Abstract
Ginseng, an ancient and famous medicinal herb in the Orient, has been used as a valuable tonic and for the treatment of various diseases including hepatic disorders. Ginseng saponins, commonly known as ginsenosides, are principal constituents and have believed to be responsible for multiple ginseng health benefits. There are more 40 ginsenosides isolated from ginseng. To date, treatment options for common liver diseases such as cirrhosis, fatty liver, and chronic hepatitis remain problematic. In this regard, ginseng extracts and individual ginsenosides have shown a wide array of beneficial role in the regulation of regular liver functions and the treatment of liver disorders of acute/chronic hepatotoxicity, hepatitis, hepatic fibrosis/cirrhosis, hepatocellular carcinoma, and so on in various pathways and mechanisms. In this paper, we first outline the pharmacological effects of ginseng and ginsenosides on the liver functions.
1. Introduction
Botanical medicines have been applied for the treatment of various human diseases with thousands of years of history in Asia and are sharing a large market in the form of drugs, dietary supplements, and foods. In the west, botanical medicines are categorized as complementary/alternative medicines, dietary supplements, or foods. Ginseng, referred to as the root of Panax ginseng C.A. Meyer (Araliaceae), is one of the most valuable medicinal plants, particularly in Korea, China, and Japan [1].
Ginseng has been used as a valuable tonic and for the treatment of various diseases [1, 2]. Traditionally, ginseng has been processed to make white ginseng (air-drying after harvest) and red ginseng (steaming or heat process) to enhance its preservation and efficacy. In which, red ginseng is more common as an herbal medicine than white ginseng because steaming induces changes in the chemical constituents and enhances the biological activities of ginseng [1, 2]. The pharmacological properties of ginseng are mainly attributed to ginseng saponins, commonly called ginsenoisdes, the major and bioactive constituents [3, 4]. With the development of modern chromatography, there are more 40 ginsenoisdes such as ginsenoisdes Rb1, Rb2, Rg1, Rd, and Re identified from ginseng up to date [4, 5]. Except for ginsenoisde Ro and polyacetylene ginsenoisde Ro belonging to oleanane-type saponins, other ginsenoisdes are of dammarane-type saponins and classified into protopanaxadiol and protopanaxatriol groups depending on whether or not hydroxyl group at C-6 of aglycon moieties exist (Figure 1). On the other hand, ginseng and ginsenoisdes have been found to exhibit multiple pharmacological activities via different mechanisms and pathways in vitro, in vivo, and clinical models [2–4]. Having been well documented, there are hundreds of research papers as well as extensive reviews spotlighted on individual topics, that is, cardiovascular [6, 7], central nervous [8], and immune systems [9, 10]. However, the pharmacological effects of ginseng/ginsenoisdes on liver disorders have not been systematically reviewed. The purpose of this paper is to introduce the multifaceted pharmacological effects and related mechanisms of ginseng/ginsenoisdes on hepatic functions.
Figure 1.
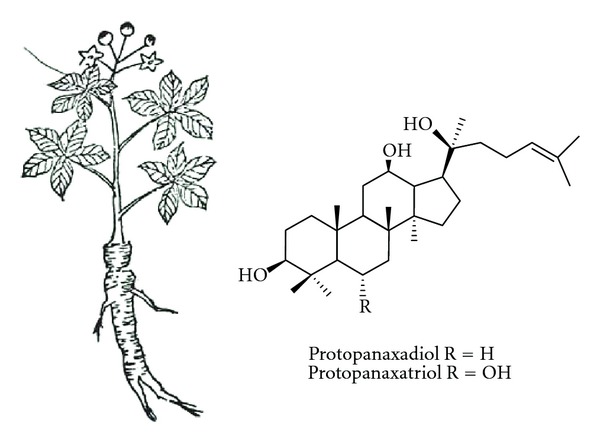
Panax ginseng C.A. Mayer and ginsenoisde skeletons.
2. Liver Diseases and Herbal Medicine in the Treatment of Liver Diseases
Liver diseases represent a major health burden worldwide, with liver cirrhosis being the ninth leading cause of death in western countries [11]. Chronic viral hepatitis B and C, alcoholic liver disease, nonalcoholic fatty liver disease, and hepatocellular carcinoma are the major entities and many problems remain unresolved. Therapies developed along the principles of western medicine are often limited in efficacy, carry the risk of adverse effects, and are often too costly, especially for the developing world. Therefore, treating liver diseases with plant-derived products which are accessible and do not require laborious pharmaceutical synthesis seems highly attractive. Furthermore, in spite of the advances in conventional medicine in the last decades, professionals and the lay public of developed countries pay increasing attention to phytomedicine. Several recent surveys from Europe and the United States have demonstrated a sharp rise in the use of botanical drugs within a few years, and up to 65% of patients with liver disease take herbal preparations [12–15].
3. Protective Effect of Ginseng Extracts and Ginsenoisdes on Hepatic Functions and Diseases
3.1. Ginseng Extracts
3.1.1. Multiple Efficacies
General Hepatotoxicity —
Acute/chronic hepatotoxicity suffers from drugs used, alcohol, contaminant, and poisoning and may be incidence of liver diseases including liver hepatitis, cirrhosis/fibrosis, and hepatocellular carcinoma (HCC). Ginseng extracts have been reported to show protective effects on hepatocytes in vitro and liver injury in various animal, and clinical models induced by a wide variety of hepatotoxins including hydrogen peroxide (H2O2) [16], alcohol [17, 18], carbon tetrachloride (CCl4) [16, 19–22], aflatoxin B1 [23, 24], fumonisins [24], tert-butylhydroperoxide (t-BHP) [25], cadmium chloride (CdCl2) [26, 27], benzo[alpha]pyrene (BP) [28], 2,3,7,8-tetrachlorodibenzo-p-dioxin [29], lipopolysaccharide [30], diethylnitrosamine [31], galactosamine (GalN) [32–34], bromobenzene [35], xenobiotics [36], and mercuric chloride [37]. Ginseng has also found to protect liver cells from radiation [38] and viral hepatitis [39].
Liver Regeneration and Transplantation —
Ginseng is beneficial in the treatment of acute graft-versus-host disease, a rare complication after liver transplantation with an extremely poor prognosis [40]. On the other hand, red ginseng is markedly effective in liver regeneration after partial hepatectomy in rats (70%) [41] and dogs (40%) [42] by increase of the liver weight and acceleration of hepatocyte proliferation.
Fatty Liver and Liver Glycogen —
Liver and adipose tissue play an important role in both lipid and sugar metabolism. It has been found that ginseng has effects on lipid and sugar when administrated to rats in vivo [43, 44]. Hepatic cholesterol and triglyceride contents are decreased and phospholipid is increased by ginseng administration in the high cholesterol diet-fed rats, supporting to improvement of the fatty liver [45]. In another study, ginseng produces a decrease of hepatic glycogen content in fat diet-fed rats [44].
Liver Cancer —
HCC is one of the most frequent tumor types worldwide. It is the fifth most common cancer and the third leading cause of cancer death [46]. Ginseng has been evaluated as a chemopreventive therapy against HCC [47–49]. Results of epidemiological studies of ginseng intake and cancer cases (4600 patients) show that those who take ginseng are less likely to contract various cancers such as cancer of the stomach, liver, and lung than those who do not take it, and that increased intake leads to a lesser ratio of danger, proving its usefulness for primary prevention [50, 51]. In a clinical survey, the prolonged administration of red ginseng extract significantly inhibits the incidence of hepatoma and also proliferation of pulmonary tumors induced by aflatoxin B1 and urethane [52]. Recently, another clinical research reported that ginseng administration induced a significant improvement in liver function tests, decreased the tumor marker levels, and decreased the viral titers in HCV (hepatitis C virus) patients, supporting powerful therapeutic effects against HCV and liver cancer [39].
3.1.2. Mechanisms of Actions
Antioxidation —
The mechanisms which provide ginseng's hepatoprotective effects are closely attributed to antioxidation properties. Ginseng enhanced the antioxidant defense mechanism [53] and increased self-antioxidant enzyme activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-S-transferase (GSH), and heme oxygenase-1 in the aged-rat liver [54] and hepatotoxins-induced liver damages in rats [20, 23, 26, 28, 30, 55, 56]. Ginseng treatments inhibited oxidative stress damage such as lipid peroxidation [16, 26, 28, 54], malondialdehyde [23], thiobarbituric acid reactive substance [23, 30, 56], alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) [25, 28, 39, 42, 57]. The protective effects have been histologically and histochemically monitored. Recently, further molecular mechanism studies found that ginseng suppresses mitogen-activated protein kinase (MAPK) signals [16], nuclear factor-kappa B (NF-kB), and inducible nitric oxide synthase (iNOS) protein expression [30, 56].
Anti-Inflammation —
Inflammatory effects of ginseng have been responsible for the liver protection. Ginseng suppressed the production of inflammatory cytokines (IL-1β, IFN-γ) and chemokines (MCP-1, MIP-2β, KC) in CCl4-treated mice [55]. Recently, ginseng was found to inhibit tumor necrosis factor alpha (TNF-α)-stimulated NF-kB activation and further suppressed the gene expression of iNOS and cyclooxygenase-2 (COX-2) in HepG-2 cells [58].
Inhibition of Cytochrome P450 (CYP) —
Ginseng and ginsenoisdes may have selectively inhibitory effects on CYP activities. In a recent animal study, ginseng was found to inhibit specifically benzo [alpha]pyrene-induced CYP1A1 activation by downregulation of the gene expression [28]. Another study also indicated that the inhibitory effects of ginseng on CYP enzymes take part in the protection of rat liver microsomes against CCl4 [21]. Further investigation into ginseng metabolism suggested that ginsenoisde metabolites like compound K, produced after oral administration, are responsible for the inhibition of the CYP-mediated metabolism rather than naturally occurring ginsenoisdes [59]. These evidence may explain some of the hepatoprotective effects of ginseng against hepatotoxins.
3.2. Ginsenoisdes
3.2.1. Ginsenoisde Rb1
Ginsenoisde Rb1, a major ginseng saponin, protects hepatocytes against t-BHP and CCl4 by regulating inducible hepatic enzymes of ALT and AST [22, 60] and may be associated with modulating liver CYP activation [61] and protein phosphorylations [62]. In a recent study, ginsenoisde Rb1 significantly inhibits liver fibrosis by inhibiting activation, proliferation, and expression of collagen, transforming growth factor-β 1 (TGF-1), matrix metalloproteinase (MMP)-2, and tissue inhibitor of metalloproteinase (TIMP)-1 in hepatic stellate cells, the major cause of liver fibrosis, at 10–80 μg/mL [63]. More recently, ginsenoisde Rb1 is found to inhibit TNF-α-mediated NF-kB transcriptional activity in HepG2 cells with IC50 of 27.45 μM and gene expression of iNOS and COX-2 inducible inflammatory enzymes [58].
Ginsenoisde Rb1 is beneficial against fatty/high cholesterol liver as well as hepatic triglycerides in rats fed on high-fat diet, supporting that ginsenoisde Rb1 may be involved in lipid metabolism by regulating the activity of microsomal CYP monooxygenase and 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase [64–66].
3.2.2. Ginsenoisde Rg1
Ginsenoisde Rg1, also one of the most abundant ginseng components of ginseng, significantly improves the extent of liver fibrosis in rat induced by thioacetamide as reducing the serum levels of fibrotic markers of ALT, AST, and alkaline phosphatase and hepatic hydroxyproline content [67]. In addition, in cultured hepatic stellate cells, ginsenoisde Rg1 markedly inhibits cell proliferation, activation and formation of reactive oxygen species, and the NF-kB activity [67]. Also, ginsenoisde Rg1 is found to inhibit TNF-α-mediated NF-kB transcriptional activity in HepG2 cells with IC50 of 28.14 μM and gene expression of iNOS and COX-2 inducible inflammatory enzymes [58].
Recently, ginsenoisde Rg1 suppresses hepatic glucose production via AMP-activated protein kinase (AMPK) activation in HepG2 cells [68].
3.2.3. Ginsenoisdes Rd, Re, and Ro
Yuan et al. reported that ginsenoisde Re (40 mg/kg) attenuates alcoholic fatty liver disease through regulation of AMPK and mitogen-activated protein kinase (MAPK) pathways in alcoholic-fed ICR mice [69]. In another related study, the same group revealed ginsenoisde Re to lower blood glucose and lipid level via activation of AMPK pathway in HepG2 cells and high-fat diet fed mice, suggesting therapeutic benefits on type 2 diabetic patients with insulin resistance and dyslipidemia [70].
Ginsenoisde Rd is recently found to inhibit TNF-α-mediated NF-kB transcriptional activity in HepG2 cells with IC50 of 12.05 μM and gene expression of iNOS and COX-2 inducible inflammatory enzymes [58].
Ginsenoisde Ro, an oleanane-type ginsenoisde, (50 and 200 mg/kg, p.o.) shows the protective effects on GalN and CCl4-induced acute hepatitis rats as monitored by the level of inducible hepatic enzymes of glutamic oxaloacetic transaminase and glutamic pyruvic transaminase [32].
3.2.4. Ginsenoisdes Rg2, Rg3, and M1
Ginsenoisdes Rg2 and Rg3 represent major components of red ginseng [5]. Ginsenoisde M1 or compound K is an intestine bacterial metabolite of the ginseng protopanaxadiol-type saponins. In a recent study, ginsenoisde Rg2 significantly inhibits hepatic glucose production in HepG2 cells via activation of AMPK pathway like ginsenoisde Re [71]. Meanwhile, ginsenoisdes Rg3 and M1 have well evaluated for their cancer chemopreventative against several cancer cell lines and tumors in vitro and in vivo including leukemia, colon, and HCC [51, 72–74]. More recently, ginsenoisde Rg3 at 25–200 μg/mL shows significantly antiproliferative effects on liver cancer cells (SMMC-7721, Hep1-6, and HepG2) and hepatocellular tumor growth in vivo by inhibiting cancer cell proliferation and promoting cancer cell apoptosis [75, 76]. In another research, ginsenoisde M1 exhibits antihepatocellular carcinoma activity by augmenting apoptosis via Bid-mediated mitochondrial pathways [77]. On the other hand, ginsenoisde M1 has beneficial role against hyperlipidemia and obesity by attenuating hepatic lipid accumulation via AMPK activation in human hepatoma cells [78]. In addition, ginsenoisdes Rg3 and M1 are also involved in general hepatoprotection against hepatotoxins [60, 79].
4. Application of Ginseng to Hepatic Diseases
Liver disorders are among the most serious sicknesses in the population. Together with healthy lifestyle recommendation, therapies for hepatic diseases are necessary and need be more effective and economical [80]. Botanical medicines have been used traditionally worldwide for the prevention and treatment of liver disease. By now, herbal products are increasingly consumed, mainly in chronic liver disease. Turmeric (Curcuma longa), green tea (Camellia sinensis), garlic (Allium sativa), licorice (Glycyrrhiza spp.), especially milk thistle (Silybum marianum) with have been well researched and recognized as phytotherapies for use in a wide variety of prevention and treatment of hepatic diseases [81, 82].
Based on compelling experimental evidence, ginseng has an interesting hepatoprotective profile. From a therapeutic standpoint, ginseng could provide an alternative phytomedicine and another option for the preventative therapy and treatments of common liver diseases such as cirrhosis, fatty liver, and chronic hepatitis in addition to a well-known tonic and in form
s of healthy foods.
5. Conclusions
Ginseng and its principal components, ginsenoisdes, have shown a wide array of pharmacological activities including beneficial role in the regulation of liver functions and the treatment of liver disorders of acute/chronic hepatotoxicity, hepatitis, hepatic fibrosis/cirrhosis, liver hepatectomy, liver transplantation, and even liver failure and HCC. The possible activity pathways of the actions have been also investigated. There is increasing attention to the effects of ginseng on the liver functions. However, more detailed molecular mechanisms of the activities of ginseng/ginsenoisdes as well as further efficacy and safety studies remain to be explored.
It is another important area for further research and development to combine ginseng with other liver active drugs to investigate their possible synergic efficacy and preferably pharmacological properties.
Taken together, accumulating evidence supports the potential of ginseng in the treatment of the hepatic diseases and further studies will facilitate their application so far.
Acknowledgment
N. H. Tung is supported by JSPS (the Japan Society for the Promotion of Science).
References
- 1.Yun TK. Brief introduction of Panax ginseng C.A. Meyer. Journal of Korean Medical Science. 2001;16(supplement):S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JD, Rhee DK, Lee YH. Biological activities and chemistry of saponins from Panax ginseng C. A. Meyer. Phytochemistry Reviews. 2005;4(2-3):159–175. [Google Scholar]
- 3.Ernst E. Panax ginseng: an overview of the clinical evidence. Journal of Ginseng Research. 2010;34(4):259–263. [Google Scholar]
- 4.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacologica Sinica. 2008;29(9):1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 5.Qi LW, Wang CZ, Yuan CS. Isolation and analysis of ginseng: advances and challenges. Natural Product Reports. 2011;28(3):467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karmazyn M, Moey M, Gan XT. Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs. 2011;71(15):1989–2008. doi: 10.2165/11594300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Buettner C, Yeh GY, Phillips RS, Mittleman MA, Kaptchuk TJ. Systematic review of the of ginseng on cardiovascular risk factors. Annals of Pharmacotherapy. 2006;40(1):83–95. doi: 10.1345/aph.1G216. [DOI] [PubMed] [Google Scholar]
- 8.Radad K, Gille G, Liu L, Rausch WD. Use of ginseng in medicine with emphasis on neurodegenerative disorders. Journal of Pharmacological Sciences. 2006;100(3):175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- 9.Lee DCW, Lau ASY. Effects of Panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules. 2011;16(4):2802–2816. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochemical Pharmacology. 1999;58(11):1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 11.Kim WR, Brown RS, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36(1):227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 12.De Smet PAGM. Herbal remedies. The New England Journal of Medicine. 2002;347(25):2046–2056. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC, Davis RB, Foster DF, et al. Long-term trends in the use of complementary and alternative medical therapies in the United States. Annals of Internal Medicine. 2001;135(4):262–268. doi: 10.7326/0003-4819-135-4-200108210-00011. [DOI] [PubMed] [Google Scholar]
- 14.Strader DB, Bacon BR, Lindsay KL, et al. Use of complementary and alternative medicine in patients with liver disease. American Journal of Gastroenterology. 2002;97(9):2391–2397. doi: 10.1111/j.1572-0241.2002.05993.x. [DOI] [PubMed] [Google Scholar]
- 15.Flora KD, Rosen HR, Benner KG. The use of naturopathic remedies for chronic liver disease. American Journal of Gastroenterology. 1996;91(12):2654–2655. [PubMed] [Google Scholar]
- 16.Bak MJ, Jun M, Jeong WS. Antioxidant and hepatoprotective of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. International Journal of Molecular Sciences. 2012;13(2):2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuin M, Battezzati PM, Camisasca M, Riebenfeld D, Podda M. Effects of a preparation containing a standardized ginseng extract combined with trace elements and multivitamins against hepatotoxin-induced chronic liver disease in the elderly. Journal of International Medical Research. 1987;15(5):276–281. doi: 10.1177/030006058701500503. [DOI] [PubMed] [Google Scholar]
- 18.Park HM, Go HK, Mun AR, et al. n red ginseng attenuate an ethanol-induced hepatocyte toxicity, by increasing the level of Mg2+ and suppression of the MAPK pathway. Proceedings of the Spring International Ginseng Conference; April 2012; Jeju, Korea. The Korean Society of Ginseng; p. p. 93. [Google Scholar]
- 19.El Denshary ES, Al-Gahazali MA, Mannaa FA, Salem HA, Hassan NS, Abdel-Wahhab MA. Dietary honey and ginseng protect against carbon tetrachloride-induced hepatonephrotoxicity in rats. doi: 10.1016/j.etp.2011.01.012. Experimental and Toxicologic Pathology, http://dx.doi.org/10.1016/j.etp.2011.01.012. In press. [DOI] [PubMed] [Google Scholar]
- 20.Chang HF, Lin YH, Chu CC, Wu SJ, Tsai YH, Chao JCJ. Protective effects of Ginkgo biloba, Panax ginseng, and Schizandra chinensis extract on liver injury in rats. American Journal of Chinese Medicine. 2007;35(6):995–1009. doi: 10.1142/S0192415X07005466. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Chun YJ, Park JD, Kim SI, Roh JK, Jeong TC. Protection of rat liver microsomes against carbon tetrachloride-lnduced lipid peroxidation by red ginseng. Planta Medica. 1997;63(5):415–418. doi: 10.1055/s-2006-957724. [DOI] [PubMed] [Google Scholar]
- 22.Jeong TC, Kim HJ, Park JI, et al. Protective effects of red ginseng saponins against carbon tetrachloride-induced hepatotoxicity in Sprague Dawley rats. Planta Medica. 1997;63(2):136–140. doi: 10.1055/s-2006-957630. [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Kim YH, Noh JR, Cho ES, Park JH, Son HY. Protective effect of korean red ginseng against aflatoxin B1-induced hepatotoxicity in rat. Journal of Ginseng Research. 2011;35(2):243–249. doi: 10.5142/jgr.2011.35.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Wahhab MA, Hassan NS, El-Kady AA, et al. Red ginseng extract protects against aflatoxin B1 and fumonisins-induced hepatic pre-cancerous lesions in rats. Food and Chemical Toxicology. 2010;48(2):733–742. doi: 10.1016/j.fct.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Kim JH, Lee SY, Park JH, Hwang GS. Processed ginseng protects t-BHP-induced oxidative damage in HepG2 cells. Proceedings of the Spring International Ginseng Conference; April 2012; Jeju, Korea. The Korean Society of Ginseng; p. p. 99. [Google Scholar]
- 26.Shukla R, Kumar M. Role of Panax ginseng as an antioxidant after cadmium-induced hepatic injuries. Food and Chemical Toxicology. 2009;47(4):769–773. doi: 10.1016/j.fct.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Karadeniz A, Cemek M, Simsek N. The effects of Panax ginseng and Spirulina platensis on hepatotoxicity induced by cadmium in rats. Ecotoxicology and Environmental Safety. 2009;72(1):231–235. doi: 10.1016/j.ecoenv.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Gum SI, Jo SJ, Ahn SH, et al. The potent protective effect of wild ginseng (Panax ginseng C.A. Meyer) against benzo[α]pyrene-induced toxicity through metabolic regulation of CYP1A1 and GSTs. Journal of Ethnopharmacology. 2007;112(3):568–576. doi: 10.1016/j.jep.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Lee HC, Hwang SG, Lee YG, et al. In vivo effects of Panax ginseng extracts on the cytochrome P450-dependent monooxygenase system in the liver of 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed guinea pig. Life Sciences. 2002;71(7):759–769. doi: 10.1016/s0024-3205(02)01742-3. [DOI] [PubMed] [Google Scholar]
- 30.Kang KS, Yamabe N, Kim HY, Yokozawa T. Effect of sun ginseng methanol extract on lipopolysaccharide-induced liver injury in rats. Phytomedicine. 2007;14(12):840–845. doi: 10.1016/j.phymed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Wu XG, Zhu DH, Li X. Anticarcinogenic effect of red ginseng on the development of liver cancer induced by diethylnitrosamine in rats. Journal of Korean Medical Science. 2001;16:S61–65. doi: 10.3346/jkms.2001.16.S.S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda H, Samukawa KI, Kubo M. Anti-hepatitic activity of ginsenoside Ro. Planta Medica. 1991;57(6):523–526. [PubMed] [Google Scholar]
- 33.Hikino H, Kiso Y, Kinouchi J, Sanada S, Shoji J. Antihepatotoxic actions of ginsenosides from Panax ginseng roots. Planta Medica. 1985;51(1):62–64. [PubMed] [Google Scholar]
- 34.Song JH, Park MJ, Kim E, Kim YC. Effects of Panax ginseng on galactosamine-induced cytotoxicity in primary cultured rat hepatocytes. Yakhak Hoeji. 1990;34(5):341–347. [Google Scholar]
- 35.Huh K, Jang BS, Park JM. Protective effect of ginseng on bromobenzene-induced hepatotoxicity in mice. Korean Journal of Ginseng Science. 1988;12(2):114–120. [Google Scholar]
- 36.Lee FC, Park JK, Kim EK, Ko JH, Lee JS, Kim KY. The role of Panax ginseng in detoxification of xenobiotics. Proceedings of the 4th International Ginseng Symposium; 1984; Seoul, Korea. Korean Society of Ginseng; [Google Scholar]
- 37.Saxena PN, Mahour K. Analysis of hepatoprotection by Panax ginseng following mercuric chloride intoxication in albino rat. Proceedings of the 9th International Ginseng Symposium; 2006; Chungnam, Korea. Korean Society of Ginseng; [Google Scholar]
- 38.Kim TS, Kim YJ, Jang SA, Yang KH, Seung NK, Sohn EH. Protective effects of red ginseng against radiation-induced hepatotoxicity in mice. Proceedings of the Spring International Ginseng Conference; April 2012; Jeju, Korea. The Korean Society of Ginseng; p. p. 100. [Google Scholar]
- 39.Abdel-Wahhab MA, Gamil K, El-Kady AA, El-Nekeety AA, Naguib KM. Therapeutic effects of Korean red ginseng extract in Egyptian patients with chronic liver diseases. Journal of Ginseng Research. 2011;35(1):69–79. [Google Scholar]
- 40.Xu X, Ling Q, Wei Q, et al. Korean red ginseng: a new approach for the treatment of graft-versus-host disease after liver transplantation. Transplantation Proceedings. 2011;43(7):2651–2655. doi: 10.1016/j.transproceed.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Kwon YS, Jang KH. The effect of Korean red ginseng on liver regeneration after 70% hepatectomy in rats. Journal of Veterinary Medical Science. 2004;66(2):193–195. doi: 10.1292/jvms.66.193. [DOI] [PubMed] [Google Scholar]
- 42.Kwon YS, Jang KH, Jang IH. The effects of Korean red ginseng (Ginseng Radix Rubra) on liver regeneration after partial hepatectomy in dogs. Journal of Veterinary Science (Suwon-si, Korea) 2003;4(1):83–92. [PubMed] [Google Scholar]
- 43.Yokozawa T, Seno H, Oura H. Effect of ginseng extract on lipid and sugar metabolism. I. Metabolic correlation between liver and adipose tissue. Chemical and Pharmaceutical Bulletin. 1975;23(12):3095–3100. doi: 10.1248/cpb.23.3095. [DOI] [PubMed] [Google Scholar]
- 44.Yokozawa T, Kanai K, Takefuji M, Oura H. Effect of Ginseng saponin on liver glycogen content. Chemical and Pharmaceutical Bulletin. 1976;24(12):3202–3204. doi: 10.1248/cpb.24.3202. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto M, Uemura T, Nakama S, Uemiya M, Kumagai A. Serum HDL-cholesterol-increasing and fatty liver-improving actions of Panax ginseng in high cholesterol diet-fed rats with clinical effect on hyperlipidemia in man. American Journal of Chinese Medicine. 1983;11(1–4):96–101. doi: 10.1142/S0192415X83000161. [DOI] [PubMed] [Google Scholar]
- 46.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 47.Helms S. Cancer prevention and therapeutics: Panax ginseng . Alternative Medicine Review. 2004;9(3):259–274. [PubMed] [Google Scholar]
- 48.Ginseng-HCC Chemopreventive Study Osaka Group. Study on chemoprevention of hepatocellular carcinoma by ginseng: an introduction to the protocol. Journal of Korean Medical Science. 2001;16(supplement):S70–S74. doi: 10.3346/jkms.2001.16.S.S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishino H, Tokuda H, Ii T, et al. Cancer chemoprevention by ginseng in mouse liver and other organs. Journal of Korean Medical Science. 2001;16(supplement):S66–69. doi: 10.3346/jkms.2001.16.S.S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamangar F, Gao YT, Shu XO, et al. Ginseng intake and gastric cancer risk in the Shanghai women’s health study cohort. Cancer Epidemiology Biomarkers and Prevention. 2007;16(3):629–630. doi: 10.1158/1055-9965.EPI-06-1009. [DOI] [PubMed] [Google Scholar]
- 51.Yun TK. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutation Research. 2003;523-524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 52.Yun TK, Yun YS, Han IW. Anticarcinogenic effect of long-term oral administration of red ginseng on newborn mice exposed to various chemical carcinogens. Cancer Detection and Prevention. 1983;6(6):515–525. [PubMed] [Google Scholar]
- 53.Kim HG, Yoo SR, Park HJ, et al. Antioxidant effects of Panax ginseng C.A. Meyer in healthy subjects: a randomized, placebo-controlled clinical trial. Food and Chemical Toxicology. 2011;49(9):2229–2235. doi: 10.1016/j.fct.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 54.Ramesh T, Kim SW, Sung JH, et al. Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Experimental Gerontology. 2012;47(1):77–84. doi: 10.1016/j.exger.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Shim JY, Kim MH, Kim HD, Ahn JY, Yun YS, Song JY. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory response. Toxicology and Applied Pharmacology. 2010;242(3):318–325. doi: 10.1016/j.taap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Yokozawa T, Kang KS, Yamabe N, Kim HY. Therapeutic potential of heat-processed Panax ginseng with respect to oxidative tissue damage. Drug Discovery & Therapeutics. 2007;1(1):30–44. [PubMed] [Google Scholar]
- 57.Voces J, Alvarez AI, Vila L, Ferrando A, Cabral De Oliveira C, Prieto JG. Effects of administration of the standardized Panax ginseng extract G115 on hepatic antioxidant function after exhaustive exercise. Comparative Biochemistry and Physiology C. 1999;123(2):175–184. doi: 10.1016/s0742-8413(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 58.Song SB, Tung NH, Quang TH, Ngan NTT, Kim KE, Kim YH. Inhibition of TNF-α-mediated NF-κB transcriptional activity in HepG2 cells by dammarane-type saponins from Panax ginseng leaves. Journal of Ginseng Research. 2012;36(2):146–152. doi: 10.5142/jgr.2012.36.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Zhang JW, Li W, et al. Ginsenoside metabolites, rather than naturally occurring ginsenosides, lead to inhibition of human cytochrome P450 enzymes. Toxicological Sciences. 2006;91(2):356–364. doi: 10.1093/toxsci/kfj164. [DOI] [PubMed] [Google Scholar]
- 60.Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver International. 2005;25(5):1069–1073. doi: 10.1111/j.1478-3231.2005.01068.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Ye X, Ma Z, et al. Induction of cytochrome P450 1A1 expression by ginsenoside Rg1 and Rb1 in HepG2 cells. European Journal of Pharmacology. 2008;601(1–3):73–78. doi: 10.1016/j.ejphar.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 62.Park HJ, Park KM, Rhee MH, et al. Effect of ginsenoside RB1 on rat liver phosphoproteins induced by carbon tetrachloride. Biological and Pharmaceutical Bulletin. 1996;19(6):834–838. doi: 10.1248/bpb.19.834. [DOI] [PubMed] [Google Scholar]
- 63.Lo YT, Tsai YH, Wu SJ, Chen JR, Chao JC. Ginsenoside Rb1 inhibits cell activation and liver fibrosis in rat hepatic stellate cells. Journal of Medicinal Food. 2011;14(10):1135–1143. doi: 10.1089/jmf.2010.1485. [DOI] [PubMed] [Google Scholar]
- 64.Sakakibara K, Shibata Y, Higashi T, Sanada S, Shoji J. Effect of Ginseng saponins on cholesterol metabolism. I. The level and the synthesis of serum and liver cholesterol in rats treated with ginsenosides. Chemical and Pharmaceutical Bulletin. 1975;23(5):1009–1016. doi: 10.1248/cpb.23.1009. [DOI] [PubMed] [Google Scholar]
- 65.Ikehara M, Shibata Y, Higashi T. Effect of ginseng saponins on cholesterol metabolism. III. Effect of ginsenoside-Rb1 on cholesterol synthesis in rats fed on high-fat diet. Chemical and Pharmaceutical Bulletin. 1978;26(9):2844–2849. doi: 10.1248/cpb.26.2844. [DOI] [PubMed] [Google Scholar]
- 66.Park KH, Shin HJ, Song YB, et al. Possible role of ginsenoside Rb1 on regulation of rat liver triglycerides. Biological and Pharmaceutical Bulletin. 2002;25(4):457–460. doi: 10.1248/bpb.25.457. [DOI] [PubMed] [Google Scholar]
- 67.Geng J, Peng W, Huang Y, Fan H, Li S. Ginsenoside-Rg1 from Panax notoginseng prevents hepatic fibrosis induced by thioacetamide in rats. European Journal of Pharmacology. 2010;634(1–3):162–169. doi: 10.1016/j.ejphar.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 68.Kim SJ, Yuan HD, Chung SH. Ginsenoside Rg1 suppresses hepatic glucose production via AMP-activated protein kinase in HepG2 cells. Biological and Pharmaceutical Bulletin. 2010;33(2):325–328. doi: 10.1248/bpb.33.325. [DOI] [PubMed] [Google Scholar]
- 69.Yuan HD, Kim SJ, Quan HY, Kim DY, Kim GW, Chung SH. Ginsenoside Re attenuates alcoholic fatty liver disease via regulation of AMPK and MAPK pathways in alcohol-fed ICR mice. Proceedings of the Spring International Ginseng Conference; April 2012; Jeju, Korea. The Korean Society of Ginseng; p. p. 114. [Google Scholar]
- 70.Quan HY, Yuan HD, Jung MS, Ko SK, Park YG, Chung SH. Ginsenoside Re lowers blood glucose and lipid levels via activation of AMP-activated protein kinase in HepG2 cells and high-fat diet fed mice. International Journal of Molecular Medicine. 2012;29(1):73–80. doi: 10.3892/ijmm.2011.805. [DOI] [PubMed] [Google Scholar]
- 71.Yuan HD, Kim DY, Quan HY, Kim SJ, Jung MS, Chung SH. Ginsenoside Rg2 induces orphan nuclear receptor SHP gene expression and inactivates GSK3β via AMP-activated protein kinase to inhibit hepatic glucose production in HepG2 cells. Chemico-Biological Interactions. 2012;195(1):35–42. doi: 10.1016/j.cbi.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Cho SH, Chung KS, Choi JH, Kim DH, Lee KT. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer. 2009;9, article 149:1–13. doi: 10.1186/1471-2407-9-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He BC, Gao JL, Luo X, et al. Ginsenoside Rg3 inhibits colorectal tumor growth through the down-regulation of Wnt/β-catenin signaling. International Journal of Oncology. 2011;38(2):437–445. doi: 10.3892/ijo.2010.858. [DOI] [PubMed] [Google Scholar]
- 74.Luo X, Wang CZ, Chen J, et al. Characterization of gene expression regulated by American ginseng and ginsenoside Rg3 in human colorectal cancer cells. International Journal of Oncology. 2008;32(5):975–983. [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang JW, Chen XM, Chen XH, Zheng SS. Ginsenoside Rg3 inhibits hepatocellular carcinoma growth via intrinsic apoptotic pathway. World Journal of Gastroenterology. 2011;17(31):3605–3613. doi: 10.3748/wjg.v17.i31.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C, Liu L, Yu Y, Chen B, Tang C, Li X. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Molecular Medicine Reports. 2012;5(5):1295–1298. doi: 10.3892/mmr.2012.808. [DOI] [PubMed] [Google Scholar]
- 77.Song G, Guo S, Wang W, et al. Intestinal metabolite compound K of ginseng saponin potently attenuates metastatic growth of hepatocellular carcinoma by augmenting apoptosis via a bid-mediated mitochondrial pathway. Journal of Agricultural and Food Chemistry. 2010;58(24):12753–12760. doi: 10.1021/jf103814f. [DOI] [PubMed] [Google Scholar]
- 78.Kim DY, Yuan HD, Chung IK, Chung SH. Compound k, intestinal metabolite of ginsenoside, attenuates hepatic lipid accumulation via AMPK Activation in human hepatoma cells. Journal of Agricultural and Food Chemistry. 2009;57(4):1532–1537. doi: 10.1021/jf802867b. [DOI] [PubMed] [Google Scholar]
- 79.Lee HU, Bae EA, Myung JH, Kim DH. Hepatoprotective effect of 20(S)-ginsenosides Rg3 and its metabolite 20(S)-ginsenoside Rh2 on tert-butyl hydroperoxide-induced liver injury. Biological and Pharmaceutical Bulletin. 2005;28(10):1992–1994. doi: 10.1248/bpb.28.1992. [DOI] [PubMed] [Google Scholar]
- 80.Nobili V, Carter-Kent C, Feldstein AE. The role of lifestyle changes in the management of chronic liver disease. BMC Medicine. 2011;9, article 70:1–7. doi: 10.1186/1741-7015-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scott-Luper ND. A review of plants used in the treatment of liver disease: part 1. Alternative Medicine Review. 1998;3(6):410–421. [PubMed] [Google Scholar]
- 82.Loguercio C, Festi D. Silybin and the liver: from basic research to clinical practice. World Journal of Gastroenterology. 2011;17(18):2288–2301. doi: 10.3748/wjg.v17.i18.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]


