Post-transcriptional modification of the tRNA anticodon loop is critical for translation. Yeast Trm7 is required for 2′-O-methylation of C32 and N34 of tRNAPhe, tRNATrp, and tRNALeu(UAA) to form Cm32 and Nm34, and trm7-Δ mutants have severe growth and translation defects, but the reasons for these defects are not known. The authors show here that overproduction of tRNAPhe suppresses the growth defect of trm7-Δ mutants, suggesting that the crucial biological role of Trm7 is the modification of tRNAPhe.
Keywords: RTT10, TRM7, tRNAPhe, wyebustosine, YMR259c
Abstract
Post-transcriptional modification of the tRNA anticodon loop is critical for translation. Yeast Trm7 is required for 2′-O-methylation of C32 and N34 of tRNAPhe, tRNATrp, and tRNALeu(UAA) to form Cm32 and Nm34, and trm7-Δ mutants have severe growth and translation defects, but the reasons for these defects are not known. We show here that overproduction of tRNAPhe suppresses the growth defect of trm7-Δ mutants, suggesting that the crucial biological role of Trm7 is the modification of tRNAPhe. We also provide in vivo and in vitro evidence that Trm7 interacts with ORF YMR259c (now named Trm732) for 2′-O-methylation of C32, and with Rtt10 (named Trm734) for 2′-O-methylation of N34 of substrate tRNAs and provide evidence for a complex circuitry of anticodon loop modification of tRNAPhe, in which formation of Cm32 and Gm34 drives modification of m1G37 (1-methylguanosine) to yW (wyebutosine). Further genetic analysis shows that the slow growth of trm7-Δ mutants is due to the lack of both Cm32 and Nm34, and the accompanying loss of yW, because trm732-Δ trm734-Δ mutants phenocopy trm7-Δ mutants, whereas each single mutant is healthy; nonetheless, TRM732 and TRM734 each have distinct roles, since mutations in these genes have different genetic interactions with trm1-Δ mutants, which lack m2,2G26 in their tRNAs. We speculate that 2′-O-methylation of the anticodon loop may be important throughout eukaryotes because of the widespread conservation of Trm7, Trm732, and Trm734 proteins, and the corresponding modifications, and because the putative human TRM7 ortholog FTSJ1 is implicated in nonsyndromic X-linked mental retardation.
INTRODUCTION
Post-transcriptional modifications of tRNA are numerous, widely conserved in nature, and critical for proper tRNA function (Agris et al. 2007; Phizicky and Hopper 2010). In the yeast Saccharomyces cerevisiae, 25 chemically distinct modifications are found among the 28 unique cytoplasmic tRNA species (of 42 total) that have been characterized for modifications, and at least 19 of these modifications are also known to occur in humans. Yeast cytoplasmic tRNAs have an average of 12.6 modifications, with 2.6 modifications occurring within the anticodon loop (N32–N38) or its immediate vicinity (N31, N39, and N40), and with the remaining 10 modifications in the main body of the tRNA, remote from the anticodon loop (Juhling et al. 2009).
Modifications in the main body of the tRNA have a number of different crucial roles in the cell. Lack of certain body modifications can provoke either of two quality-control pathways that degrade specific tRNAs at different stages of biosynthesis. Thus, a nuclear surveillance pathway targets pre-tRNAiMet lacking 1-methyladenosine (m1A58) for 3′-exonucleolytic degradation by the TRAMP complex, Rrp6, and the nuclear exosome (Kadaba et al. 2004, 2006; LaCava et al. 2005; Vanacova et al. 2005; Schneider et al. 2007), whereas a rapid tRNA decay (RTD) pathway targets several mature hypomodified tRNA species for 5′ exonulceolytic degradation by Xrn1 and Rat1, due in large measure to destabilization of the acceptor and T-stem (Alexandrov et al. 2006; Chernyakov et al. 2008; Whipple et al. 2011). In addition to these quality control pathways, lack of m1A9 leads to misfolding of human mitochondrial tRNALys (Helm et al. 1999), and lack of G-1 leads to the accumulation of uncharged tRNAHis (Gu et al. 2005).
Modifications in and around the anticodon loop of tRNA play several crucial roles in translation (Agris et al. 2007; Phizicky and Hopper 2010). Some anticodon modifications have a demonstrated role in ensuring charging fidelity; thus, for example, the presence of m1G37 on tRNAAsp prevents mischarging by yeast arginyl-tRNA synthetase (ArgRS) (Putz et al. 1994), and the presence of lysidine on C34 of E. coli tRNAIle promotes charging by IleRS and prevents mischarging by MetRS (Muramatsu et al. 1988).
Many anticodon loop modifications appear to affect decoding efficiency and/or accuracy, presumably by altering codon:anticodon interactions in the ribosome (Ogle et al. 2001; Murphy and Ramakrishnan 2004; Murphy et al. 2004; Selmer et al. 2006). Thus, in yeast, mcm5U34 (5-methoxycarbonylmethyluridine) and ncm5U34 (5-carbamoylmethyluridine) promote the reading of codons ending in G, whereas mcm5s2U34 (5-methoxycarbonylmethyl-2-thiouridine) promotes reading of codons ending in either A or G (Johansson et al. 2008). Furthermore, these decoding properties likely explain the multiple phenotypes of yeast strains lacking the cm5 moiety of U34, which are ascribed to reduced function of tRNALys(UUU) and tRNAGln(UUG) (Esberg et al. 2006), and the lethality of yeast strains lacking the s2 and mcm5 moieties of mcm5s2U34, which is ascribed to reduced function of tRNALys(UUU) (Bjork et al. 2007). In addition, yeast mutants lacking i6A37 (N6-isopentenyladenosine) in their tRNAs have reduced nonsense suppression (Laten et al. 1978; Dihanich et al. 1987).
Several modifications in and near the anticodon loop also affect reading-frame maintenance. Thus, in bacteria, increased +1 frameshifting occurs with hypomodified tRNAs that have s2U34 or mnm5U34 (5-methylaminomethyluridine) instead of mnm5s2U34, G37 instead of m1G37, A37 or i6A37 instead of ms2io6A37 (2-methylthio-N6-(cis-hydroxyisopentenyl) adenosine), and U38 and U39 instead of Ψ38 and Ψ39 (pseudouridine) (Urbonavicius et al. 2001). Similarly, in yeast, mutants with m1G37 instead of yW37 (wyebutosine) in their tRNAPhe have increased −1 frameshifting (Waas et al. 2007), mutants lacking t6A37 (N6-threonylcarbamoyladenosine) in their tRNAs have increased −1 frameshifting and +1 frameshifting, as well as increased recognition of GUG as an initiation codon (El Yacoubi et al. 2011), and mutants lacking Ψ38 and Ψ39 in their tRNAs have increased +1 and −1 frameshifting (Lecointe et al. 2002; Bekaert and Rousset 2005).
In a number of cases, however, it is not clear exactly which tRNAs are affected by lack of specific anticodon loop modifications, how the lack of modifications explains the observed phenotypes, and exactly how the modifications influence individual steps of translation. One such case is 2′-O-methylation of N32 and N34 to form Nm32 and Nm34 in the anticodon loop, which is critical for tRNA function, but largely unexplored. In yeast, 2′-O-methylation occurs on C32 and N34 of tRNAPhe, tRNALeu(UAA), and tRNATrp (Fig. 1A) and requires Trm7 methyltransferase, mutants of which have a severe growth defect, presumably due to a 70% reduction in translation (Pintard et al. 2002). Trm7 and its modifications are of particular interest for two additional reasons. First, 2′-O-methylation of the anticodon loop is highly conserved in eukaryotes. Thus, all five sequenced eukaryotic tRNATrp species have Cm32 and Cm34, and all 17 sequenced eukaryotic tRNAPhe genes have Cm32, 16 of which also have Gm34, including that of humans (Juhling et al. 2009). Moreover, Nm modifications in the anticodon loop appear to be widespread in other eukaryotic tRNA species since, for example, five of the 15 other sequenced human tRNA species contain Nm32 or Nm34 (Juhling et al. 2009). Second, Trm7 and its modifications are associated with human disorders. Thus, mutations in the putative TRM7 homolog FTSJ1, which result in truncated polypeptides, have been strongly associated with nonsyndromic X-linked mental retardation in multiple studies (Freude et al. 2004; Ramser et al. 2004; Froyen et al. 2007; Takano et al. 2008), and tRNAPhe from neuroblastoma cells and Ehrlich ascites tumors has a significant portion of its tRNAPhe lacking Cm32 and Gm34, and containing m1G37 instead of yW (Kuchino et al. 1982).
FIGURE 1.
tRNAPhe is the Trm7 substrate that is important for healthy growth. (A) Schematic of anticodon loops of Trm7 substrate tRNAs. (B) Overexpression of tRNAPhe suppresses slow growth of the trm7-Δ strain. Strains as indicated containing a [URA3 TRM7] plasmid or a [URA3] vector control, and a [LEU2] plasmid as indicated, were grown overnight in SD-Leu medium at 30°C, diluted to OD600 of ∼0.5 in H2O, and serially diluted 10-fold in H2O, and then 2 μL was spotted onto SD-Leu media containing 5-FOA to select for the LEU2 plasmid and against the URA3 plasmid. Cells were then grown for 2 d at 30°C. (C) tRNAPhe levels in trm7-Δ mutants are not decreased. Wild-type and trm7-Δ mutant strains were grown at 30°C in YPD media, and RNA was isolated and analyzed by Northern blot as described in Materials and Methods. Numbers below lanes are levels of RNA normalized to 5S rRNA, and then normalized to wild type, itself normalized to 5S rRNA. (D) trm7-Δ mutants do not have an obvious tRNAPhe charging defect. Strains were grown as in C, and RNA was isolated under acidic conditions and then analyzed by Northern blot as described in Materials and Methods. Control sample was treated with base prior to PAGE to deacylate the tRNA. Upper and lower arrows denote charged and uncharged tRNA species. Numbers indicate percentage of aminoacylated tRNAPhe.
Because of the severe growth defect of trm7-Δ mutants, the widespread conservation of 2′-O-methylation of the anticodon loop, and the likely importance of Trm7 and its modifications in humans, we sought to define the biology of Trm7 and its modifications in yeast. We demonstrate here that tRNAPhe is the Trm7 target that affects growth and translation and provide evidence for a complex circuitry for anticodon loop modifications in tRNAPhe, in which Trm7 recruits one partner to methylate N32 and another to methylate N34 (both of which are important for different reasons), and in which lack of either of these modifications reduces formation of yW from m1G37.
RESULTS
The growth defect of trm7-Δ mutants is primarily due to hypomodified tRNAPhe
The growth defect of trm7-Δ mutants could in principle be due to defective decoding of cognate codons by Trm7 tRNA substrates, to defective decoding of noncognate codons by Trm7 tRNA substrates, to a defect in some other step of translation, or to some unknown second function of Trm7 unrelated to tRNA modification. We reasoned that if the trm7-Δ growth defect was due to poor decoding by one or more Trm7 tRNA substrates (or to defects upstream of decoding), then overexpression of those tRNAs might suppress the slow-growth phenotype, as shown previously for phenotypes of other modification mutants (Alexandrov et al. 2006; Esberg et al. 2006). We tested this hypothesis by constructing a trm7-Δ strain harboring a URA3 plasmid expressing TRM7 (relevant genotype: trm7-Δ [TRM7 URA3]), and testing for growth after introduction of a high-copy (2μ) LEU2 plasmid containing tRNA genes, and subsequent selection against the [TRM7 URA3] plasmid on media containing 5-fluoroorotic acid (5-FOA). We find that overexpression of tRNAPhe efficiently suppresses the slow growth of the trm7-Δ [TRM7 URA3] strain when plated to media containing 5-FOA, whereas overexpression of tRNALeu(UAA) or tRNATrp has no observable effect (Fig. 1B), suggesting that tRNAPhe is the biologically important target of Trm7. Furthermore, we find that overexpression of tRNAPhe completely suppresses the paromomycin sensitivity of trm7-Δ mutants (data not shown), which further suggests that the trm7-Δ growth defect is due to poor reading of Phe codons.
In this particular case, defective decoding of Phe codons in trm7-Δ mutants could be due to reduced decoding of Phe codons by hypomodified tRNAPhe and/or to increased decoding of Phe codons by near-cognate tRNALeu(UAA), which is also a Trm7 substrate. Indeed, it has been speculated that the 2′-O-methyl group of U34 might prevent tRNALeu(UAA) from reading UUX codons not ending in A (Johansson et al. 2008), suggesting that tRNALeu(UAA) in trm7-Δ mutants might decode the near-cognate UUU or UUC Phe codons (in addition to its cognate UUA Leu codon), which could then cause slow growth. However, since a trm7-Δ [2μ LEU2 tRNALeu(UAA)] strain does not grow more poorly than a trm7-Δ [2μ LEU2 vector] strain on SD–Leu media with 5-FOA (Fig. 1B), the slow growth of trm7-Δ mutants is not due to misreading of Phe codons by hypomodified tRNALeu(UAA). Consistent with this interpretation, overexpression of tRNALeu(UAA) does not abrogate suppression of the growth defect of trm7-Δ mutants caused by overexpression of tRNAPhe (Fig. 1B). Thus, we conclude that the growth defect of trm7-Δ mutants is due to poor decoding of Phe codons by hypomodified tRNAPhe, or to some earlier step in the production of functional tRNA for translation. Since Northern analysis shows that trm7-Δ mutants have normal amounts of tRNAPhe (Fig. 1C), and charged tRNAPhe (Fig. 1D), we infer that the defect in trm7-Δ mutants occurs at some step between charging and decoding in the ribosome A-site.
Trm7 requires YMR259c to form Cm32 and Rtt10 to form Nm34 in vivo, and both are required for efficient synthesis of yW37 from m1G37 of tRNAPhe
Since high-throughput studies suggested that Trm7 physically interacts with ORF YMR259c and Rtt10 (Krogan et al. 2006), we explicitly examined these interactions, and their consequences on 2′-O-methylation. Consistent with the high-throughput results, we find that Trm7 interacts with ORF YMR259c and with Rtt10, since affinity purification of Trm7 from a chromosomal TRM7-cMORF strain (carrying a C-terminal fusion of TRM7 to a tandem affinity purification tag derived from the MORF collection) (Gelperin et al. 2005) results in substantial copurification of chromosomally tagged YMR259c-9myc (Fig. 2A, lane 5) or Rtt10-9myc (Fig. 2B, lane 5), whereas no copurification of either protein is observed with affinity-purified Trm8-cMORF (Fig. 2A, lane 6; Fig. 2B, lane 6).
FIGURE 2.
Trm7 forms a complex with ORF YMR259c and with Rtt10. (A) Chromosomally expressed YMR259c-9Myc and Trm7-cMORF form a complex. Soluble crude extract was prepared from the indicated strains, and proteins were purified using IgG-sepharose beads, followed by treatment with 3C protease as described in Materials and Methods. Extracts (input) were subjected to immunoblot analysis with anti-protein A and anti-(c-myc) antibody, and 3C-treated immunoprecipitates (IP) were analyzed by immunoblot with anti-(c-myc) antibody. C-terminal cMORF-tagged fusion proteins consist of ORF-His6-HA-3C site-ZZ domain of protein A. (B) Chromosomally expressed Rtt10-9Myc and Trm7-cMORF form a complex. Indicated strains were analyzed as in A. The c-myc reactive band of molecular weight >200 kDa in the input (*) is likely Rtt10 aggregates.
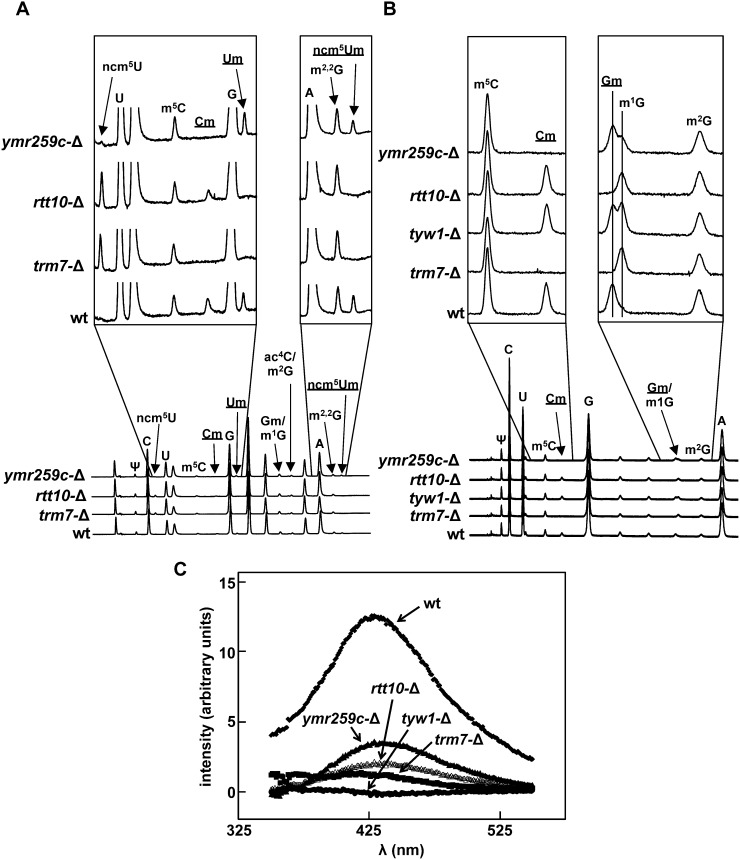
To determine the effect of YMR259c and Rtt10 on 2′-O-methylation, we purified tRNAs from trm7-Δ, rtt10-Δ, and ymr259c-Δ mutants, and evaluated their nucleoside content by HPLC. As expected, we find that tRNALeu(UAA) from wild-type cells has Cm and ncm5Um (from residues 32 and 34, respectively), as well as Um (which presumably arises from incomplete modification of ncm5Um34), whereas tRNALeu(UAA) from trm7-Δ mutants lacks any detectable Cm, ncm5Um, or Um, and has instead ncm5U (Fig. 3A; Table 1). Strikingly, however, we find that tRNALeu(UAA) from ymr259c-Δ mutants has no detectable Cm (expected from residue 32), but has nearly the same amount of ncm5Um +Um (0.64 + 0.5 moles/mole) as found in wild-type cells at residue 34 (1.16 + 0.3 moles/mole). Conversely, tRNALeu(UAA) from rtt10-Δ mutants has no detectable ncm5Um (expected from residue 34), but instead has ncm5U (1.0 moles/mole) and has retained substantial amounts of Cm compared with that found in wild-type cells at residue 32 (0.36 vs. 0.61 moles/mole). Thus, it appears that YMR259c is required for formation of Cm32 on tRNALeu(UAA), and that Rtt10 is required for formation of ncm5Um34 or Um34. Consistent with this conclusion, we find that tRNATrp from trm7-Δ mutants lacks detectable Cm from Cm32 and Cm34, and that tRNATrp from rtt10-Δ mutants has ∼50% of the normal amounts Cm (data not shown).
FIGURE 3.
ORF YMR259c is required for 2′-O-methylation of C32, and Rtt10 is required for 2′-O-methylation of N34 in vivo. (A) HPLC traces of tRNALeu(UAA) from ymr259c-Δ cells and rtt10-Δ cells. tRNALeu(UAA) isolated from the indicated strains was digested to nucleosides and analyzed by HPLC as described in Materials and Methods. 2′-O-methylated nucleosides are underlined. (B) HPLC traces of tRNAPhe from ymr259c-Δ cells and rtt10-Δ cells. tRNAPhe was analyzed as in A. (C) tRNAPhe from trm7-Δ mutants lacks yW. Emission spectra of tRNAPhe purified from wild-type cells, tyw1-Δ mutants, trm7-Δ mutants, rtt10-Δ mutants, or ymr259c-Δ mutants were determined after excitation at 320 nm.
TABLE 1.
Quantification of HPLC analysis of tRNALeu(UAA)

Surprisingly, examination of tRNAPhe from trm7-Δ mutants reveals an additional 0.89 moles/mole of m1G, in addition to the expected lack of detectable Cm and Gm (Fig. 3B; Table 2). m1G is not normally present in tRNAPhe, but might be present as the precursor to yW37 (wyebutosine), which is normally generated from G37 by Trm5-catalyzed methylation to form m1G (Bjork et al. 2001), followed by the successive action of Tyw1, Tyw2, Tyw3, and Tyw4 (Noma et al. 2006). Consistent with this interpretation, we find that tRNAPhe from trm7-Δ mutants lacks yW fluorescence at 425 nm, which is normally observed in wild-type tRNAPhe (Fig. 3C); that tRNAPhe from tyw1-Δ mutants contains both Gm (0.78 moles/mole) and m1G (1.0 mole/mole) (Fig. 3B; Table 2) and lacks detectable yW fluorescence (Fig. 3C); and that tRNAPhe from trm7-Δ tyw1-Δ mutants has the same amount of m1G as trm7-Δ mutants (data not shown). Since mass spectrometry analysis shows explicitly that m1G37 is present in tRNAPhe from trm7-Δ mutants, trm7-Δ tyw1-Δ mutants, and tyw1-Δ mutants, whereas yW37 is present in tRNAPhe from wild-type strains (data not shown), we conclude that the m1G observed in HPLC analysis of tRNAPhe from trm7-Δ mutants derives from the failure to complete yW synthesis after formation of the m1G37 intermediate.
TABLE 2.
Quantification of HPLC analysis of tRNAPhe
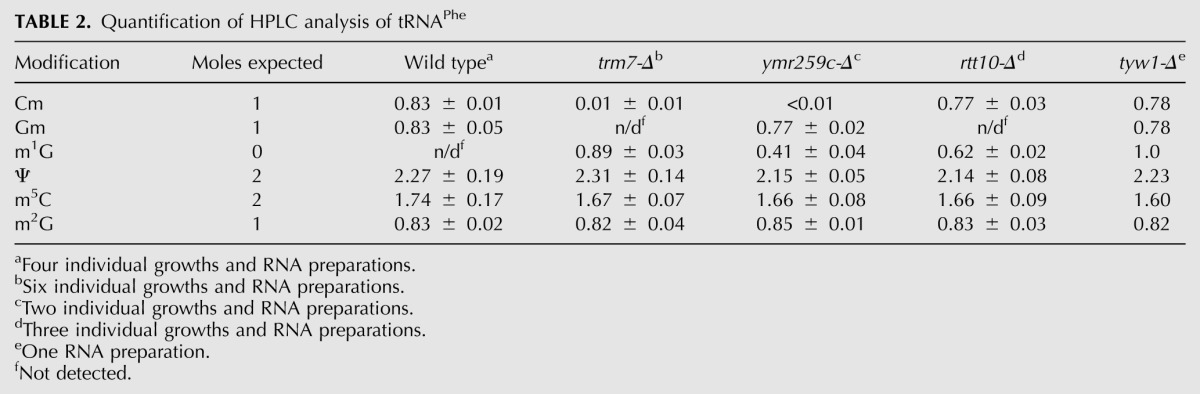
With this interpretation of the m1G levels in tRNAPhe of trm7-Δ mutants, our results show that YMR259c and Rtt10 have similar effects on 2′-O-methylation of tRNAPhe to those observed with tRNALeu(UAA). Thus, tRNAPhe from ymr259c-Δ mutants lacks any detectable Cm (expected from residue 32) (Fig. 3B; Table 2), but has comparable levels of Gm to that in wild-type cells at residue 34 (0.77 compared with 0.83 moles/mole), and in addition, has moderate levels of m1G (0.41 moles/mole), consistent with its comparable reduced fluorescence from yW (Fig. 3C). Similarly, tRNAPhe from rtt10-Δ mutants has nearly the same amount of Cm (0.77 moles/mole) as found in wild-type cells at residue 32 (0.83 moles/mole), but lacks any detectable Gm, and has 0.62 moles of m1G and substantially reduced levels of yW (Fig. 3B,C; Table 2). Our data therefore show that YMR259c is required with Trm7 to 2′-O-methylate C32 of substrate tRNAs, and Rtt10 is required with Trm7 to modify N34, and suggest that yW formation requires Gm34 more than it requires Cm32. Moreover, the demonstration that Trm7 can independently 2′-O-methylate C32 with YMR259c, and N34 with Rtt10, suggests that these protein pairs might act independently in vivo.
Consistent with independent function for each Trm7 partner protein, our data suggest that Trm7 forms independent complexes with YMR259c and Rtt10. Thus, we find that Rtt10-9myc does not copurify with chromosomally tagged YMR259c-cMORF (Fig. 4, lane 5), whereas Trm7-9myc does copurify, as expected (Fig. 4, lane 6). Furthermore, no complex is detected between YMR259c-9myc and Rtt10-MORF when Rtt10-MORF is overexpressed 600-fold on a 2μ plasmid under control of the galactose promoter (data not shown). These results indicate that it is unlikely that a ternary Trm732/Trm7/Trm734 complex exists, unless it is inherently unstable under our purification conditions.
FIGURE 4.
Chromosomally expressed YMR259c-cMORF interacts with Trm7-9myc, but not with Rtt10-9myc. Indicated strains were analyzed as in Figure 2A. The c-myc reactive band of molecular weight >200 kDa in the input (*) is likely Rtt10 aggregates.
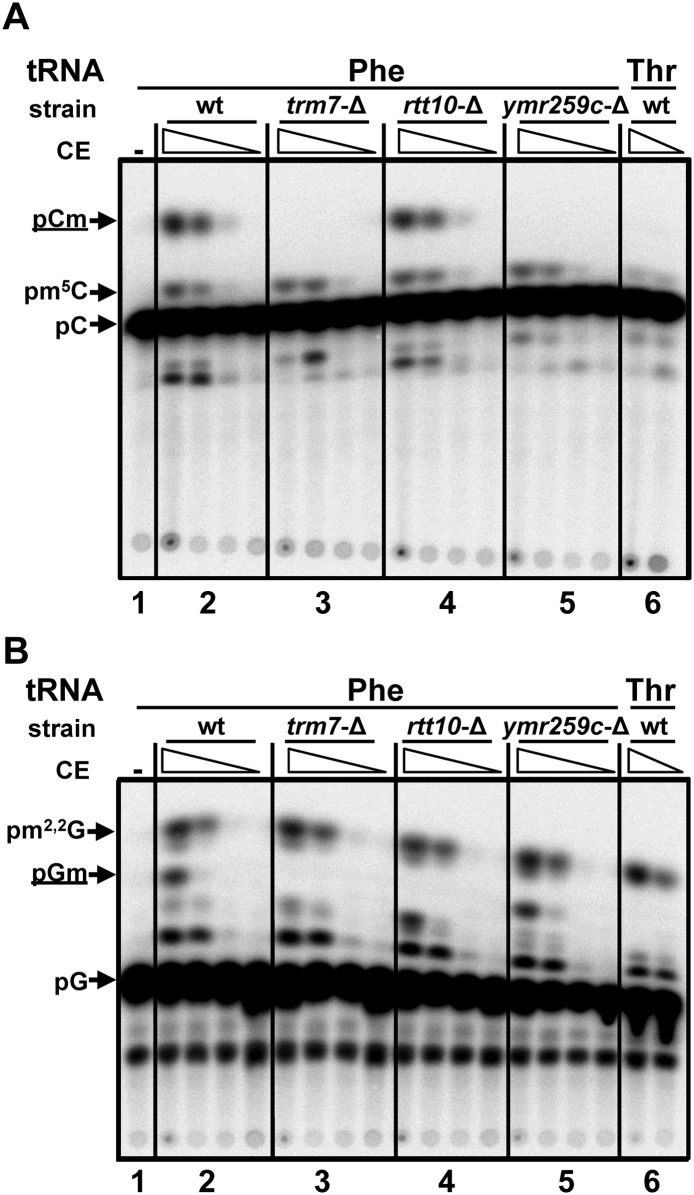
Trm7 requires YMR259c to catalyze Cm32 formation and Rtt10 to catalyze Nm34 formation in vitro
To more rigorously evaluate the effect of YMR259c and Rtt10 on Trm7 modification activity, we examined extracts from strains deleted for each of these components. As expected, extracts from wild-type cells catalyze formation of both Cm and Gm on tRNAPhe (Fig. 5A,B, panel 2), and extracts from trm7-Δ cells do not catalyze formation of either Cm or Gm (Fig. 5A,B, panel 3), although trm7-Δ extracts are fully functional for formation of m5C and m2,2G. Consistent with in vivo results, ymr259c-Δ extracts do not catalyze any detectable Cm formation on tRNAPhe (Fig. 5A, panel 5), whereas rtt10-Δ extracts have nearly identical Cm formation activity to that of wild type extracts (Fig. 5A, cf. panels 2 and 4). As expected for specific modification activity, no Cm formation is observed with tRNAThr(IGU) (Fig. 5A, panel 6), which does not normally have Cm. Similarly, rtt10-Δ extracts do not catalyze detectable formation of Gm (Fig. 5B, panel 4), whereas ymr259c-Δ extracts have very similar Gm formation activity to that of wild-type extracts (Fig. 5B, cf. panels 2 and 5).
FIGURE 5.
ORF YMR259c is required for formation of Cm32 and Rtt10 is required for Gm34, on substrate tRNAPhe in vitro. (A) ORF YMR259c is required for formation of Cm on tRNAPhe in yeast extracts. A total of 50 μg of crude extracts from the indicated strains were 10-fold serially diluted and assayed for Cm methyltransferase activity with in vitro-transcribed [α-32P]CTP-labeled tRNAPhe, and the products were digested and analyzed by thin layer chromatography as described in Materials and Methods. (B) Rtt10 is required for formation of Gm on tRNAPhe in yeast extracts. Crude extracts were assayed for Gm methyltransferase activity using [α-32P]GTP-labeled tRNAPhe as described in A.
Thus, we conclude that Trm7 requires YMR259c (now named Trm732) for Cm32 formation, and requires Rtt10 (now named Trm734) for Nm34 formation both in vivo and in vitro, and our data suggest that the two activities are independent of each other.
Slow growth of trm7-Δ mutants is due to lack of both Cm32 and Nm34
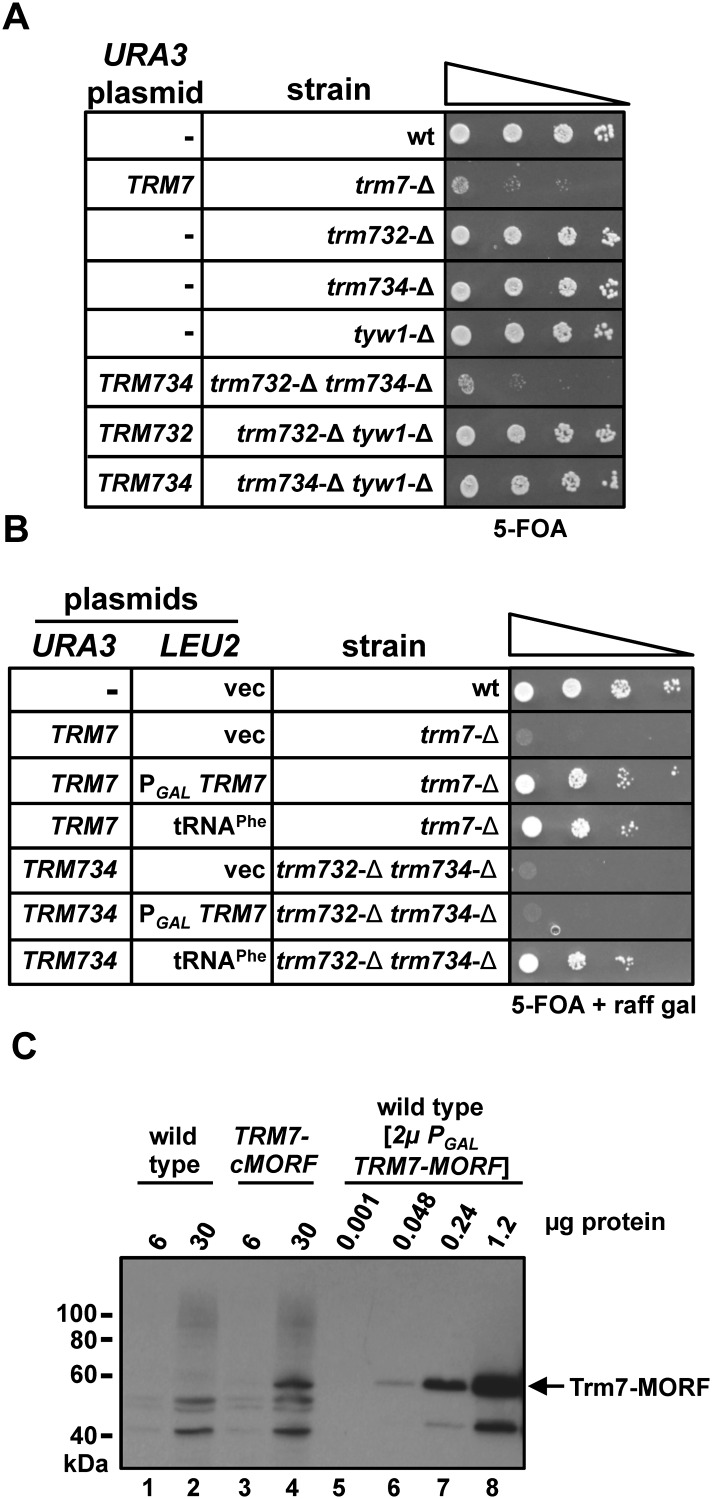
The finding that Cm32 formation requires Trm732 (YMR259c) and that Nm34 formation requires Trm734 (Rtt10) allowed us to determine the contribution of each 2′-O-methylated residue to the slow growth phenotype of trm7-Δ mutants by examining the phenotypes of each reconstructed single mutant and the corresponding double mutant. We find that neither trm732-Δ mutants nor trm734-Δ mutants have any obvious growth defect on media containing 5-FOA (Fig. 6A), whereas the trm732-Δ trm734-Δ [TRM734 URA3] strain grows as poorly as the trm7-Δ [TRM7 URA3] strain when plated to media containing 5-FOA (Fig. 6A). Control experiments demonstrate that tyw1-Δ mutants (Noma et al. 2006), trm732-Δ tyw1-Δ mutants, and trm734-Δ tyw1-Δ mutants are each as healthy as wild-type cells (Fig. 6A). These results show that, in effect, the slow growth of trm7-Δ mutants is actually a negative synthetic effect due to the lack of Cm32 and Gm34, accompanied by lack of yW37, on tRNAPhe, all due to lack of Trm7.
FIGURE 6.
A trm732-Δ trm734-Δ mutant phenocopies the growth phenotypes of a trm7-Δ mutant. (A) Lack of both Cm32 and Nm34 are required for slow growth of trm7-Δ mutants. Strains were grown overnight at 30°C in YPD medium, adjusted to an OD600 of ∼0.5, and serially diluted 10-fold; then, 2 μL was spotted onto media containing 5-FOA and incubated for 2 d at 30°C. (B) Overexpression of tRNAPhe suppresses the slow growth of trm732-Δ trm734-Δ mutants. Strains with plasmids as indicated were grown overnight in S-Leu medium containing raffinose and galactose and analyzed by spotting to S media containing 5-FOA, raffinose, and galactose, and incubated for 2 d at 30°C. (C) Trm7 is overproduced more than 100-fold when expressed from a [2μ PGAL] plasmid. Soluble crude extract was prepared from indicated strains and then subjected to immunoblot analysis with anti-protein A antibody.
Consistent with our conclusion that the trm732-Δ trm734-Δ strain phenocopies the trm7-Δ mutant strain we find that overexpression of tRNAPhe efficiently suppresses the slow growth of a trm732-Δ trm734-Δ mutant (Fig. 6B). In contrast, overproduction of Trm7 does not suppress the slow growth of a trm732-Δ trm734-Δ mutant. Since Trm7 levels are about 100-fold greater when expressed from the [2μ PGAL TRM7] plasmid than from the chromosomal copy (Fig. 6C, cf. lane 4 with lanes 5–8), we infer that Trm732 and Trm734 provide specific functions to promote modification that cannot be bypassed by Trm7 alone.
The synthetic phenotype of a trm7-Δ trm1-Δ strain is due to lack of Nm34, but not Cm32
Since previous high-throughput experiments suggested that trm7-Δ trm1-Δ mutants, which also lack m2,2G26 on their tRNAs (Ellis et al. 1986), have a synthetic growth defect (Wilmes et al. 2008), we examined this defect to explore its cause. Consistent with the earlier report, we find that trm7-Δ trm1-Δ [TRM7 URA3] mutants do not detectably grow when plated on media containing 5-FOA, whereas trm1-Δ mutants are healthy (Fig. 7). Strikingly, we find strong evidence that the contribution of the trm7-Δ mutation to the trm7-Δ trm1-Δ lethal phenotype is almost entirely due to lack of Nm34. Thus, trm734-Δ trm1-Δ [TRM1 URA3] mutants are extremely sick when plated on media containing 5-FOA, whereas trm732-Δ trm1-Δ [TRM1 URA3] mutants and tyw1-Δ trm1-Δ [TRM1 URA3] mutants have no obvious growth defect. If, as seems likely, the lethal phenotype of trm7-Δ trm1-Δ mutants is primarily due to lack of m2,2G26 and Nm34 (and possibly the accompanying substantial reduction in yW37), this finding also demonstrates that Cm32 and Nm34 have separate functions, even though they appear to make equivalent contributions to the trm7-Δ mutant phenotype (Fig. 6A).
FIGURE 7.

A trm7-Δ trm1-Δ mutation is lethal primarily due to the lack of Nm34 and m2,2G26. Strains with plasmids as indicated were grown overnight at 30°C in YPD medium, and analyzed as in Figure 6A.
DISCUSSION
We have shown here that trm7-Δ mutants grow poorly primarily because of decreased tRNAPhe function (Fig. 1B). Our data suggest a complex mechanism of anticodon loop modification of tRNAPhe, in which the Trm7 methyltransferase interacts with Trm732 for 2′-O-methylation of C32 and with Trm734 for 2′-O-methylation of G34, and these modifications in turn drive modification of m1G37 to yW (Fig. 8). Furthermore, we have provided strong evidence that the slow growth phenotype of trm7-Δ mutants (Pintard et al. 2002) requires lack of both Cm32 and Gm34 of tRNAPhe (Fig. 6A), although the specific functions of Cm32 and Gm34 are different, based on differential genetic interactions with trm1-Δ mutants (Fig. 7).
FIGURE 8.
Summary of the proteins required for 2′-O-methylation of the anticodon loop of yeast tRNAPhe. A schematic of the anticodon loop of tRNAPhe is shown, together with the proteins required for formation of Cm32 and Gm34. Arrows indicate that yW37 formation requires Gm34, and to a lesser extent Cm32.
Our observation that Gm34 of tRNAPhe (and to a lesser extent Cm32) drives formation of yW37 from m1G explains previous systems biology data showing that trm7-Δ mutants have reduced levels of yW (Chan et al. 2010). These results suggest strongly that Tyw1 requires Gm34 and Cm32 for the next catalytic step in yW formation from m1G37 (Noma et al. 2006). This dependence of yW37 formation on the presence of Gm34 and Cm32 is consistent with the circuitous tRNAPhe maturation pathway, which involves splicing in the cytoplasm, followed by Trm7 modification in the cytoplasm, retrograde tRNA nuclear import (Murthi et al. 2010), m1G37 modification by Trm5, and subsequent re-export for yW37 formation (Ohira and Suzuki 2011). Furthermore, the requirement of Gm34 and Cm32 modification for yW modification of tRNAPhe represents the first documented example in eukaryotes of a tRNA modification on one residue directing modification on a different residue, similar to an earlier report describing a tRNA modification network in Thermus thermophilus that is dependent on m7G (Tomikawa et al. 2010).
The Trm7 requirement for Trm732 to form Cm32 and for Trm734 to form Nm34 (Figs. 3, 5; Tables 1, 2) adds to an emerging theme of complexes that are required for apparently simple tRNA methylation reactions; in yeast, these include Trm6/Trm61 for formation of m1A58 (Anderson et al. 1998), Trm8/Trm82 for formation of m7G46 (Alexandrov et al. 2002), Trm11/Trm112 for formation of m2G10 (Purushothaman et al. 2005), and Trm9/Trm112 for the terminal methylation of mcm5U34 (Studte et al. 2008). In the case of Trm7 methylation, it is clear both in vivo and in vitro that Trm732 and Trm734 impart specificity to Trm7 for methylation of the corresponding residues (Figs. 3, 5; Tables 1, 2), much as Pho85 kinase specificity is modulated by partner proteins (Dephoure et al. 2005; Huang et al. 2007). Indeed, the requirement for different Trm7 partners for each modification may be explained in part by the fact that the 2′-O-methyl groups of C32 and G34 are 12.6 Å apart in the tRNAPhe crystal structure (PDB 1EHZ), with that of C32 buried, and that of G34 solvent exposed (Shi and Moore 2000). However, we note that each subunit of the Trm7 complexes may also contribute in other ways, as shown for Trm6 and Trm61 variants in m1A modification (Ozanick et al. 2007).
It is unclear why 2′-O-methylation of C32 and G34 of tRNAPhe is critical for healthy growth of yeast. Since overexpression of tRNAPhe suppresses the slow-growth phenotype of trm7-Δ mutants, but levels of charged tRNAPhe appear normal (Fig. 1D), a critical defect of trm7-Δ mutants is likely at a step after charging, and at or before ribosome A-site decoding, since if the defect was in translocation, P-site interactions, or E-site interactions, additional tRNAPhe copies would not be expected to overcome the defect. In principle, Cm32 and/or Gm34 of tRNAPhe might influence any step of tRNAPhe function during the transfer of charged tRNA to EF-1A, during delivery to the ribosome, or at any translation step before peptide bond formation (Gromadski et al. 2007; Zaher and Green 2009). We note that since the trm7-Δ slow-growth phenotype is really a synthetic genetic interaction that requires loss of both Cm32 and Nm34 (and yW), and since each 2′-O-methylation has a distinct role, only one defect of trm7-Δ mutants needs to be corrected by overexpression of tRNAPhe to overcome the slow-growth phenotype.
It is not immediately obvious how Cm32 and Gm34 contribute to tRNAPhe function. Although 2′-O-methylation is known to stabilize the C3′-endo form of uridine (Kawai et al. 1992) and to increase stacking (Drake et al. 1974), it is unclear how these properties contribute to tRNAPhe anticodon loop function. One possible role of Cm32 in tRNAPhe function could be to influence the interaction of Cm32 with A38. In a number of tRNA structures, including that of yeast tRNAPhe, a bifurcated hydrogen bond is formed between O2 of C32 (or U32, or the spatially equivalent O4 of Ψ) and the exocyclic amine of N38 (Auffinger and Westhof 1999). Furthermore, the identity of the N32:N38 pair influences codon:anticodon recognition in prokaryotes (Olejniczak et al. 2005) and affects translational fidelity (Ledoux et al. 2009; Murakami et al. 2009). Thus, it is possible that 2′-O-methylation of C32 influences the C32:A38 pair to affect one or more of the steps of translation before peptide bond formation (Zaher and Green 2009).
One possible role of Gm34 of tRNAPhe might be to promote cognate codon:anticodon interactions in the yeast ribosome A-site, perhaps due to its improved stacking and pairing properties, although no specific role is ascribed to the 2′-oxygen of G34 in the crystal structure of bacterial ribosomes complexed with the yeast anticodon stem–loop (Ogle et al. 2001). Gm34 might also conceivably affect interactions of tRNAPhe with its synthetase PheRS, since G34 is a known determinant for charging (Sampson et al. 1989), and since completely unmodified tRNAPhe is less efficiently charged than fully modified tRNAPhe in vitro (Sampson and Uhlenbeck 1988), although our northern analysis indicates that levels of charged tRNAPhe are nearly normal in trm7-Δ mutants (Fig. 1D).
It is unclear from our results how 2′-O-methylation of the anticodon loops of tRNALeu(UAA) and tRNATrp by Trm7 contributes to their function. Since overexpression of tRNAPhe completely suppresses the slow growth phenotype of trm7-Δ mutants (Fig. 1B), 2′-O-methylation of tRNALeu(UAA) and tRNATrp is not measurably important under these growth conditions. Although it is possible that the Cm32 and Nm34 modifications of tRNALeu(UAA) and tRNATrp have an important role under other conditions, it is also possible that these tRNAs are modified by Trm7 due to overlapping substrate specificity with tRNAPhe rather than because of an inherent biological necessity, as has been speculated for tRNA modification phenotypes associated with the rapid tRNA decay pathway and the nuclear surveillance pathway (Phizicky and Alfonzo 2010), and appears to explain the multiple phenotypes associated with mutations in the ELP complex (Esberg et al. 2006; Chen et al. 2011).
It remains to be determined whether the other documented phenotypes of trm734-Δ mutants are related to tRNA biology. Since both trm734-Δ (rtt10-Δ) mutants and trm7-Δ mutants were identified in a screen for mutated genes that promote Ty1 transposition (Nyswaner et al. 2008), it is plausible that hypomodified tRNA causes a decrease in expression of specific Phe-rich genes important for Ty1 transposition. Trm734 (Rtt10, Ere2) was also recently identified as having a role in retromer-dependent endoplasmic recycling, because trm734-Δ (rtt10-Δ, ere2-Δ) mutants have increased canavanine resistance due to a defect in Can1 recycling (Shi et al. 2011). It is unclear at present whether the Trm734 2′-O-methylation function is responsible for this phenotype, or if this phenotype represents a second role of Trm734.
It also remains to be determined the extent to which the Trm7 anticodon loop modification circuitry described here is conserved in other eukaryotes and the extent to which Trm7 modifications are important for tRNAPhe or other tRNAs in other eukaryotes. Available evidence suggests that Trm7 and Trm734 are widely conserved in eukaryotes and that Trm734 contains WD40 repeats (Feder et al. 2003; Shi et al. 2011), and our BLAST (http://blast.ncbi.nlm.nih.gov/) and InterProScan (http://ebi.ac.uk/Tools/pfa/iprscan/) analyses indicate that Trm732 is widely conserved, contains a DUF2428 domain (domain of unknown function), and contains Armadillo repeats (data not shown). Since both WD40 repeats (Stirnimann et al. 2010) and Armadillo repeats (Tewari et al. 2010) are typically found in scaffolding proteins, it seems plausible that these domains are important for interactions with Trm7 in eukaryotes. In addition, 16 of 17 sequenced eukaryotic tRNAPhe species are known to have Cm32 and Gm34, all five sequenced eukaryotic tRNATrp species have Cm32 and Cm34 (Juhling et al. 2009), and a substantial portion of the tRNAPhe from neuroblastoma cells and Ehrlich ascites tumors lacks Cm32 and Gm34, and has m1G37 instead of yW37 (Kuchino et al. 1982). Furthermore, high-throughput analysis suggests that the putative Schizosaccharomyces pombe TRM7 gene is essential (Kim et al. 2010), and numerous studies implicate the putative human homolog FTSJ1 in nonsyndromic X-linked mental retardation (Freude et al. 2004; Ramser et al. 2004; Froyen et al. 2007; Takano et al. 2008). Based on these observations, it is tempting to speculate that the circuitry for Trm7 modification of the anticodon loop is conserved and that these modifications are widely important for tRNAPhe function.
MATERIALS AND METHODS
Yeast strains
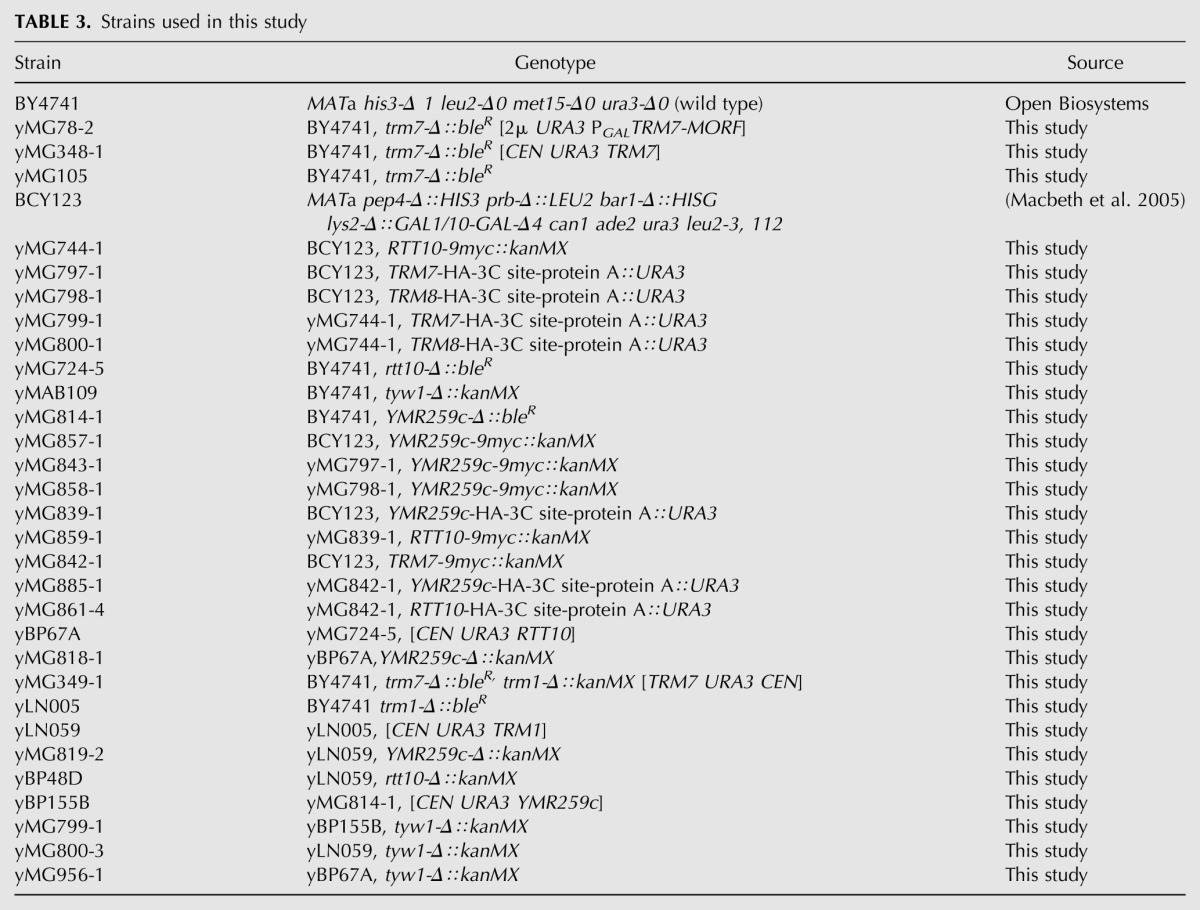
Yeast strains are listed in Table 3. The BY4741 trm7-Δ∷bleR [2μ URA3 PGALTRM7-MORF] strain (yMG78-2) and the trm7-Δ∷bleR [TRM7 URA3 CEN] strain (yMG348-1) were constructed by standard methods (Chernyakov et al. 2008). All double-mutant trm7-Δ strains were constructed by PCR amplification of DNA from the appropriate YKO collection kanMX strain (Open Biosystems), followed by transformation of the DNA into a trm7-Δ∷bleR [URA3 TRM7] strain. Other double-mutant strains were constructed similarly. C-terminally tagged ORF-9myc strains were generated by transformation of parent strain BCY123 with a gene-specific PCR product of the appropriate fragment of the pYM18 9myc∷kanMX cassette (Janke et al. 2004). C-terminally tagged ORF-cMORF strains (tagged with a cassette comprised of a His6-HA-3C site-ZZ domain of protein A from the MORF collection) (Gelperin et al. 2005) were generated in a similar fashion from the cMORF∷URA3 cassette of pAVA0258. All strain constructions were verified by PCR confirmation using appropriate oligonucleotides.
TABLE 3.
Strains used in this study
Plasmids
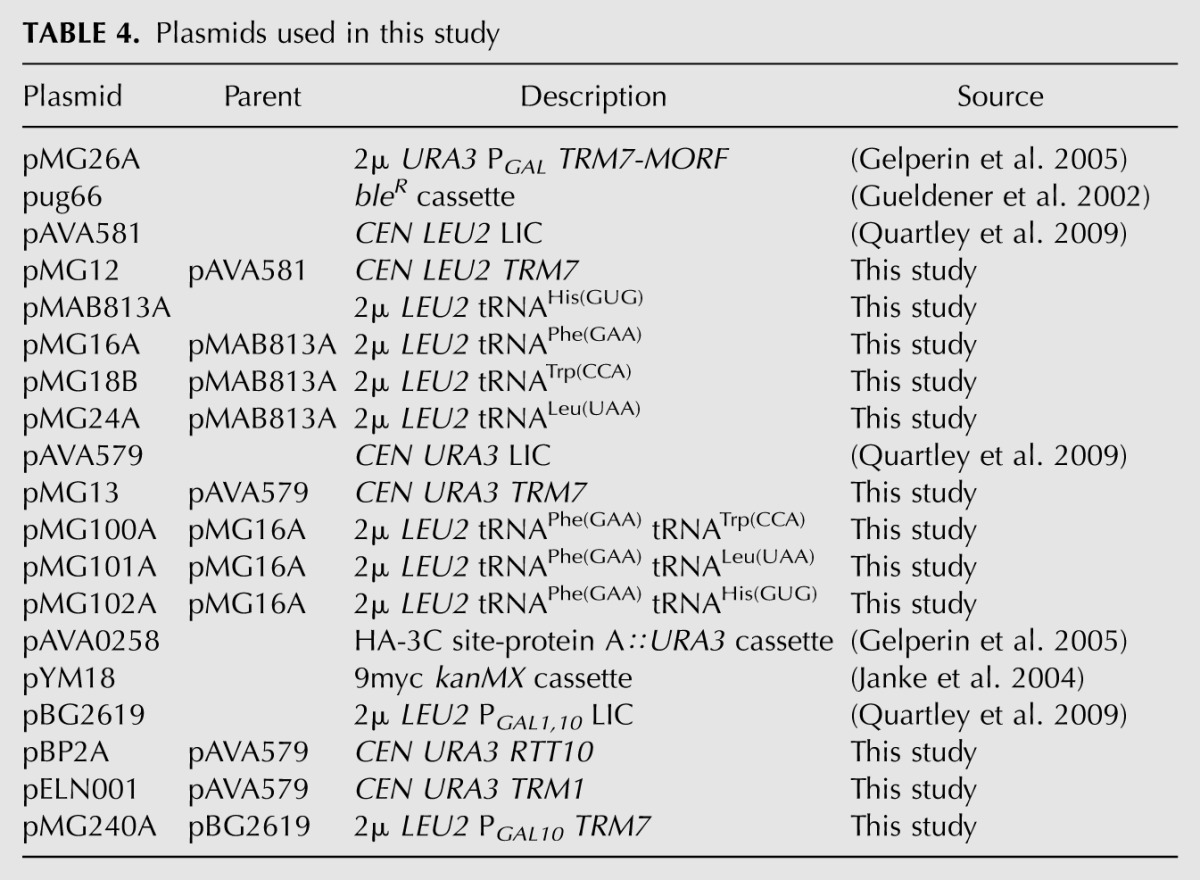
Plasmids used in this study are listed in Table 4. CEN plasmids were constructed by ligation-independent cloning (LIC) of appropriate DNA fragments into pAVA581 (LEU2) and pAVA579 (URA3) (Quartley et al. 2009). [2μ LEU2 tRNA] expression plasmids were constructed by insertion of the appropriate tRNA sequence into the XhoI BglII site of a tRNA expression plasmid (pMAB813A), which harbors a BamH1 fragment containing the tRNAHis(GUG) gene (tH(GUG)G2) and flanking sequences, with a XhoI site 22 bp 5′ of the +1 site of the mature tRNA sequence, and a BglII site 21 bp 3′ of residue 73. Dual tRNA expression plasmids were constructed by PCR amplification of the appropriate tRNA fragment and ligation into the multicloning site of the [2μ LEU2 tRNAPhe] plasmid (pMG16A) derived from pMAB813A. Plasmids for ORF expression in yeast are [2μ PGAL1,10] dual ORF expression vectors, designed to express ORFs under PGAL1 control with a C-terminal PT tag (ORF-3C site-HA epitope-His6-ZZ domain of protein A), cloned by LIC after digestion with BbrP1 and PacI, and to express ORFs under PGAL10 control with no tag, cloned by LIC after digestion with SwaI, essentially as described previously (Quartley et al. 2009). All plasmids were confirmed by sequencing before use.
TABLE 4.
Plasmids used in this study
Northern blot analysis
Yeast strains were grown to an OD600 of ∼1 and RNA was prepared using the hot phenol method, or under acidic conditions to preserve aminoacylation, and RNA was then analyzed by Northern blot as previously described (Alexandrov et al. 2006) using appropriate probes.
Growth and lysis of strains for biochemical analysis
Yeast strains were grown in YPD to an OD600 of ∼2, or if they harbored a [2μ PGAL1,10] expression plasmid, were grown in S dropout media containing raffinose and galactose to an OD600 of ∼1. Yeast crude extracts were prepared by bead beating in the presence of protease inhibitors as described previously (Quartley et al. 2009).
Affinity purification of MORF-tagged proteins
cMORF-tagged proteins were purified by affinity purification using IgG sepharose, elution with GST-3C protease, and then removal of the protease with glutathione sepharose resin as described previously (Quartley et al. 2009).
Immunoblot analysis
Yeast crude extracts and affinity-purified samples were subjected to SDS-PAGE and proteins were transferred to nitrocellulose membrane (Bio-Rad), and probed with the appropriate antibodies. The 9myc tag was detected with mouse monoclonal anti-[c-myc] (Roche), followed by incubation with goat anti-mouse IgG-HRP (Bio-Rad), and visualization with Amersham ECL Plus (GE Healthcare). MORF-tagged constructs were detected with rabbit polyclonal anti-protein A (Sigma), followed by goat anti-rabbit IgG-HRP (Bio-Rad), and visualization.
Isolation and purification of tRNA
Yeast strains were grown at 30°C in YPD to mid-log phase. Bulk low-molecular weight RNA was extracted from 300 OD pellets, and the appropriate 5′ biotinylated oligonucleotides were used to purify tRNA as previously described (Jackman et al. 2003).
HPLC analysis of tRNA
Purified tRNA from yeast was digested with P1 nuclease and phosphatase as previously described (Jackman et al. 2003), and nucleosides were subjected to HPLC analysis essentially as previously described (Jackman et al. 2003) for tRNALeu(UAA) and tRNATrp. tRNAPhe was analyzed similarly, except that the HPLC buffers were at pH 7.0 and the gradients were adjusted to a maximize separation of Gm and m1G. At a flow rate of 0.75 mL/min, the gradient was as follows: 100% buffer A (10 mM (NH4)H2PO4, 2.5% methanol) for 14.4 min; a gradient to achieve 10% buffer B (10mM (NH4)H2PO4, 20% methanol) at 24 min; and a gradient to achieve 25% buffer B at 45 min.
Detection of wye base by fluorescence
tRNAPhe purified from appropriate strains was diluted to 10 μg/mL in 10 mM Tris-HCl (pH 8.0), 1 mM ethylenediaminetetraacetic acid (EDTA), and emission from 350 to 550 nm was measured in 1-nm increments after excitation at 320 nm, and plotted after subtracting the spectrum from tRNATrp.
In vitro transcription and methyltransferase assays
Plasmids containing tRNAPhe (pEMP1577) and tRNAThr(IGU) (pEMP1568) were digested with BstN1 and transcribed with T7 polymerase in the presence of 50 μCi [α32P]CTP or [α32P]GTP (3000 Ci/mmol, Perkin Elmer), and tRNA was purified by PAGE and assayed for methyltransferase activity with crude extract, followed by P1 nuclease digestion (Jackman et al. 2003), and resolution of nucleotides by cellulose thin-layer chromatography developed in isobutyric acid:ammonia:H2O (66:1:33).
ACKNOWLEDGMENTS
We thank E. Grayhack for numerous discussions and helpful insights, as well as other members of the Phizicky and Grayhack labs for discussions. We thank L. Nemeth for help in constructing yeast strains and plasmids and M. Dumont (University of Rochester) for antibodies and help in measuring yW fluorescence. This research was supported by NIH grants GM52347 to E.M.P. and GM27930 to A.K.H., and NSF grant CHE-0910751 to P.A.L. M.P.G. was supported by a NIH postdoctoral training grant NCI T32 CA09363 and M.A.P. was supported by a NIH predoctoral training grant 5T32 GM068411.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.035287.112.
REFERENCES
- Agris PF, Vendeix FA, Graham WD 2007. tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol 366: 1–13 [DOI] [PubMed] [Google Scholar]
- Alexandrov A, Martzen MR, Phizicky EM 2002. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA 8: 1253–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM 2006. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21: 87–96 [DOI] [PubMed] [Google Scholar]
- Anderson J, Phan L, Cuesta R, Carlson BA, Pak M, Asano K, Bjork GR, Tamame M, Hinnebusch AG 1998. The essential Gcd10p–Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev 12: 3650–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffinger P, Westhof E 1999. Singly and bifurcated hydrogen-bonded base-pairs in tRNA anticodon hairpins and ribozymes. J Mol Biol 292: 467–483 [DOI] [PubMed] [Google Scholar]
- Bekaert M, Rousset JP 2005. An extended signal involved in eukaryotic −1 frameshifting operates through modification of the E site tRNA. Mol Cell 17: 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork GR, Jacobsson K, Nilsson K, Johansson MJ, Bystrom AS, Persson OP 2001. A primordial tRNA modification required for the evolution of life? EMBO J 20: 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork GR, Huang B, Persson OP, Bystrom AS 2007. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA 13: 1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Dyavaiah M, DeMott MS, Taghizadeh K, Dedon PC, Begley TJ 2010. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet 6: e1001247 doi: 10.1371/journal.pgen.1001247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Huang B, Eliasson M, Ryden P, Bystrom AS 2011. Elongator complex influences telomeric gene silencing and DNA damage response by its role in wobble uridine tRNA modification. PLoS Genet 7: e1002258 doi: 10/1371/journal.pgen.1002258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM 2008. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev 22: 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N, Howson RW, Blethrow JD, Shokat KM, O'Shea EK 2005. Combining chemical genetics and proteomics to identify protein kinase substrates. Proc Natl Acad Sci 102: 17940–17945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihanich ME, Najarian D, Clark R, Gillman EC, Martin NC, Hopper AK 1987. Isolation and characterization of MOD5, a gene required for isopentenylation of cytoplasmic and mitochondrial tRNAs of Saccharomyces cerevisiae. Mol Cell Biol 7: 177–184 [Published erratum appears in Mol Cell Biol 1987 May;7(5):2035.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AF, Mason SF, Trim AR 1974. Optical studies of the base-stacking properties of 2′-O-methylated dinucleoside monophosphates. J Mol Biol 86: 727–739 [DOI] [PubMed] [Google Scholar]
- El Yacoubi B, Hatin I, Deutsch C, Kahveci T, Rousset JP, Iwata-Reuyl D, Murzin AG, de Crecy-Lagard V 2011. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J 30: 882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SR, Morales MJ, Li JM, Hopper AK, Martin NC 1986. Isolation and characterization of the TRM1 locus, a gene essential for the N2,N2-dimethylguanosine modification of both mitochondrial and cytoplasmic tRNA in Saccharomyces cerevisiae. J Biol Chem 261: 9703–9709 [PubMed] [Google Scholar]
- Esberg A, Huang B, Johansson MJ, Bystrom AS 2006. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol Cell 24: 139–148 [DOI] [PubMed] [Google Scholar]
- Feder M, Pas J, Wyrwicz LS, Bujnicki JM 2003. Molecular phylogenetics of the RrmJ/fibrillarin superfamily of ribose 2′-O-methyltransferases. Gene 302: 129–138 [DOI] [PubMed] [Google Scholar]
- Freude K, Hoffmann K, Jensen LR, Delatycki MB, des Portes V, Moser B, Hamel B, van Bokhoven H, Moraine C, Fryns JP, et al. 2004. Mutations in the FTSJ1 gene coding for a novel S-adenosylmethionine-binding protein cause nonsyndromic X-linked mental retardation. Am J Hum Genet 75: 305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froyen G, Bauters M, Boyle J, Van Esch H, Govaerts K, van Bokhoven H, Ropers HH, Moraine C, Chelly J, Fryns JP, et al. 2007. Loss of SLC38A5 and FTSJ1 at Xp11.23 in three brothers with non-syndromic mental retardation due to a microdeletion in an unstable genomic region. Hum Genet 121: 539–547 [DOI] [PubMed] [Google Scholar]
- Gelperin DM, White MA, Wilkinson ML, Kon Y, Kung LA, Wise KJ, Lopez-Hoyo N, Jiang L, Piccirillo S, Yu H, et al. 2005. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev 19: 2816–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromadski KB, Schummer T, Stromgaard A, Knudsen CR, Kinzy TG, Rodnina MV 2007. Kinetics of the interactions between yeast elongation factors 1A and 1Bα, guanine nucleotides, and aminoacyl-tRNA. J Biol Chem 282: 35629–35637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Hurto RL, Hopper AK, Grayhack EJ, Phizicky EM 2005. Depletion of Saccharomyces cerevisiae tRNAHis guanylyltransferase Thg1p leads to uncharged tRNAHis with additional m5C. Mol Cell Biol 25: 8191–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res 30: e23. doi: 10.1093/nar/30.6.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm M, Giege R, Florentz C 1999. A Watson-Crick base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry 38: 13338–13346 [DOI] [PubMed] [Google Scholar]
- Huang D, Friesen H, Andrews B 2007. Pho85, a multifunctional cyclin-dependent protein kinase in budding yeast. Mol Microbiol 66: 303–314 [DOI] [PubMed] [Google Scholar]
- Jackman JE, Montange RK, Malik HS, Phizicky EM 2003. Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. RNA 9: 574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Magiera MM, Rathfelder N, Taxis C, Reber S, Maekawa H, Moreno-Borchart A, Doenges G, Schwob E, Schiebel E, et al. 2004. A versatile toolbox for PCR-based tagging of yeast genes: New fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21: 947–962 [DOI] [PubMed] [Google Scholar]
- Johansson MJ, Esberg A, Huang B, Bjork GR, Bystrom AS 2008. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol 28: 3301–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J 2009. tRNAdb 2009: Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res 37: D159–D162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J 2004. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev 18: 1227–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaba S, Wang X, Anderson JT 2006. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA 12: 508–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai G, Yamamoto Y, Kamimura T, Masegi T, Sekine M, Hata T, Iimori T, Watanabe T, Miyazawa T, Yokoyama S 1992. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2′-hydroxyl group. Biochemistry 31: 1040–1046 [DOI] [PubMed] [Google Scholar]
- Kim DU, Hayles J, Kim D, Wood V, Park HO, Won M, Yoo HS, Duhig T, Nam M, Palmer G, et al. 2010. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol 28: 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, et al. 2006. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440: 637–643 [DOI] [PubMed] [Google Scholar]
- Kuchino Y, Borek E, Grunberger D, Mushinski JF, Nishimura S 1982. Changes of post-transcriptional modification of wye base in tumor-specific tRNAPhe. Nucleic Acids Res 10: 6421–6432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D 2005. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121: 713–724 [DOI] [PubMed] [Google Scholar]
- Laten H, Gorman J, Bock RM 1978. Isopentenyladenosine deficient tRNA from an antisuppressor mutant of Saccharomyces cerevisiae. Nucleic Acids Res 5: 4329–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecointe F, Namy O, Hatin I, Simos G, Rousset JP, Grosjean H 2002. Lack of pseudouridine 38/39 in the anticodon arm of yeast cytoplasmic tRNA decreases in vivo recoding efficiency. J Biol Chem 277: 30445–30453 [DOI] [PubMed] [Google Scholar]
- Ledoux S, Olejniczak M, Uhlenbeck OC 2009. A sequence element that tunes E. coli tRNAGGCAla to ensure accurate decoding. Nat Struct Mol Biol 16: 359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth MR, Schubert HL, Vandemark AP, Lingam AT, Hill CP, Bass BL 2005. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science 309: 1534–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Ohta A, Suga H 2009. Bases in the anticodon loop of tRNAAlaGGC prevent misreading. Nat Struct Mol Biol 16: 353–358 [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S 1988. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature 336: 179–181 [DOI] [PubMed] [Google Scholar]
- Murphy FV 4th, Ramakrishnan V 2004. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol 11: 1251–1252 [DOI] [PubMed] [Google Scholar]
- Murphy FV 4th, Ramakrishnan V, Malkiewicz A, Agris PF 2004. The role of modifications in codon discrimination by tRNALysUUU. Nat Struct Mol Biol 11: 1186–1191 [DOI] [PubMed] [Google Scholar]
- Murthi A, Shaheen HH, Huang HY, Preston MA, Lai TP, Phizicky EM, Hopper AK 2010. Regulation of tRNA bidirectional nuclear-cytoplasmic trafficking in Saccharomyces cerevisiae. Mol Biol Cell 21: 639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A, Kirino Y, Ikeuchi Y, Suzuki T 2006. Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J 25: 2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyswaner KM, Checkley MA, Yi M, Stephens RM, Garfinkel DJ 2008. Chromatin-associated genes protect the yeast genome from Ty1 insertional mutagenesis. Genetics 178: 197–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle JM, Brodersen DE, Clemons WM Jr, Tarry MJ, Carter AP, Ramakrishnan V 2001. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 292: 897–902 [DOI] [PubMed] [Google Scholar]
- Ohira T, Suzuki T 2011. Retrograde nuclear import of tRNA precursors is required for modified base biogenesis in yeast. Proc Natl Acad Sci 108: 10502–10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejniczak M, Dale T, Fahlman RP, Uhlenbeck OC 2005. Idiosyncratic tuning of tRNAs to achieve uniform ribosome binding. Nat Struct Mol Biol 12: 788–793 [DOI] [PubMed] [Google Scholar]
- Ozanick SG, Bujnicki JM, Sem DS, Anderson JT 2007. Conserved amino acids in each subunit of the heteroligomeric tRNA m1A58 Mtase from Saccharomyces cerevisiae contribute to tRNA binding. Nucleic Acids Res 35: 6808–6819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky EM, Alfonzo JD 2010. Do all modifications benefit all tRNAs? FEBS Lett 584: 265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky EM, Hopper AK 2010. tRNA biology charges to the front. Genes Dev 24: 1832–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintard L, Lecointe F, Bujnicki JM, Bonnerot C, Grosjean H, Lapeyre B 2002. Trm7p catalyses the formation of two 2′-O-methylriboses in yeast tRNA anticodon loop. EMBO J 21: 1811–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman SK, Bujnicki JM, Grosjean H, Lapeyre B 2005. Trm11p and Trm112p are both required for the formation of 2-methylguanosine at position 10 in yeast tRNA. Mol Cell Biol 25: 4359–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz J, Florentz C, Benseler F, Giege R 1994. A single methyl group prevents the mischarging of a tRNA. Nat Struct Biol 1: 580–582 [DOI] [PubMed] [Google Scholar]
- Quartley E, Alexandrov A, Mikucki M, Buckner FS, Hol WG, DeTitta GT, Phizicky EM, Grayhack EJ 2009. Heterologous expression of L. major proteins in S. cerevisiae: A test of solubility, purity, and gene recoding. J Struct Funct Genomics 10: 233–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramser J, Winnepenninckx B, Lenski C, Errijgers V, Platzer M, Schwartz CE, Meindl A, Kooy RF 2004. A splice site mutation in the methyltransferase gene FTSJ1 in Xp11.23 is associated with non-syndromic mental retardation in a large Belgian family (MRX9). J Med Genet 41: 679–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JR, Uhlenbeck OC 1988. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci 85: 1033–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JR, DiRenzo AB, Behlen LS, Uhlenbeck OC 1989. Nucleotides in yeast tRNAPhe required for the specific recognition by its cognate synthetase. Science 243: 1363–1366 [DOI] [PubMed] [Google Scholar]
- Schneider C, Anderson JT, Tollervey D 2007. The exosome subunit Rrp44 plays a direct role in RNA substrate recognition. Mol Cell 27: 324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmer M, Dunham CM, Murphy FVt, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V 2006. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313: 1935–1942 [DOI] [PubMed] [Google Scholar]
- Shi H, Moore PB 2000. The crystal structure of yeast phenylalanine tRNA at 1.93 A resolution: A classic structure revisited. RNA 6: 1091–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Stefan CJ, Rue SM, Teis D, Emr SD 2011. Two novel WD40 domain-containing proteins, Ere1 and Ere2, function in the retromer-mediated endosomal recycling pathway. Mol Biol Cell 22: 4093–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnimann CU, Petsalaki E, Russell RB, Muller CW 2010. WD40 proteins propel cellular networks. Trends Biochem Sci 35: 565–574 [DOI] [PubMed] [Google Scholar]
- Studte P, Zink S, Jablonowski D, Bar C, von der Haar T, Tuite MF, Schaffrath R 2008. tRNA and protein methylase complexes mediate zymocin toxicity in yeast. Mol Microbiol 69: 1266–1277 [DOI] [PubMed] [Google Scholar]
- Takano K, Nakagawa E, Inoue K, Kamada F, Kure S, Goto Y 2008. A loss-of-function mutation in the FTSJ1 gene causes nonsyndromic X-linked mental retardation in a Japanese family. Am J Med Genet B Neuropsychiatr Genet 147B: 479–484 [DOI] [PubMed] [Google Scholar]
- Tewari R, Bailes E, Bunting KA, Coates JC 2010. Armadillo-repeat protein functions: Questions for little creatures. Trends Cell Biol 20: 470–481 [DOI] [PubMed] [Google Scholar]
- Tomikawa C, Yokogawa T, Kanai T, Hori H 2010. N7-Methylguanine at position 46 (m7G46) in tRNA from Thermus thermophilus is required for cell viability at high temperatures through a tRNA modification network. Nucleic Acids Res 38: 942–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbonavicius J, Qian Q, Durand JM, Hagervall TG, Bjork GR 2001. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J 20: 4863–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacova S, Wolf J, Martin G, Blank D, Dettwiler S, Friedlein A, Langen H, Keith G, Keller W 2005. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol 3: e189 doi: 10.1371/journal.pbio.0030189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waas WF, Druzina Z, Hanan M, Schimmel P 2007. Role of a tRNA base modification and its precursors in frameshifting in eukaryotes. J Biol Chem 282: 26026–26034 [DOI] [PubMed] [Google Scholar]
- Whipple JM, Lane EA, Chernyakov I, D'Silva S, Phizicky EM 2011. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev 25: 1173–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmes GM, Bergkessel M, Bandyopadhyay S, Shales M, Braberg H, Cagney G, Collins SR, Whitworth GB, Kress TL, Weissman JS, et al. 2008. A genetic interaction map of RNA-processing factors reveals links between Sem1/Dss1-containing complexes and mRNA export and splicing. Mol Cell 32: 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher HS, Green R 2009. Fidelity at the molecular level: Lessons from protein synthesis. Cell 136: 746–762 [DOI] [PMC free article] [PubMed] [Google Scholar]