The structural and functional integrity of tRNA is crucial for translation. In the yeast Saccharomyces cerevisiae, certain aberrant pre-tRNA species are subject to nuclear surveillance, leading to 3′ exonucleolytic degradation, and certain mature tRNA species are subject to rapid tRNA decay (RTD) if they are appropriately hypomodified or bear specific destabilizing mutations, leading to 5′-3′ exonucleolytic degradation by Rat1 and Xrn1. For example, trm8-Δ trm4-Δ strains are temperature sensitive due to lack of m7G46 and m5C and the consequent RTD of tRNAVal(AAC). It is unknown how the RTD pathway interacts with translation and other cellular processes, and how generally this pathway acts on hypomodified tRNAs. The authors provide evidence here that elongation factor 1A (EF-1A) competes with the RTD pathway for substrate tRNAs, since its overexpression suppresses the tRNA degradation and the growth defect of strains subject to RTD.
Keywords: TEF1, TRM1, EF-1A, tRNA turnover, Saccharomyces cerevisiae
Abstract
The structural and functional integrity of tRNA is crucial for translation. In the yeast Saccharomyces cerevisiae, certain aberrant pre-tRNA species are subject to nuclear surveillance, leading to 3′ exonucleolytic degradation, and certain mature tRNA species are subject to rapid tRNA decay (RTD) if they are appropriately hypomodified or bear specific destabilizing mutations, leading to 5′-3′ exonucleolytic degradation by Rat1 and Xrn1. Thus, trm8-Δ trm4-Δ strains are temperature sensitive due to lack of m7G46 and m5C and the consequent RTD of tRNAVal(AAC), and tan1-Δ trm44-Δ strains are temperature sensitive due to lack of ac4C12 and Um44 and the consequent RTD of tRNASer(CGA) and tRNASer(UGA). It is unknown how the RTD pathway interacts with translation and other cellular processes, and how generally this pathway acts on hypomodified tRNAs. We provide evidence here that elongation factor 1A (EF-1A) competes with the RTD pathway for substrate tRNAs, since its overexpression suppresses the tRNA degradation and the growth defect of strains subject to RTD, whereas reduced levels of EF-1A have the opposite effect. We also provide evidence that RTD acts on a variety of tRNAs lacking one or more different modifications, since trm1-Δ trm4-Δ mutants are subject to RTD of tRNASer(CGA) and tRNASer(UGA) due to lack of m2,2G26 and m5C, and since trm8-Δ, tan1-Δ, and trm1-Δ single mutants are each subject to RTD. These results demonstrate that RTD interacts with the translation machinery and acts widely on hypomodified tRNAs.
INTRODUCTION
The structural and functional integrity of tRNA is crucial for translation in all cells. Efficient and accurate translation requires that tRNAs have four features. First, to ensure their participation in translation with more or less equal efficiency, all tRNAs require a similar overall shape and structure, including the familiar clover leaf secondary structure, and tertiary interactions that are important for efficient folding of the tRNA into its usual L-shape (Kim et al. 1974; Jack et al. 1976; Westhof et al. 1985; Basavappa and Sigler 1991). Second, to ensure specific recognition by their cognate tRNA synthetases (Ling et al. 2009), all tRNAs require unique features, including the anticodon (N34-N36), the discriminator base (N73), several modifications, and a number of residues throughout the tRNA (Muramatsu et al. 1988; Musier-Forsyth and Schimmel 1992; Giege et al. 1998). Third, to ensure high-accuracy translation (Zaher and Green 2009; Kramer et al. 2010), all tRNAs require features to uniquely decode their cognate codon, including an extensive set of anticodon loop modifications for highly specific codon:anticodon pairing (Murphy and Ramakrishnan 2004; Murphy et al. 2004; Agris et al. 2007), and other sequence elements (Cochella and Green 2005; Ledoux et al. 2009). Fourth, all tRNAs are required to be rugged enough to survive constant use in translation but flexible enough to withstand the bending and contortions that occur during ribosome transit (Valle et al. 2003; Yarus et al. 2003; Schmeing et al. 2009). The approximately 3 × 106 molecules of tRNA in an actively growing yeast cell require synthesis of about two tRNAs/second/locus (Waldron and Lacroute 1975) and long tRNA lifetimes, which in turn require mechanisms to ensure that tRNAs have the proper stability (Phizicky and Hopper 2010).
tRNA is subject to two quality-control steps during and after its biogenesis. During processing, a nuclear surveillance pathway acts on pre-tRNAiMet lacking 1-methyladenosine at A58 (m1A58) and on pre-tRNA species that are aberrantly processed at their 3′ ends; this occurs by oligoadenylation of the 3′ end of the tRNA by the TRAMP complex, followed by 3′ exonucleolytic degradation by Rrp6 and the nuclear exosome (Kadaba et al. 2004, 2006; LaCava et al. 2005; Vanacova et al. 2005; Copela et al. 2008; Wang et al. 2008; Ozanick et al. 2009). After processing, a rapid tRNA decay (RTD) pathway acts on several specific mature tRNA species that lack certain modifications; this is mediated by the 5′-3′ exonucleases Rat1 and Xrn1 and by Met22, which likely regulates Rat1 and Xrn1 activity indirectly, through inhibition by its substrate pAp (Dichtl et al. 1997; Alexandrov et al. 2006; Chernyakov et al. 2008; Whipple et al. 2011). Thus, trm8-Δ trm4-Δ strains are temperature sensitive due to lack of m7G46 and m5C in their tRNAs and the consequent RTD of tRNAVal(AAC) (Alexandrov et al. 2006), and tan1-Δ trm44-Δ strains are temperature sensitive due to lack of ac4C12 and Um44 in their tRNAs and the consequent RTD of tRNASer(CGA) and tRNASer(UGA), as well as tRNALeu(GAG) (Chernyakov et al. 2008; Kotelawala et al. 2008). RTD may act on charged tRNAs, since RTD of tRNAVal(AAC) in trm8-Δ trm4-Δ strains appears to be selective for the charged species and since suppressor mutations in MET22, RAT1, and XRN1 that prevent RTD also restore charging (Alexandrov et al. 2006; Chernyakov et al. 2008).
We have recently shown that the RTD pathway also acts on tRNAs bearing mutations that destabilize the combined acceptor and T-stems of tRNASer or tRNATyr (Whipple et al. 2011). To understand how the RTD pathway selects certain substrate tRNA species lacking modifications, but not other tRNAs with the same modifications, we developed a genetic screen to examine tRNASer(CGA) variants. From analysis of 43 tRNASer variants, we showed that RTD substrate recognition in vivo correlates strongly with reduced predicted stability of the acceptor and T-stems, but not of the anticodon stem, and does not necessarily require hypomodified tRNA, since fully modified tRNAs are subject to RTD if appropriately destabilized. Moreover, our biochemical evidence shows a strong correlation between the increased degradation by purified Xrn1 and the weaker predicted stability of the acceptor and T-stems or the weaker stability due to lack of ac4C12 and Um44 (Whipple et al. 2011). Remarkably, tRNASer species subject to RTD also have an increased population of molecules in which the 3′ ends have CCACCA or oligo(A) instead of the usual CCA terminus (Wilusz et al. 2011).
A number of features of the RTD pathway are currently unclear, including how the RTD pathway interacts with the translation machinery and with other cellular processes, as well as the true scope of the RTD pathway with respect to the number of different modification defects that can trigger RTD and the number of different tRNAs that are subject to RTD. To gain a better understanding of these factors, we selected and characterized high-copy suppressors of the RTD phenotype of trm8-Δ trm4-Δ strains and examined the role of RTD in another tRNA modification mutant. We find evidence that the translation elongation factor 1A (EF-1A) is in competition with the RTD pathway for charged tRNA substrates. We also find that RTD is triggered by trm1-Δ trm4-Δ mutants lacking m2,2G and m5C, due to loss of tRNASer(CGA) and tRNASer(UGA), and provide evidence that the lack of a number of different individual modifications triggers RTD of the same tRNA species found for the corresponding double mutants. These results significantly broaden the scope of the RTD pathway and define an important interaction of this pathway with the translation apparatus.
RESULTS
RTD competes with EF-1A for tRNA substrates
To further define components of RTD, we screened the MORF library of yeast expression plasmids for genes that suppress the temperature-sensitive phenotype of trm8-Δ trm4-Δ mutants, when expressed at high levels under the control of the PGAL promoter from a high-copy plasmid (Gelperin et al. 2005). In this way we found several high-copy suppressors (Fig. 1A), including TEF1 and TEF2, which each encode identical copies of EF-1A.
FIGURE 1.
Overproduction of EF-1A suppresses RTD in a trm8-Δ trm4-Δ strain. (A) Overexpression of TEF1 or TEF2 suppresses the temperature sensitivity of a trm8-Δ trm4-Δ strain. A trm8-Δ trm4-Δ strain was transformed with MORF plasmids expressing the indicated ORFs under PGAL control, transformants were grown overnight in S -Ura media containing raffinose, and serial 10-fold dilutions were plated on YP media containing raffinose and galactose and incubated for 2 d at the indicated temperatures. (B) Overproduction of EF-1A prevents degradation of tRNAVal(AAC) in trm8-Δ trm4-Δ mutants. Strains containing plasmids as indicated were grown at 28°C in S -Ura media containing raffinose and galactose to OD600 0.6, thiolutin (TL) was added, cells were shifted to 34°C for the times indicated, and RNA was extracted, resolved by 10% PAGE, transferred to a membrane, and hybridized to the indicated probes, as described in the Materials and Methods. RNA levels were quantified by normalizing to 5S RNA levels and then normalizing to the values in the wild type (BY4741) at time zero. Diamonds indicate wild type; squares, trm8-Δ trm4-Δ; open triangles, trm8-Δ trm4-Δ [TEF1]; and open circles, trm8-Δ trm4-Δ [TEF2].
To determine if overexpression of EF-1A suppresses the temperature-sensitive phenotype of trm8-Δ trm4-Δ mutants by preventing RTD of tRNAVal(AAC), we examined tRNA levels after thiolutin treatment, which inhibits further transcription by RNA polymerases including polymerase III (Jimenez et al. 1973), thereby freezing the number of tRNA species before the temperature shift. We find that, under these conditions, overexpression of TEF1 or TEF2 markedly prevents degradation of tRNAVal(AAC) in trm8-Δ trm4-Δ mutants undergoing RTD at 34°C (Fig. 1B).
To determine the generality of EF-1A effects, we examined suppression of the growth defect of tan1-Δ trm44-Δ mutants, which are also subject to RTD and have different tRNA substrates (Chernyakov et al. 2008). Since overexpression of either TEF1 or TEF2 also suppresses the temperature sensitivity of tan1-Δ trm44-Δ mutants (Fig. 2A) and since overexpression of TEF1 prevents RTD of its known substrates tRNASer(CGA) and tRNASer(UGA) after thiolutin treatment and a temperature shift to 36°C (Fig. 2B), we conclude that the overexpression of EF-1A generally prevents RTD.
FIGURE 2.
Overproduction of EF-1A suppresses RTD in a tan1-Δ trm44-Δ mutant. (A) Overproduction of TEF1 or TEF2 partially suppresses the temperature-sensitive phenotype of tan1-Δ trm44-Δ mutants. Strains with MORF plasmids expressing TEF1 or TEF2 (or a vector control) were grown and plated on YP media containing raffinose and galactose as described in Figure 1A. (B) Overproduction of TEF1 prevents degradation of tRNASer(CGA) and tRNASer(UGA) in tan1-Δ trm44-Δ mutants. Strains were grown at 28°C, treated with thiolutin (TL), and shifted to 36°C for the times indicated, and RNA was extracted, resolved by PAGE, transferred, and hybridized. RNA levels were quantified by normalizing to 5.8 S RNA levels and then normalizing to the values in the wild type (BY4741) at time zero. Diamonds indicate wild type; open squares, tan1-Δ trm44-Δ; and triangles, tan1-Δ trm44-Δ [TEF1].
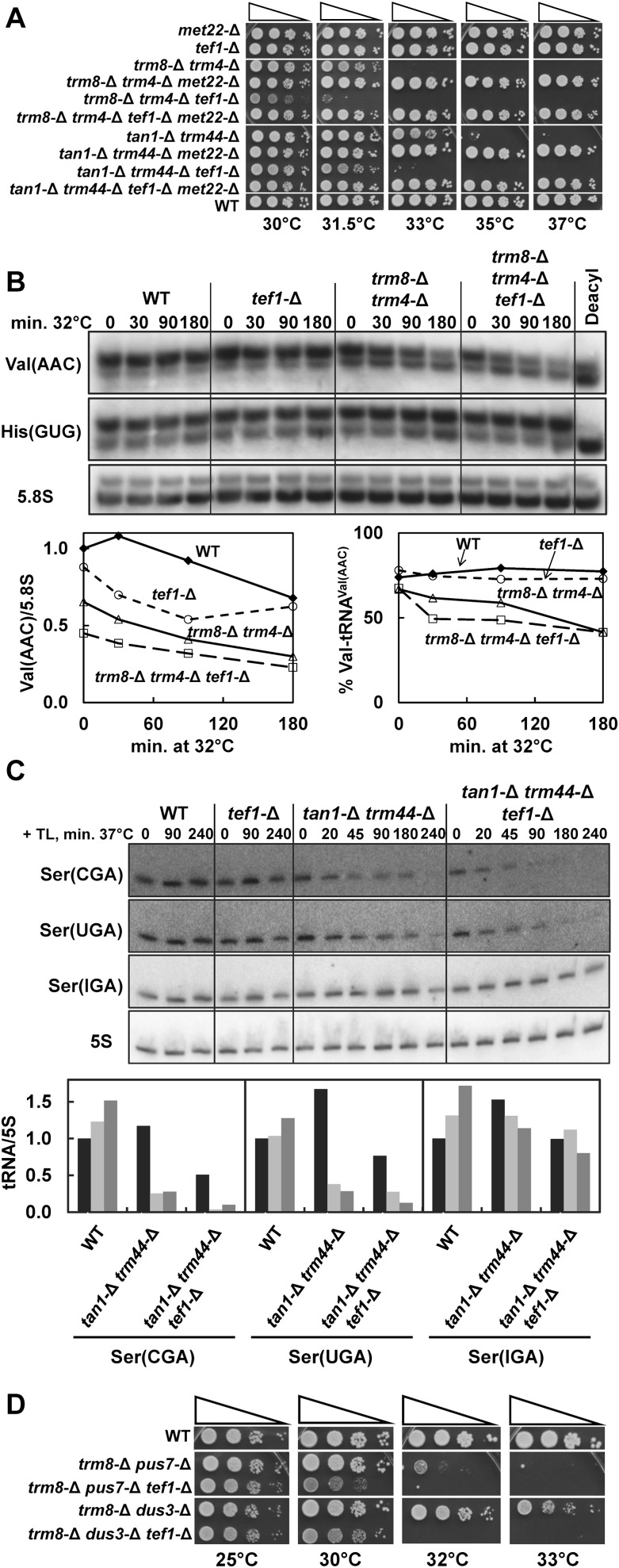
One plausible mechanism by which RTD could be suppressed by overexpression of EF-1A is if there is competition between EF-1A and the RTD pathway for charged tRNAs. It is well established that EF-1A binds charged tRNA (Gromadski et al. 2007), and our evidence suggests that RTD of tRNAVal(AAC) preferentially degrades the charged species (Alexandrov et al. 2006; Chernyakov et al. 2008). If competition between the RTD pathway and EF-1A occurs, then reduced levels of EF-1A ought to make cells more temperature sensitive, due to enhanced RTD. Consistent with this hypothesis, we find that a tef1-Δ mutation significantly enhances the temperature sensitivity of a trm8-Δ trm4-Δ mutant or a tan1-Δ trm44-Δ mutant (Fig. 3A). Since tef1-Δ mutants are not themselves temperature sensitive (Fig. 3A) and since the enhanced temperature sensitivity of a trm8-Δ trm4-Δ tef1-Δ mutant or a tan1-Δ trm44-Δ tef1-Δ mutant is completely suppressed by introduction of a met22-Δ mutation to prevent RTD (Chernyakov et al. 2008), we infer that the enhanced temperature sensitivity is due to the enhanced RTD occurring in tef1-Δ–derivative strains. Consistent with this explanation, we find that total tRNAVal(AAC) levels are reduced in a trm8-Δ trm4-Δ tef1-Δ mutant relative to a trm8-Δ trm4-Δ mutant prior to a shift from 28°C and are degraded at the same rate in both strains after a temperature shift and that levels of charged tRNAVal(AAC) are reduced at an accelerated rate after a shift to 32°C (Fig. 3B) or after a shift to 30.5°C or 35°C (data not shown). Similarly, we observe somewhat accelerated and more complete RTD of tRNASer(CGA) and tRNASer(UGA) in a tan1-Δ trm44-Δ tef1-Δ mutant relative to that in a tan1-Δ trm44-Δ mutant, after thiolutin treatment and a shift to 37°C (Fig. 3C).
FIGURE 3.
Reduced levels of EF-1A exacerbate the temperature sensitivity of strains subject to RTD and result in reduced levels of tRNAs subject to RTD. (A) A tef1-Δ mutation exacerbates the temperature sensitivity of trm8-Δ trm4-Δ mutants and tan1-Δ trm44-Δ mutants, and this growth defect is fully suppressed by a met22-Δ mutation. Strains were grown overnight in YPD media, serially 10-fold diluted, plated on YPD plates, and incubated for 2 d at the indicated temperatures. (B) trm8-Δ trm4-Δ tef1-Δ mutants have reduced levels of tRNAVal(AAC) and its charged species during RTD, relative to trm8-Δ trm4-Δ mutants. Strains were grown at 28°C and shifted to 32°C, cells were harvested at the times indicated, and RNA was isolated under acidic conditions to preserve charging. Then RNAs were resolved by 6.5% acidic PAGE as described in the Materials and Methods, transferred, and hybridized to probes as indicated. RNA levels were quantified as described in Figure 2B. Quantification of charging was done for each individual tRNAVal(AAC) sample. (C) tan1-Δ trm44-Δ tef1-Δ mutants have reduced levels of tRNASer(CGA) and tRNASer(UGA) relative to tan1-Δ trm44-Δ mutants during RTD. Strains were grown at 28°C, treated with thiolutin (TL), and shifted to 37°C, and RNA was analyzed at the times indicated and quantified as described in Figure 1B. Bars in black indicate 0 min; light gray, 90 min; and dark gray, 180 min. (D) A tef1-Δ mutation exacerbates the temperature sensitivity of trm8-Δ pus7-Δ mutants and trm8-Δ dus3-Δ mutants. Strains were grown, diluted, and plated on YPD plates as described in A.
These results are consistent with competition between EF-1A and the RTD pathway for charged tRNAs in the cell, which is possible because cellular levels of EF-1A approach those of tRNAs (Thiele et al. 1985). As predicted from these results, we also find that a tef1-Δ mutation enhances the temperature sensitivity of a trm8-Δ pus7-Δ mutant and a trm8-Δ dus3-Δ mutant (Fig. 3D), which are each subject to RTD (Chernyakov et al. 2008). Based on these results, we conclude that the enhanced sensitivity of tef1-Δ–derivative strains to RTD is a general feature of the pathway, presumably due to the relative lack of competing EF-1A, just as the enhanced protection from RTD caused by overexpression of EF-1A is likely due to the relative increase in competing EF-1A.
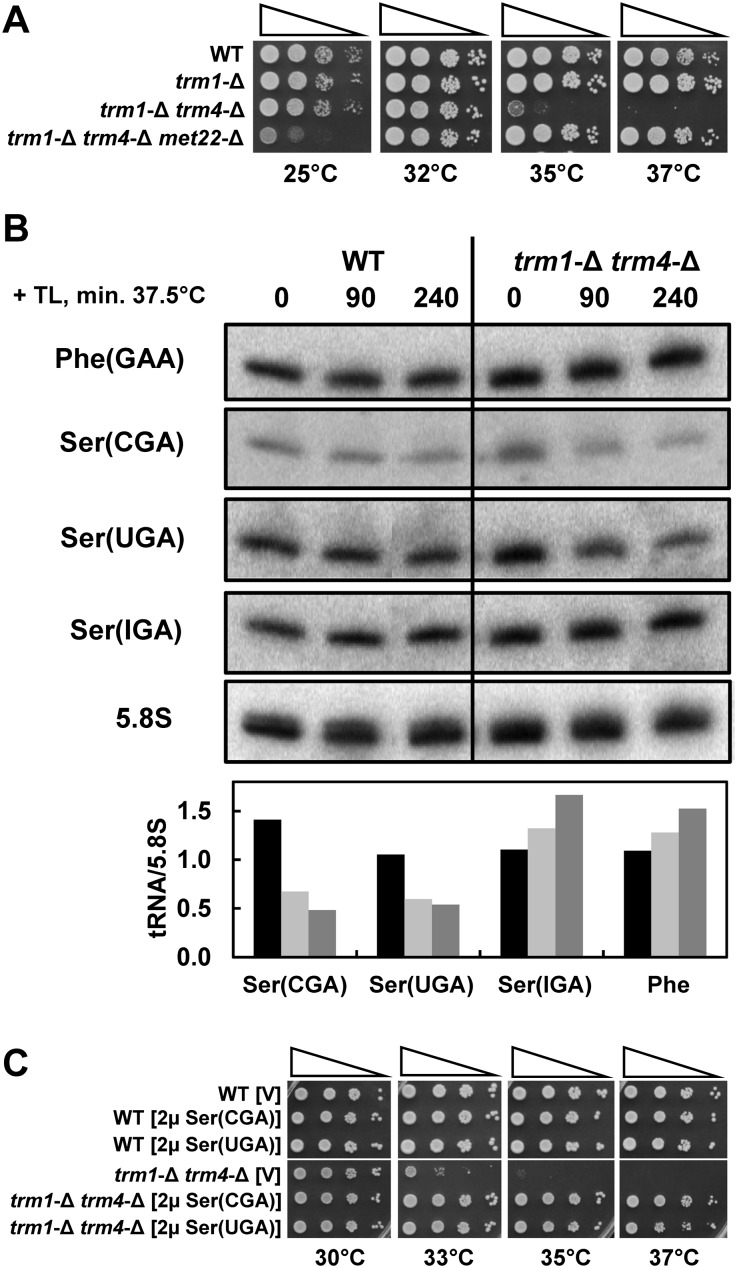
trm1-Δ trm4-Δ mutants are subject to RTD of tRNASer(CGA) and tRNASer(UGA) due to the lack of m2,2G and m5C
To further explore the potential of RTD to occur with different modification mutants, we focused on double mutants generated from trm1-Δ mutants since trm1-Δ mutants, like trm8-Δ mutants (Alexandrov et al. 2005), have a subtle growth defect at high temperature that is significantly exacerbated in double mutants (Gustavsson and Ronne 2008). Thus, we find that trm1-Δ trm4-Δ mutants are temperature sensitive and that this temperature sensitivity is suppressed by a met22-Δ mutation, indicative of an RTD phenotype (Fig. 4A). Northern analysis demonstrates reduced levels of only tRNASer(CGA) and tRNASer(UGA) of nine tRNA species examined (of 16 total) that are known to have both of these modifications (Fig. 4B; data not shown). Consistent with the idea that these tRNAs are the targets of RTD in trm1-Δ trm4-Δ mutants, we find that the temperature sensitivity of trm1-Δ trm4-Δ mutants is nearly fully suppressed by overexpression of tRNASer(CGA) or tRNASer(UGA) (Fig. 4C).
FIGURE 4.
trm1-Δ trm4-Δ mutants are subject to RTD of tRNASer(CGA) and tRNASer(UGA). (A) The temperature sensitivity of trm1-Δ trm4-Δ mutants is suppressed by a met22-Δ mutation. Strains were grown, diluted, and plated on YPD plates as described in Figure 3A. (B) trm1-Δ trm4-Δ mutants have reduced levels of tRNASer(CGA) and tRNASer(UGA). Wild-type or trm1-Δ trm4-Δ strains were grown in YPD media at 28°C, treated with thiolutin (TL), and shifted to 37.5°C, and RNA from cells was analyzed by hybridization at the times indicated. Quantification of RNA levels was done as described in Figure 2B. Bars in black indicate 0 min; light gray, 90 min; and dark gray, 240 min. (C) The temperature sensitivity of trm1-Δ trm4-Δ mutants is suppressed by overproduction of tRNASer(CGA) or tRNASer(UGA). Wild-type and trm1-Δ trm4-Δ strains bearing [2μ LEU2 tRNASer(CGA)], [2μ LEU2 tRNASer(UGA)], or a plasmid vector control were grown overnight in SD-Leu media, serially diluted, plated on SD-Leu media, and incubated for 4 d at the indicated temperatures.
RTD occurs in trm8-Δ, tan1-Δ, and trm1-Δ single-mutant strains, but with reduced efficiency
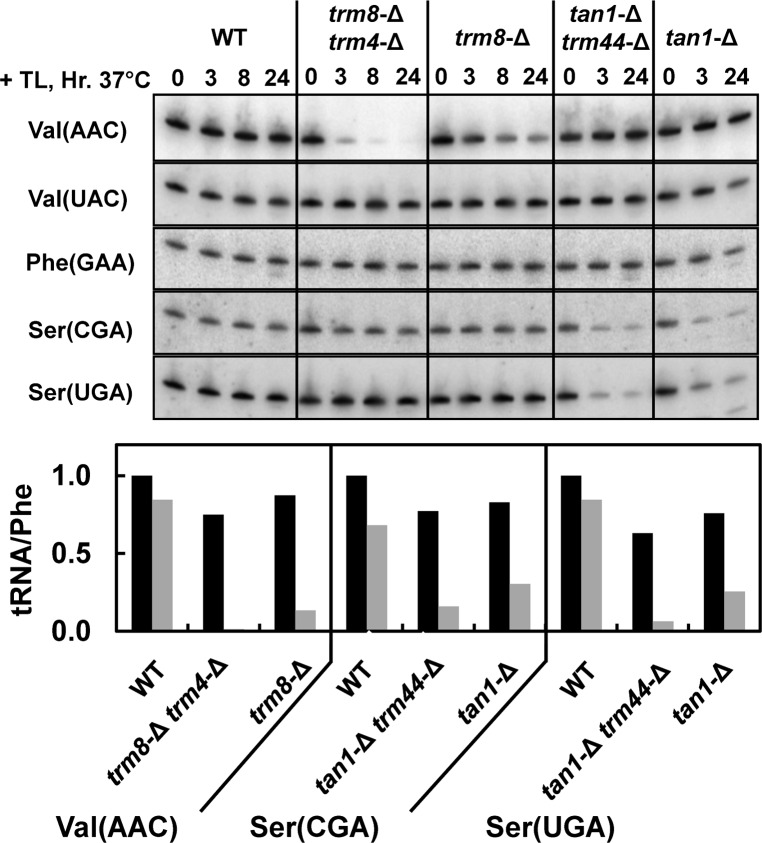
We speculated that RTD might also occur in single mutants because RTD occurs in several double mutants with a common trm8-Δ mutation (trm8-Δ trm4-Δ, trm8-Δ pus7-Δ, and trm8-Δ dus3-Δ) (Alexandrov et al. 2006; Chernyakov et al. 2008) and because our analysis shows that RTD occurs with varying efficiency that corresponds to the extent of destabilization of the acceptor and T-stems (Whipple et al. 2011). Consistent with this idea, we find that RTD can be triggered by single-modification mutants, albeit with reduced efficiency relative to that in double mutants. Thus, a temperature shift of a trm8-Δ mutant to 37°C after thiolutin treatment results in a distinct loss of tRNAVal(AAC) levels within 8–24 h, albeit at a much reduced rate relative to that in trm8-Δ trm4-Δ mutants, but there is no reduction in levels of tRNAPhe, which also has m7G (Fig. 5). Similarly, a temperature shift of a tan1-Δ mutant to 37°C after thiolutin treatment results in significant loss of tRNASer(CGA) and tRNASer(UGA) within 3–24 h, but not as severely as in tan1-Δ trm44-Δ mutants (Fig. 5). In both strains, tRNALeu(GAG) is lost to the same extent, consistent with the fact that tRNALeu(GAG) is not modified by Trm44 (data not shown).
FIGURE 5.
trm8-Δ mutants undergo slow degradation of tRNAVal(AAC), and tan1-Δ mutants undergo slow degradation of tRNASer(CGA) and tRNASer(UGA). Strains were grown at 28°C, treated with thiolutin, and shifted to 37°C, and RNAs from cells were analyzed at the times indicated, as described in Figure 1B. RNA levels were quantified by normalizing to tRNAPhe levels, and then by normalizing to the values in wild type (BY4741) at time zero. Bars in black indicate 0 h; gray, 24 h.
As we found for double mutants, we find that single mutants have a detectable RTD growth phenotype that is exacerbated by reduced EF-1A levels. Thus, we find that tef1-Δ derivatives of trm8-Δ, tan1-Δ, and trm1-Δ single-mutant strains are each more temperature sensitive than the corresponding single-mutant strains on YP (yeast extract, peptone) media containing glycerol (YPG) (Fig. 6A) or glucose (YPD) (Fig. 6B,C), and that a met22-Δ mutation completely suppresses the temperature sensitivity of trm8-Δ, tan1-Δ, and trm1-Δ single-mutant strains, as well as of the corresponding tef1-Δ–derivative strains (Fig. 6B,C).
FIGURE 6.
The RTD phenotypes of single-modification mutants are exacerbated by a tef1-Δ mutation. (A) A tef1-Δ mutation exacerbates the temperature sensitivity of trm8-Δ mutants, tan1-Δ mutants, and trm1-Δ mutants. Strains were grown overnight in YPD media, serially 10-fold diluted, plated on YP plates containing 3% glycerol (YPG), and incubated for 4 d at the indicated temperatures. (B) A met22-Δ mutation fully suppresses the temperature sensitivity of trm8-Δ mutants and trm8-Δ tef1-Δ mutants. Strains were grown, diluted, and plated on YPD plates as described in Figure 3A. Similar results are observed in YPG media (data not shown). (C) A met22-Δ mutation fully suppresses the temperature sensitivity of tan1-Δ and trm1-Δ mutants, and their tef1-Δ–derivative strains. Strains were grown, diluted, and plated on YPD plates as described in Figure 3A. Similar results are observed in YPG media (data not shown).
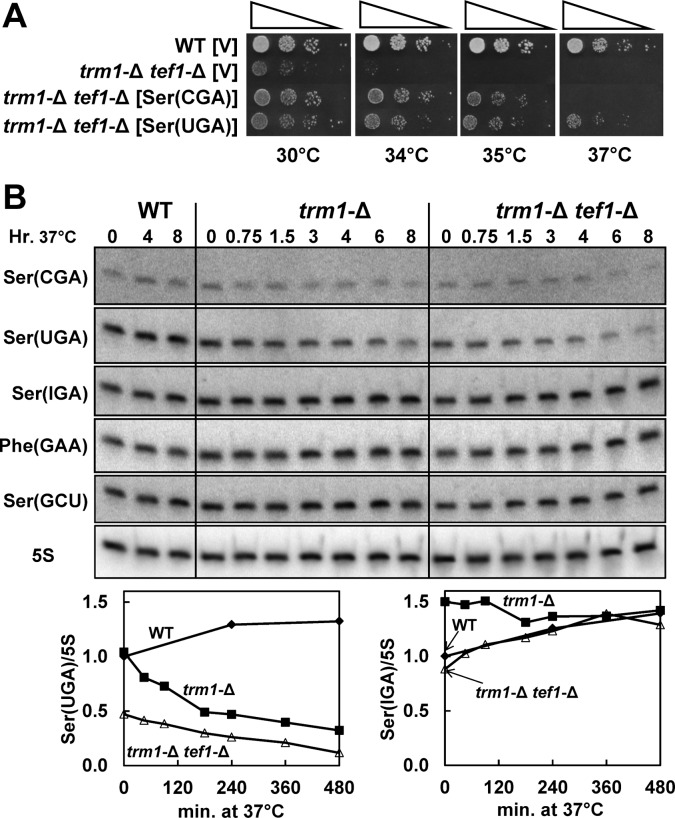
To identify the tRNAs subject to RTD in a trm1-Δ tef1-Δ strain, we examined suppression of the growth defect by overexpression of individual tRNA species, and examined tRNA degradation. Our evidence suggests that the temperature sensitivity of trm1-Δ tef1-Δ mutants is associated primarily with loss of tRNASer(CGA) and tRNASer(UGA), since overexpression of either of these tRNA species substantially suppresses the temperature sensitivity on YPG media (Fig. 7A) and since Northern analysis demonstrates loss of these same tRNA species after a temperature shift to 37°C in YPG media (Fig. 7B). We note that the trm1-Δ single-mutant strain also undergoes RTD of tRNASer(CGA) and tRNASer(UGA) under these conditions, in the absence of thiolutin treatment, albeit at a reduced rate relative to that observed in the trm1-Δ tef1-Δ strain. The observation of RTD in a trm1-Δ strain in the absence of thiolutin treatment emphasizes the substantial amount of RTD occurring in this single-mutant strain under these conditions.
FIGURE 7.
A trm1-Δ tef1-Δ strain is subject to RTD of tRNASer(CGA) and tRNASer(UGA). (A) The temperature sensitivity of a trm1-Δ tef1-Δ strain is substantially suppressed by overproduction of tRNASer(CGA) or tRNASer(UGA). Wild-type and trm1-Δ tef1-Δ strains bearing [2μ LEU2 tRNASer(CGA)], [2μ LEU2 tRNASer(UGA)], or a plasmid vector control were grown overnight in SD-Leu media, serially diluted, plated on YPG media, and incubated for 3 d at the indicated temperatures. (B) trm1-Δ tef1-Δ mutants undergo more rapid degradation of tRNASer(UGA) than trm1-Δ mutants. Strains were grown in YPG media to OD600 0.4 and shifted to 37°C, and RNA from cells was analyzed as described in Figure 1B. Diamonds indicate wild type; squares, trm1-Δ; and open triangles, trm1-Δ tef1-Δ.
DISCUSSION
We have provided substantial evidence here for a model in which the RTD machinery is in competition with EF-1A for access to tRNA substrates. We have shown that elevated levels of EF-1A suppress the temperature sensitivity of trm8-Δ trm4-Δ strains and tan1-Δ trm44-Δ strains and prevent degradation of the corresponding tRNAVal(AAC) and tRNASer(CGA) (and tRNASer(UGA)) species. Furthermore, the reduction of EF-1A levels by a tef1-Δ mutation leads to exacerbated temperature sensitivity of the trm8-Δ trm4-Δ strains and tan1-Δ trm44-Δ strains (each of which is suppressed by a met22-Δ mutation) and results in reduced levels of tRNAVal(AAC) and tRNASer(CGA) (and tRNASer(UGA)), respectively, before and during the temperature shift.
These effects of EF-1A extend to a number of strains subject to RTD. Thus, compared with trm1-Δ strains, trm1-Δ tef1-Δ strains are more temperature sensitive and undergo more complete degradation of tRNASer(CGA) and tRNASer(UGA), and the temperature sensitivity is suppressed by a met22-Δ mutation. Similarly, trm8-Δ tef1-Δ and tan1-Δ tef1-Δ strains are each more temperature sensitive than the corresponding trm8-Δ and tan1-Δ strains, and their temperature sensitivity is suppressed by a met22-Δ mutation. In addition, trm8-Δ pus7-Δ tef1-Δ and trm8-Δ dus3-Δ tef1-Δ strains are each more temperature sensitive than their TEF1+ derivatives.
The apparent competition between EF-1A and the RTD pathway is consistent with the known specificity of EF-1A for charged tRNA (Gromadski et al. 2007) and the observed selective loss of charged tRNAVal(AAC) during RTD in trm8-Δ trm4-Δ mutants, which suggests that charged tRNAVal(AAC) may be the substrate for RTD (Chernyakov et al. 2008). Indeed, since bacterial EF-Tu, which corresponds to eukaryotic EF-1A, binds charged tRNAs with a different affinity based on the identity of the amino acid and specific base pairs in the T-stem (LaRiviere et al. 2001; Sanderson and Uhlenbeck 2007; Schrader et al. 2009), differential competition between EF-1A and RTD for specific tRNA species may explain why certain tRNAs are substrates for RTD, but other identically modified tRNA species are not, despite the reduced predicted stability of their acceptor and T-stems (Whipple et al. 2011).
Our findings also significantly extend the known range of modification defects subject to RTD. We had previously reported that the RTD pathway acts in trm8-Δ trm4-Δ mutants, trm8-Δ pus7-Δ mutants, and trm8-Δ dus3-Δ mutants (each targeting tRNAVal(AAC)); in tan1-Δ trm44-Δ mutants (targeting tRNASer(CGA), tRNASer(UGA), and tRNALeu(GAG)); and in wild-type strains bearing mutations in tRNASer and tRNATyr that disrupt the predicted stability of the acceptor and T-stems (Alexandrov et al. 2006; Chernyakov et al. 2008; Whipple et al. 2011). The results described here extend the scope of RTD (based on Northern analysis and met22-Δ suppression of the temperature sensitivity) to trm1-Δ trm4-Δ mutants (lacking m2,2G26 and m5C) targeting tRNASer(CGA) and tRNASer(UGA) and to three single modification mutants: trm8-Δ mutants, targeting tRNAVal(AAC), and tan1-Δ mutants and trm1-Δ mutants, each targeting tRNASer(CGA) and tRNASer(UGA).
Since each of the trm8-Δ, tan1-Δ, and trm1-Δ single mutants target the same tRNA species that are targeted in the corresponding double-mutant strains but in each case the RTD effect (as measured by Northern and/or growth phenotype) is more mild than in the corresponding double-mutant strains, we speculate that RTD arises in numerous other cases of hypomodified or mutated tRNAs, even under conditions where there is little, if any, distinct growth phenotype, as is especially true for trm8-Δ mutants (Alexandrov et al. 2005) and trm1-Δ mutants (Gustavsson and Ronne 2008). Indeed, it seems likely that RTD is continually monitoring the integrity of tRNA by monitoring the spectrum of modifications to the body of the tRNA, and mutations generated during transcription that impair stability of the acceptor and T-stems and the tertiary fold (Whipple et al. 2011).
It is curious that trm1-Δ mutants, like tan1-Δ mutants, only appear to target tRNASer(CGA) and tRNASer(UGA), since m2,2G26 is known to be present on 19 tRNA species, and is likely also present on most of the five unsequenced tRNA species with a G26 residue. The sensitivity of these two tRNASer species to RTD in both trm1-Δ mutants and tan1-Δ mutants is consistent with the fact that these tRNA species are predicted to have among the least stable combined acceptor and T-stems among the 42 cytoplasmic tRNA species (Whipple et al. 2011), and suggests that these tRNASer species are poised at the edge of permissible stability for function in the cell, such that lack of any of several modifications targets them for destruction by RTD. Furthermore, the specificity of both trm1-Δ mutants and trm1-Δ trm4-Δ mutants for tRNASer(CGA) and tRNASer(UGA) are additional examples of cases in which a small specific subset of tRNAs is the main apparent biological target of a modification mutant with a distinct phenotype (Phizicky and Alfonzo 2010). This is also observed in trm8-Δ trm4-Δ mutants, which target only tRNAVal(AAC) for RTD and not the three other tRNAs with the same modifications in the same locations (Alexandrov et al. 2006), and in tan1-Δ trm44-Δ mutants, which target tRNASer(CGA) and tRNASer(UGA) for RTD and not the two other tRNASer species with the same modifications (Kotelawala et al. 2008). A similar result is observed in trm6-504 mutants (which lack m1A58), which target pre-tRNAiMet for degradation, and not the other 17 species known to have m1A58 (Anderson et al. 1998; Vanacova et al. 2005; Schneider et al. 2007). Likewise, yeast strains carrying mutations in the ELP complex (which lack the cm5U moiety of mcm5U34, ncm5U34, ncm5Um34, and mcm5s2U34), have several distinctive phenotypes that are due only to tRNALys(UUU) and tRNAGln(UUG) and not the nine other tRNA species with these modifications (Esberg et al. 2006; Johansson et al. 2008), and the lethal phenotype of elp3-Δ tuc1-Δ mutants (which lack both the cm5U and the s2U moieties of mcm5s2U34) is due to defects in tRNALys(UUU) and not the two other tRNA species with this modification (Bjork et al. 2007).
In summary, the results described here and previously (Whipple et al. 2011) provide substantial support for a model in which RTD acts widely on tRNAs lacking one or more of several different modifications implicated in stabilizing tertiary structure or on tRNAs with mutations that destabilize their combined acceptor and T-stems. In such strains, RTD occurs constitutively before a temperature shift, resulting in reduced initial levels of tRNAs for substrate species. As the temperature increases in the strain, the 5′ ends of RTD substrate tRNAs are increasingly exposed to attack by Xrn1 and Rat1 exonucleases, and RTD occurs at a faster rate. The increased expression of EF-1A can compete with the RTD pathway for binding of charged tRNAs and prevent RTD, until temperatures are high enough that the tRNAs not bound by EF-1A have increased likelihood of RTD due to their more exposed 5′ ends, whereas reduced levels of EF-1A cause more RTD at lower temperatures because the tRNA is less bound to EF-1A and is therefore more available for attack. Future experiments will be required to further clarify how the RTD pathway competes with EF-1A for specific tRNA substrates and how RTD interacts with other components of the translation machinery, and other cellular processes.
MATERIALS AND METHODS
Yeast strains and plasmids
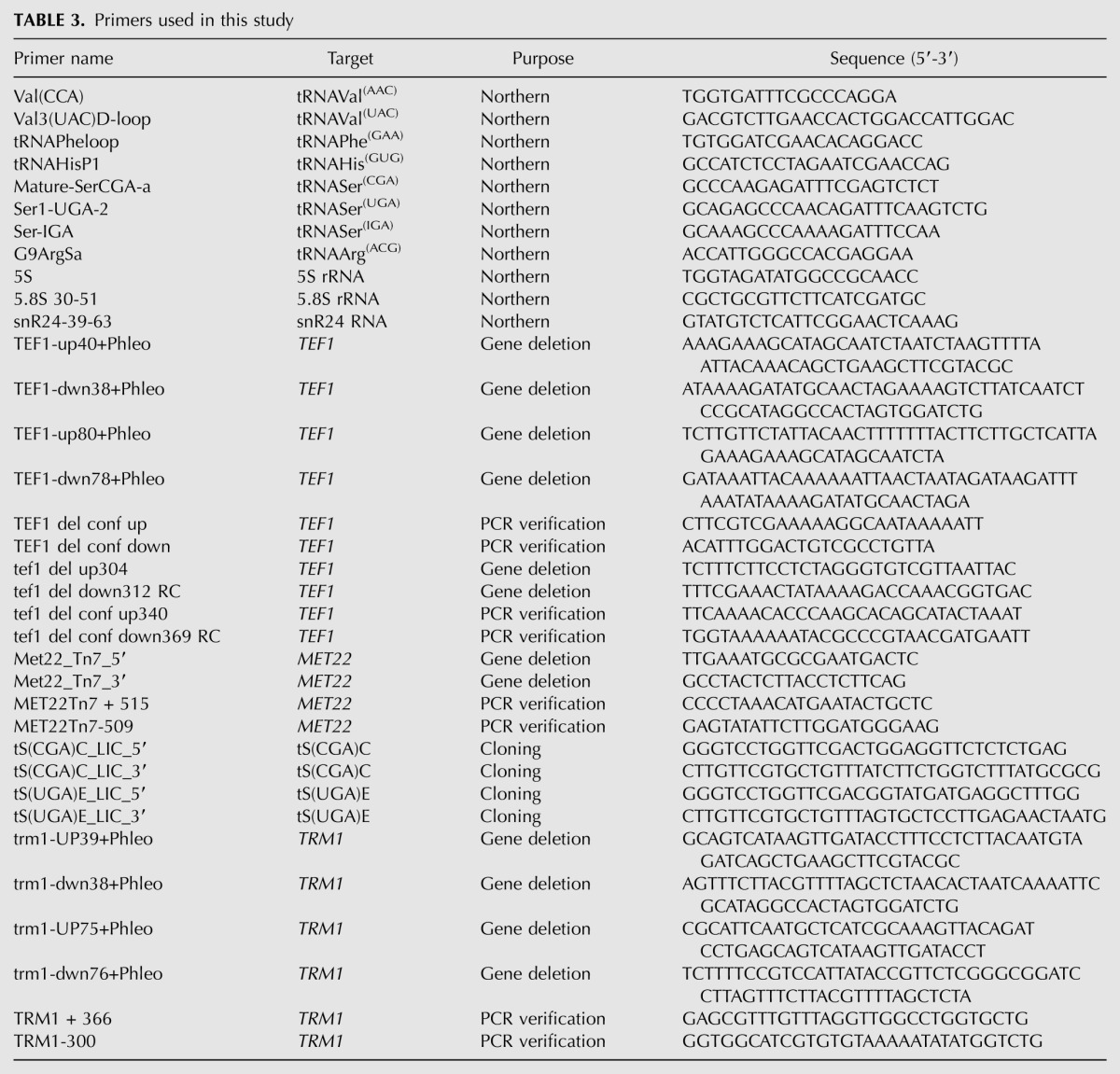
Strains used for these experiments are all derived from BY4741 and BY4742 and are listed in Table 1. Deletion strains were constructed using standard methods with appropriate knockout marker cassettes (Gueldener et al. 2002; Johnston et al. 2002), after PCR amplification of the cassette with gene specific primers or after PCR amplification of the flanking region of an existing knockout strain (Giaever et al. 2002), followed by linear transformation and PCR confirmation of insertion. Plasmids are listed in Table 2 and were constructed using similar PCR methods with appropriate primers. Primers are listed in Table 3.
TABLE 1.
Parent yeast strains used in this study

TABLE 2.
Plasmids used in this study
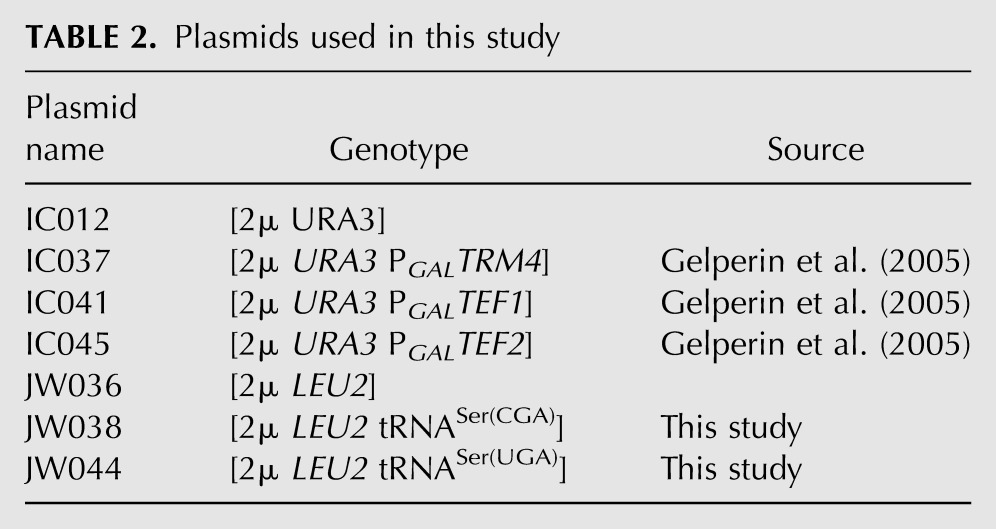
TABLE 3.
Primers used in this study
Isolation of high-copy suppressors of the temperature-sensitive phenotype of trm8-Δ trm4-Δ strains
Pools of DNA from the MORF collection of yeast ORFs (Gelperin et al. 2005) were transformed into a trm8-Δ trm4-Δ strain, plated on synthetic (S) media containing 2% raffinose, and transformants were replica plated to S media containing 2% raffinose and 2% galactose at 33°C. Plasmids were isolated and sequenced to identify the ORF associated with suppression, and plasmids were then retested by retransformation.
Northern blot analysis
Strains were grown in media as indicated at 28°C, the culture was shifted to restrictive temperature, and ∼2 OD of cells were harvested at the time indicated, washed, and quick frozen. Where indicated, thiolutin was added to 5 μg/mL, 10 min before the temperature shift. Bulk RNA was prepared using glass beads (Elder et al. 1983) or hot phenol (Alexandrov et al. 2006), and ∼2 μg RNA was separated by 10% PAGE in 8 M urea and TBE buffer, transferred to Hybond N+ membrane, cross-linked with UV, and hybridized to 5′ 32P-labeled DNA probes (Table 2) according to the method previously described (Alexandrov et al. 2006), followed by visualization on a Typhoon PhosphorImager (GE Healthcare) and quantification using Imagequant. For acidic Northerns, RNA was prepared at pH 4.5 and was resolved on 6.5% acrylamide gels for 18 h at 4°C in 8 M urea and 0.1 M sodium acetate (pH 5) (Alexandrov et al. 2006).
ACKNOWLEDGMENTS
We thank Elizabeth Grayhack and Michael Guy for discussions and comments on the manuscript. This research was supported by NIH grant GM52347 to E.M.P. J.M.D. was partially supported by NIH Training Grant in Cellular, Biochemical, and Molecular Sciences 5T32 GM068411.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.033654.112.
REFERENCES
- Agris PF, Vendeix FA, Graham WD 2007. tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol 366: 1–13 [DOI] [PubMed] [Google Scholar]
- Alexandrov A, Grayhack EJ, Phizicky EM 2005. tRNA m7G methyltransferase Trm8p/Trm82p: Evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. RNA 11: 821–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM 2006. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21: 87–96 [DOI] [PubMed] [Google Scholar]
- Anderson J, Phan L, Cuesta R, Carlson BA, Pak M, Asano K, Bjork GR, Tamame M, Hinnebusch AG 1998. The essential Gcd10p–Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev 12: 3650–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavappa R, Sigler PB 1991. The 3 Å crystal structure of yeast initiator tRNA: Functional implications in initiator/elongator discrimination. EMBO J 10: 3105–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork GR, Huang B, Persson OP, Bystrom AS 2007. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA 13: 1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM 2008. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev 22: 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochella L, Green R 2005. An active role for tRNA in decoding beyond codon:anticodon pairing. Science 308: 1178–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copela LA, Fernandez CF, Sherrer RL, Wolin SL 2008. Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation. RNA 14: 1214–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichtl B, Stevens A, Tollervey D 1997. Lithium toxicity in yeast is due to the inhibition of RNA processing enzymes. EMBO J 16: 7184–7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder RT, Loh EY, Davis RW 1983. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc Natl Acad Sci 80: 2432–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esberg A, Huang B, Johansson MJ, Bystrom AS 2006. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol Cell 24: 139–148 [DOI] [PubMed] [Google Scholar]
- Gelperin DM, White MA, Wilkinson ML, Kon Y, Kung LA, Wise KJ, Lopez-Hoyo N, Jiang L, Piccirillo S, Yu H, et al. 2005. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev 19: 2816–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, et al. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391 [DOI] [PubMed] [Google Scholar]
- Giege R, Sissler M, Florentz C 1998. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res 26: 5017–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromadski KB, Schummer T, Stromgaard A, Knudsen CR, Kinzy TG, Rodnina MV 2007. Kinetics of the interactions between yeast elongation factors 1A and 1Bα, guanine nucleotides, and aminoacyl-tRNA. J Biol Chem 282: 35629–35637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res 30: e23 doi: 10.1093/nar/30.6.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson M, Ronne H 2008. Evidence that tRNA modifying enzymes are important in vivo targets for 5-fluorouracil in yeast. RNA 14: 666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A, Ladner JE, Klug A 1976. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol 108: 619–649 [DOI] [PubMed] [Google Scholar]
- Jimenez A, Tipper DJ, Davies J 1973. Mode of action of thiolutin, an inhibitor of macromolecular synthesis in Saccharomyces cerevisiae. Antimicrob Agents Chemother 3: 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MJ, Esberg A, Huang B, Bjork GR, Bystrom AS 2008. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol 28: 3301–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M, Riles L, Hegemann JH 2002. Gene disruption. Methods Enzymol 350: 290–315 [DOI] [PubMed] [Google Scholar]
- Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J 2004. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev 18: 1227–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaba S, Wang X, Anderson JT 2006. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA 12: 508–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Suddath FL, Quigley GJ, McPherson A, Sussman JL, Wang AH, Seeman NC, Rich A 1974. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science 185: 435–440 [DOI] [PubMed] [Google Scholar]
- Kotelawala L, Grayhack EJ, Phizicky EM 2008. Identification of yeast tRNA Um44 2′-O-methyltransferase (Trm44) and demonstration of a Trm44 role in sustaining levels of specific tRNASer species. RNA 14: 158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EB, Vallabhaneni H, Mayer LM, Farabaugh PJ 2010. A comprehensive analysis of translational missense errors in the yeast Saccharomyces cerevisiae. RNA 16: 1797–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D 2005. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121: 713–724 [DOI] [PubMed] [Google Scholar]
- LaRiviere FJ, Wolfson AD, Uhlenbeck OC 2001. Uniform binding of aminoacyl-tRNAs to elongation factor Tu by thermodynamic compensation. Science 294: 165–168 [DOI] [PubMed] [Google Scholar]
- Ledoux S, Olejniczak M, Uhlenbeck OC 2009. A sequence element that tunes Escherichia coli tRNAAlaGGC to ensure accurate decoding. Nat Struct Mol Biol 16: 359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Reynolds N, Ibba M 2009. Aminoacyl-tRNA synthesis and translational quality control. Annu Rev Microbiol 63: 61–78 [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S 1988. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature 336: 179–181 [DOI] [PubMed] [Google Scholar]
- Murphy FV 4th, Ramakrishnan V 2004. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol 11: 1251–1252 [DOI] [PubMed] [Google Scholar]
- Murphy FV 4th, Ramakrishnan V, Malkiewicz A, Agris PF 2004. The role of modifications in codon discrimination by tRNALysUUU. Nat Struct Mol Biol 11: 1186–1191 [DOI] [PubMed] [Google Scholar]
- Musier-Forsyth K, Schimmel P 1992. Functional contacts of a transfer RNA synthetase with 2′-hydroxyl groups in the RNA minor groove. Nature 357: 513–515 [DOI] [PubMed] [Google Scholar]
- Ozanick SG, Wang X, Costanzo M, Brost RL, Boone C, Anderson JT 2009. Rex1p deficiency leads to accumulation of precursor initiator tRNAMet and polyadenylation of substrate RNAs in Saccharomyces cerevisiae. Nucleic Acids Res 37: 298–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky EM, Alfonzo JD 2010. Do all modifications benefit all tRNAs? FEBS Lett 584: 265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky EM, Hopper AK 2010. tRNA biology charges to the front. Genes Dev 24: 1832–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson LE, Uhlenbeck OC 2007. The 51–63 base pair of tRNA confers specificity for binding by EF-Tu. RNA 13: 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FV 4th, Weir JR, Ramakrishnan V 2009. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 326: 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Anderson JT, Tollervey D 2007. The exosome subunit Rrp44 plays a direct role in RNA substrate recognition. Mol Cell 27: 324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader JM, Chapman SJ, Uhlenbeck OC 2009. Understanding the sequence specificity of tRNA binding to elongation factor Tu using tRNA mutagenesis. J Mol Biol 386: 1255–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D, Cottrelle P, Iborra F, Buhler JM, Sentenac A, Fromageot P 1985. Elongation factor 1α from Saccharomyces cerevisiae. Rapid large-scale purification and molecular characterization. J Biol Chem 260: 3084–3089 [PubMed] [Google Scholar]
- Valle M, Zavialov A, Li W, Stagg SM, Sengupta J, Nielsen RC, Nissen P, Harvey SC, Ehrenberg M, Frank J 2003. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryo-electron microscopy. Nat Struct Biol 10: 899–906 [DOI] [PubMed] [Google Scholar]
- Vanacova S, Wolf J, Martin G, Blank D, Dettwiler S, Friedlein A, Langen H, Keith G, Keller W 2005. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol 3: e189 doi: 10.1371/journal.pbio.0030189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron C, Lacroute F 1975. Effect of growth rate on the amounts of ribosomal and transfer ribonucleic acids in yeast. J Bacteriol 122: 855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jia H, Jankowsky E, Anderson JT 2008. Degradation of hypomodified tRNAiMet in vivo involves RNA-dependent ATPase activity of the DExH helicase Mtr4p. RNA 14: 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E, Dumas P, Moras D, Romby P 1985. Crystallographic refinement of yeast aspartic acid transfer RNA. J Mol Biol 184: 119–145 [DOI] [PubMed] [Google Scholar]
- Whipple JM, Lane EA, Chernyakov I, D'Silva S, Phizicky EM 2011. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev 25: 1173–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz JE, Whipple JM, Phizicky EM, Sharp PA 2011. tRNAs marked with CCACCA are targeted for degradation. Science 334: 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M, Valle M, Frank J 2003. A twisted tRNA intermediate sets the threshold for decoding. RNA 9: 384–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher HS, Green R 2009. Fidelity at the molecular level: Lessons from protein synthesis. Cell 136: 746–762 [DOI] [PMC free article] [PubMed] [Google Scholar]