of Maf1 followed by its physical association with Pol III at tRNA genes. Conversely, Maf1 phosphorylation occurs in favorable growth conditions and is linked with its cytoplasmic localization (Moir et al. 2006; Oficjalska-Pham et al. 2006; Roberts et al. 2006).
We have shown recently that Maf1 indirectly affects maturation of tRNA precursors (Karkusiewicz et al. 2011). End-matured intron-containing pre-tRNAs accumulate in cells lacking Maf1 due to saturation of processing machinery by the increased amounts of primary transcripts. Here we have shown that the phenotype of the trm4Δtrm8Δ mutant can be suppressed by overexpression of the Maf1-encoding gene or, more efficiently, expression of the dephosphorylated Maf1-7A mutant that constitutively binds the Pol III complex and reduce tRNA transcription (Huber et al. 2009). The suppression by overexpressed Maf1-encoding gene is accompanied by a twofold stabilization of hypomodified tRNAVal(AAC), while Maf1-7A mutant with reduced basal tRNA levels due to an increased capacity of Maf1-7A to bind and inhibit Pol III gives even stronger effect. Selection of a plasmid containing a 5′-terminal part of the RPC160 gene as an autonomous suppressor of the trm4Δtrm8Δ defect was anticipated and validates the Maf1-mediated suppression. Interaction of Maf1 with the Rpc160 subunit of Pol III has been documented both genetically and by structural analysis of Pol III with Maf1 (Pluta et al. 2001; Vannini et al. 2010). Overexpression of a 5′-terminal fragment as well as point mutations in the RPC160 gene were identified previously as suppressors of the maf1Δ growth phenotype. That suppression was accompanied by reduction of tRNA levels in maf1Δ cells (Boguta et al. 1997; Pluta et al. 2001). In view of those earlier data, the similar suppressor actions of Maf1 and the N-terminal part of Rpc160 on the trm4Δtrm8Δ growth defect were expected.
The increased stability of hypomodified tRNAVal(AAC) caused by Maf1 activation raised the idea that the observed stabilization could be related to Maf1-mediated repression of Pol III transcription. Initially this hint seemed to be inconsistent with the previous observation that cells treated with the thiolutin, Pol III inhibitor, carry out the tRNA degradation at the same rate (Chernyakov et al. 2008b). However thiolutin inhibits all RNA polymerases (Tipper 1973), exerting side effects which in turn possibly influence tRNA stabilization. Moreover, cells treated with thiolutin for a short time have stopped pre-tRNA transcription, but levels of mature tRNAs are not affected (Chernyakov et al. 2008b). In favor of coupling tRNA transcription to tRNA decay we found that tRNAVal(AAC) in trm4Δtrm8Δ cells was stabilized upon Pol III inhibition, either in a Maf1-dependent manner or when directly down-regulating general tRNA transcription by two means: by introduction of the rpc128-1007 mutation in the Pol III subunit Rpc128 or by decreasing expression of another subunit, Rpc17. Regardless of the approach used, the reduced Pol III activity and, in consequence, lower levels of total tRNA brought about a significant stabilization of the hypomodified tRNAVal(AAC).
Proposed mechanism that could account for the effect of Pol III inhibition on stabilization of tRNAVal(AAC) is that, in the context of a globally lower mature tRNA level, tRNAVal(AAC) could more efficiently compete for tRNA-interacting proteins helping it achieve proper conformation and/or protecting it from nucleases. We favor the latter interpretation because at least two of the multicopy suppressors of the trm4Δtrm8Δ mutant selected in our screen encoded tRNA-interacting proteins, eEF1A and valyl-tRNA synthetase, and overexpression of either of those genes also stabilized tRNAVal(AAC).
Taken together, our studies propose two alternative ways by which RTD degradation could be limited: a decrease in tRNA synthesis and an increase in the levels of specific tRNA-interacting proteins protecting tRNA from degradation. According to our model (Fig. 6), mature tRNAs compete with each other for interacting proteins and thus the availability of some such proteins becomes limiting. Due to the missing m7G46 and m5C49 modifications, the tertiary structure of the acceptor and T stems in tRNAVal(AAC) is imperfect or less stable (reference in Whipple et al. 2011) and, therefore, the interaction of this tRNA with proteins is compromised. It would be a poorer competitor for valyl-tRNA synthetase or eEF1A than other tRNAs. A globally decreased tRNA synthesis would relief that competition by decreasing the overall tRNA:protein ratio, thereby allowing even the imperfect, hypomodified tRNAVal(AAC) to find its match(es). Overexpression of limiting protein partner(s) would reduce the same effect and we suggest that this explains the suppression by valyl-tRNA synthetase and eEF1A. On the other hand, the altered structure of hypomodified tRNAVal(AAC) makes it more susceptible to degradation by 5′→3′ exonucleases. This instability may also lead to isomerization of the acceptor stem, which is then subject to double CCA addition and can also be polyadenylated at the 3′ end like observed for hypomodified tRNASer(CGA) (Wilusz et al. 2011).
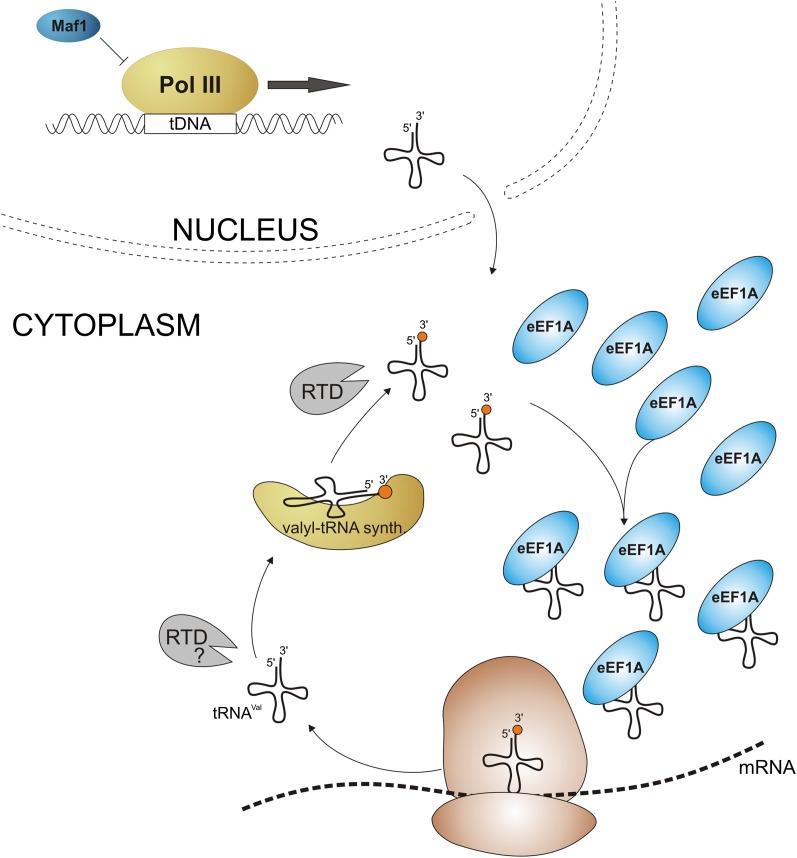
FIGURE 6.
Model for eEF1A-mediated protection of tRNAVal(AAC) from RTD degradation. Mature, hypomodified tRNAVal(AAC) pool is aminoacylated by valyl-tRNA synthetase, bound by eEF1A, and delivered to ribosomes. A shift to restrictive conditions results in degradation of hypomodified tRNAVal(AAC) by RTD pathway. It is unknown whether hypomodified tRNA can be efficiently charged and if it could be degraded prior to aminoacylation. Nevertheless, elevated population of eEF1A molecules favors their binding to tRNAVal(AAC) and prevents tRNAVal(AAC) interacting with nucleases. Increased availability of tRNA-free eEF1A molecules may be achieved by increased expression of TEF1 gene or decreased global tRNA synthesis by Pol III inhibition.
How does eEF1A prevent degradation of the unstable, hypomodified tRNAVal(AAC) by RTD degradation? eEF1A is a homolog of E. coli EF-Tu, which interacts with the acceptor stem of aminoacyl-tRNA (Stark et al. 2002; Valle et al. 2002). Here we assume that eEF1A and 5′→3′ exonucleases interact with the partially unstable tRNAVal(AAC) in a competitive manner. Fine-tuning of the competitive interaction between decay enzymes and other tRNA-binding proteins could be significant in tRNA evolution, both at the level of nucleotide sequence and nucleotide modifications. The binding of EF-Tu with aa-tRNA depends on the sequence and structure of T stem (Schrader et al. 2011). An aa-tRNA has to bind to EF-Tu with sufficient strength to form a complex with the ribosome, but weakly enough to allow the aa-tRNA to be released during decoding. Introduction of a T-stem sequence from tightly binding tRNA to a weakly binding one results in very low rate of peptide bond formation. Assuming that improper binding of tRNA with eEF1A increases the accessibility of this tRNA to 5′→3′ exonucleases, the RTD-mediated degradation could provide a positive tRNA selection in evolution.
MATERIALS AND METHODS
Strains, plasmids, and media
Yeast strains BY4741 (a his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), BY4742 (α his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0), and isogenic trm4Δ and trm8Δ were purchased from Euroscarf. The trm4Δtrm8Δ haploid and homozygous diploid mutants, ΔTT1A (α his3Δ1 leu2Δ0 lys2Δ0 met15Δ0 ura3Δ0 trm4Δ∷kanMX4 trm8Δ∷kanMX4) and ΔTT 2n (α/a his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 lysΔ0/LYS2 metΔ15/metΔ15 ura3Δ0/ura3Δ0 trm4Δ∷kanMX4/trm4Δ∷kanMX4 trm8Δ∷kanMX4/trm8Δ∷kanMX4), respectively, were obtained by genetic crosses. Single copy of endogenous MAF1 gene in ΔTT 2n was disrupted by URA3 cassette and the resulted diploid (relevant genotype MAF1/maf1Δ∷URA3 trm4Δ∷kanMX4/trm4Δ∷kanMX4 trm8Δ∷kanMX4/trm8Δ∷kanMX4) was sporulated, resulting in 2:2 segregation of viable MAF1 Ura− spores. Ura+ spores containing maf1∷URA3 allele germinated only at 18°C and showed a 12-h lag in growth at 30°C. This growth defect was complemented with MAF1 gene supplied from plasmid. To generate trm4Δtrm8Δ cells expressing Maf1-7A, trm4Δtrm8Δmaf1Δ∷URA3 spore clone was transformed with single copy plasmid pAH247 (pRS415∷MAF1-7A [S90A, S101A, S177A, s178A, S179A, S209A, S210A]; Huber et al. 2009).
BY derivatives trm44Δ and tan1Δ were purchased from Euroscarf. tan1Δtrm44Δ mutant was generated by genetic cross.
Triple rpc128-1007 trm4Δtrm8Δ mutant was isolated from among meiotic progeny of a cross rpc128-1007 (cs) with trm4Δtrm8Δ (ts). Thirteen tetrads were analyzed for kanamycin resistance and growth at 37°C and 18°C. The cs phenotype indicating rpc128-1007 segregated 2:2. Double trm4Δtrm8Δ deletions were identified among kanamycin resistance spore clones by PCR analysis of genomic DNA. All spore clones identified as trm4Δtrm8Δrpc128-1007 grew at 37°C.
The pCM189[RPC17] plasmid encoding repressible Rpc17 Pol III subunit was created in E. coli strain MH1 from pCMc17 (Ferri et al. 2000) using pCM189 (Euroscarf) as backbone. Single copy of endogenous RPC17 gene was replaced in trm4Δ trm8Δ homozygous diploid yeast strain by HIS3 cassette and the obtained strain (relevant genotype RPC17/rpc17Δ∷HIS3 trm4Δ∷kanMX4/trm4Δ∷kanMX4 trm8Δ∷kanMX4/trm8Δ∷kanMX4) was transformed with pCM189[RPC17] plasmid and sporulated. The growth of spore clones containing rpc17Δ∷HIS3 allele and pCM189[RPC17] plasmid was sensitive to doxycycline (2 μg/mL in solid YPD) but resistant to elevated temperature.
Yeast YPD or YPGly complete media contained 1% yeast extract, 2% peptone, and 2% glucose or 2% glycerol, respectively. The minimal media, SC-ura or SC-leu, containing 0.67% yeast nitrogen base and 2% glucose or glycerol were supplemented with all necessary requirements without uracil or leucine, respectively. Solid media contained 2% agar. All reagents were from Difco.
Cloning of trm4Δtrm8Δ defect suppressors
Suppressors of trm4Δtrm8Δ were cloned by complementation of the ts growth phenotype on YPD medium. trm4Δtrm8Δ strain was transformed with yeast genebank on multicopy pFL44L vector provided by F. Lacroute (Stettler et al. 1993) and plated on SC-ura. About 5000 URA+ transformants were replicated onto YPD medium and incubated at 37°C for 7 d, with growth monitored every day. POP5 was subcloned from p47 plasmid containing genomic fragment of chromosome I (coordinates 80144–84902) by ligation of NarI-NarI fragment to pFL44L empty vector.
Northern analysis
Cells were grown according to Alexandrov et al. (2006) with modification. One-hundred milliliters of culture grown at 23°C to OD600 = 1.0 was rapidly mixed with 400 mL of media preheated to 42°C. The cultures were further incubated at 37°C and harvested at time points indicated by centrifugation in a rotor preheated to 37°C. For cold-sensitive strains with rpc128-1007 mutation or overexpressed MAF1 100 mL of culture grown at 30°C to OD600 = 1.0 was rapidly mixed with 400 mL of media preheated to 40°C. Repression of RPC17 gene in liquid cultures was done by adding doxycycline (Sigma) to 2 μg/mL final concentration and further incubation for ∼24 h to OD600 = 1.0. RNA was extracted and hybridized as described previously (Graczyk et al. 2011). The following probes were employed: tRNAVal(AAC): TGGTGATTTCGCCCAGGA; tRNALeu(CAA) intron: TATTCCCACAGTTTGCGGTCA; 5.8S rRNA: GCGTTGTTCATCGATGC. After hybridization blots were washed 3 × 10 min with 1× SSC, 1% SDS and 2 × 10 min with 0.5× SSC, 0.1% SDS at 37°C, exposed to a phosphoimager screen (Fujifilm). RNA was quantified using FLA-7000 PhosphoImager (Fujifilm). Band intensities were quantified using Multi Gauge V3.0 Software. The statistical significance was computed using a t-test as implemented in the OpenOffice.org environment.
Western analysis
Cells were broken and proteins isolated as described previously (Towpik et al. 2008). Protein extracts were separated on 10% (acrylamide:bisacrylamide 33.5:0.3) SDS-PAGE and hybridized with Maf1-specific antibody as described previously (Gajda et al. 2010).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We thank Olivier Lefebvre and Damian Graczyk for correction of the manuscript and Alexandre Huber for kindly providing Maf1-7A encoding plasmid. This work was supported by National Center of Science UMO-2011/01/N/NZ1/03461 and by the European Union in the framework of European Social Fund through the Warsaw University of Technology Development Programme.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.033597.112.
REFERENCES
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM 2006. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21: 87–96 [DOI] [PubMed] [Google Scholar]
- Anderson JS, Parker RP 1998. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J 17: 1497–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguta M, Graczyk D 2011. RNA polymerase III under control: Repression and de-repression. Trends Biochem Sci 36: 451–456 [DOI] [PubMed] [Google Scholar]
- Boguta M, Czerska K, Zoladek T 1997. Mutation in a new gene MAF1 affects tRNA suppressor efficiency in Saccharomyces cerevisiae. Gene 185: 291–296 [DOI] [PubMed] [Google Scholar]
- Chernyakov I, Baker MA, Grayhack EJ, Phizicky EM 2008a. Chapter 11. Identification and analysis of tRNAs that are degraded in Saccharomyces cerevisiae due to lack of modifications. Methods Enzymol 449: 221–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM 2008b. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev 22: 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla M, Towpik J, Graczyk D, Oficjalska-Pham D, Harismendy O, Suleau A, Balicki K, Conesa C, Lefebvre O, Boguta M 2007. Maf1 is involved in coupling carbon metabolism to RNA polymerase III transcription. Mol Cell Biol 27: 7693–7702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N, Lee J, Upadhya R, Chu Y, Moir RD, Willis IM 2005. Two steps in Maf1-dependent repression of transcription by RNA polymerase III. J Biol Chem 280: 6455–6462 [DOI] [PubMed] [Google Scholar]
- Ferri ML, Peyroche G, Siaut M, Lefebvre O, Carles C, Conesa C, Sentenac A 2000. A novel subunit of yeast RNA polymerase III interacts with the TFIIB-related domain of TFIIIB70. Mol Cell Biol 20: 488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajda A, Towpik J, Steuerwald U, Muller CW, Lefebvre O, Boguta M 2010. Full repression of RNA polymerase III transcription requires interaction between two domains of its negative regulator Maf1. J Biol Chem 285: 35719–35727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk D, Debski J, Muszynska G, Bretner M, Lefebvre O, Boguta M 2011. Casein kinase II-mediated phosphorylation of general repressor Maf1 triggers RNA polymerase III activation. Proc Natl Acad Sci 108: 4926–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Bodenmiller B, Uotila A, Stahl M, Wanka S, Gerrits B, Aebersold R, Loewith R 2009. Characterization of the rapamycin-sensitive phosphoproteome reveals that Sch9 is a central coordinator of protein synthesis. Genes Dev 23: 1929–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha S, Dutta A 2009. RVB1/RVB2: Running rings around molecular biology. Mol Cell 34: 521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J 2004. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev 18: 1227–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkusiewicz I, Turowski TW, Graczyk D, Towpik J, Dhungel N, Hopper AK, Boguta M 2011. Maf1 protein, repressor of RNA polymerase III, indirectly affects tRNA processing. J Biol Chem 286: 39478–39488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelawala L, Grayhack EJ, Phizicky EM 2008. Identification of yeast tRNA Um44 2′-O-methyltransferase (Trm44) and demonstration of a Trm44 role in sustaining levels of specific tRNASer species. RNA 14: 158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai L, Fang F, Butler JS, Sherman F 2004. Polyadenylation of rRNA in Saccharomyces cerevisiae. Proc Natl Acad Sci 101: 8581–8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Kenneth NS, White RJ 2008. Elevated tRNAiMet synthesis can drive cell proliferation and oncogenic transformation. Cell 133: 78–89 [DOI] [PubMed] [Google Scholar]
- Mateyak MK, Kinzy TG 2010. eEF1A: Thinking outside the ribosome. J Biol Chem 285: 21209–21213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir RD, Lee J, Haeusler RA, Desai N, Engelke DR, Willis IM 2006. Protein kinase A regulates RNA polymerase III transcription through the nuclear localization of Maf1. Proc Natl Acad Sci 103: 15044–15049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oficjalska-Pham D, Harismendy O, Smagowicz WJ, Gonzalez de Peredo A, Boguta M, Sentenac A, Lefebvre O 2006. General repression of RNA polymerase III transcription is triggered by protein phosphatase type 2A-mediated dephosphorylation of Maf1. Mol Cell 22: 623–632 [DOI] [PubMed] [Google Scholar]
- Phizicky EM, Hopper AK 2010. tRNA biology charges to the front. Genes Dev 24: 1832–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta K, Lefebvre O, Martin NC, Smagowicz WJ, Stanford DR, Ellis SR, Hopper AK, Sentenac A, Boguta M 2001. Maf1p, a negative effector of RNA polymerase III in Saccharomyces cerevisiae. Mol Cell Biol 21: 5031–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DN, Wilson B, Huff JT, Stewart AJ, Cairns BR 2006. Dephosphorylation and genome-wide association of Maf1 with Pol III-transcribed genes during repression. Mol Cell 22: 633–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader JM, Chapman SJ, Uhlenbeck OC 2011. Tuning the affinity of aminoacyl-tRNA to elongation factor Tu for optimal decoding. Proc Natl Acad Sci 108: 5215–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H, Rodnina MV, Wieden HJ, Zemlin F, Wintermeyer W, van Heel M 2002. Ribosome interactions of aminoacyl-tRNA and elongation factor Tu in the codon-recognition complex. Nat Struct Biol 9: 849–854 [DOI] [PubMed] [Google Scholar]
- Stettler S, Chiannilkulchai N, Hermann-Le Denmat S, Lalo D, Lacroute F, Sentenac A, Thuriaux P 1993. A general suppressor of RNA polymerase I, II and III mutations in Saccharomyces cerevisiae. Mol Gen Genet 239: 169–176 [DOI] [PubMed] [Google Scholar]
- Tipper DJ 1973. Inhibition of yeast ribonucleic acid polymerases by thiolutin. J Bacteriol 116: 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towpik J, Graczyk D, Gajda A, Lefebvre O, Boguta M 2008. Derepression of RNA polymerase III transcription by phosphorylation and nuclear export of its negative regulator, Maf1. J Biol Chem 283: 17168–17174 [DOI] [PubMed] [Google Scholar]
- Upadhya R, Lee J, Willis IM 2002. Maf1 is an essential mediator of diverse signals that repress RNA polymerase III transcription. Mol Cell 10: 1489–1494 [DOI] [PubMed] [Google Scholar]
- Valle M, Sengupta J, Swami NK, Grassucci RA, Burkhardt N, Nierhaus KH, Agrawal RK, Frank J 2002. Cryo-EM reveals an active role for aminoacyl-tRNA in the accommodation process. EMBO J 21: 3557–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini A, Ringel R, Kusser AG, Berninghausen O, Kassavetis GA, Cramer P 2010. Molecular basis of RNA polymerase III transcription repression by Maf1. Cell 143: 59–70 [DOI] [PubMed] [Google Scholar]
- Whipple JM, Lane EA, Chernyakov I, D'Silva S, Phizicky EM 2011. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev 25: 1173–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ 2004. RNA polymerase III transcription and cancer. Oncogene 23: 3208–3216 [DOI] [PubMed] [Google Scholar]
- Wilusz JE, Whipple JM, Phizicky EM, Sharp PA 2011. tRNAs marked with CCACCA are targeted for degradation. Science 334: 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, Boulay J, Regnault B, Devaux F, Namane A, Seraphin B, et al. 2005. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121: 725–737 [DOI] [PubMed] [Google Scholar]