Abstract
A single catalytic site model is proposed to account for the multiphasic kinetics of oxidation of ferrocytochrome c by cytochrome c oxidase (ferrocytochrome c:oxygen oxidoreductase, EC 1.9.3.1). This model involves nonproductive binding of substrate to sites near the catalytic site on cytochrome c oxidase for cytochrome c, decreasing the binding constant for cytochrome c at the catalytic site. This substrate inhibition results in an increase in the first-order rate constant for the dissociation of the ferricytochrome c-cytochrome c oxidase complex, the rate-limiting step in the steady-state turnover of electrons between cytochrome c and cytochrome c oxidase in the spectrophotometric assay, yielding increases in the initial rate as well as the Michaelis constant--namely, multiple kinetic phases.
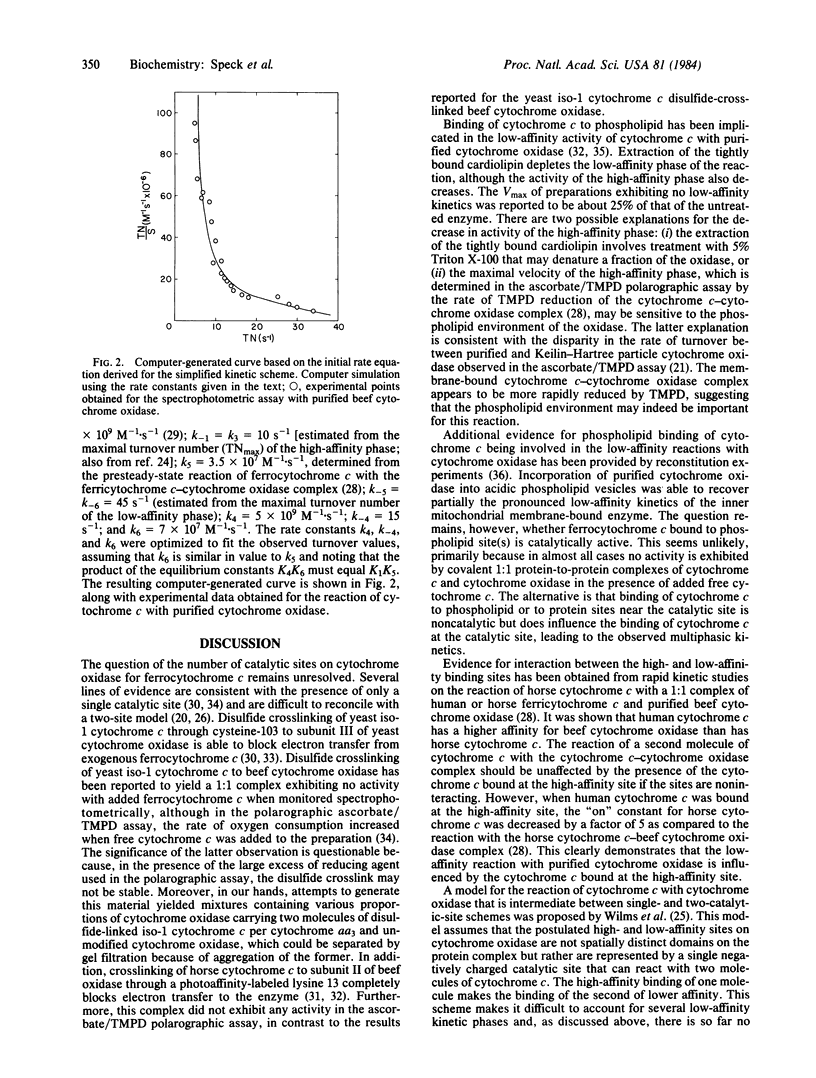
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antalis T. M., Palmer G. Kinetic characterization of the interaction between cytochrome oxidase and cytochrome c. J Biol Chem. 1982 Jun 10;257(11):6194–6206. [PubMed] [Google Scholar]
- BRODY T. M., WANG R. I. H., BAIN J. A. Intracellular distribution of diphosphopyridine nucleotide-cytochrome c reductase and cytochrome c oxidase in mammalian brain. J Biol Chem. 1952 Oct;198(2):821–826. [PubMed] [Google Scholar]
- Birchmeier W., Kohler C. E., Schatz G. Interaction of integral and peripheral membrane proteins: affinity labeling of yeast cytochrome oxidase by modified yeast cytochrome c. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4334–4338. doi: 10.1073/pnas.73.12.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson R., Azzi A., Gutweniger H., Colonna R., Montecucco C., Zanotti A. Interaction of cytochrome c with cytochrome c oxidase. Photoaffinity labeling of beef heart cytochrome c oxidase with arylazido-cytochrome c. J Biol Chem. 1978 Mar 25;253(6):1874–1880. [PubMed] [Google Scholar]
- Bisson R., Jacobs B., Capaldi R. A. Binding of arylazidocytochrome c derivatives to beef heart cytochrome c oxidase: cross-linking in the high- and low-affinity binding sites. Biochemistry. 1980 Sep 2;19(18):4173–4178. doi: 10.1021/bi00559a006. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Mitochondrial cytochrome c: preparation and activity of native and chemically modified cytochromes c. Methods Enzymol. 1978;53:128–164. doi: 10.1016/s0076-6879(78)53021-8. [DOI] [PubMed] [Google Scholar]
- CONRAD H., SMITH L. A study of the kinetics of the oxidation of cytochrome c by cytochrome c oxidase. Arch Biochem Biophys. 1956 Aug;63(2):403–413. doi: 10.1016/0003-9861(56)90055-8. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Errede B., Haight G. P., Jr, Kamen M. D. Oxidation of ferrocytochrome c by mitochondrial cytochrome c oxidase. Proc Natl Acad Sci U S A. 1976 Jan;73(1):113–117. doi: 10.1073/pnas.73.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errede B., Kamen M. D. Comparative kinetic studies of cytochromes c in reactions with mitochondrial cytochrome c oxidase and reductase. Biochemistry. 1978 Mar 21;17(6):1015–1027. doi: 10.1021/bi00599a012. [DOI] [PubMed] [Google Scholar]
- FRAJOLA W. J., VESTLING C. S. A kinetic study of cytochrome oxidase. J Biol Chem. 1954 Aug;209(2):677–686. [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976 Feb 25;251(4):1104–1115. [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J Biol Chem. 1978 Jan 10;253(1):149–159. [PubMed] [Google Scholar]
- Fuller S. D., Darley-Usmar V. M., Capaldi R. A. Covalent complex between yeast cytochrome c and beef heart cytochrome c oxidase which is active in electron transfer. Biochemistry. 1981 Nov 24;20(24):7046–7053. doi: 10.1021/bi00527a043. [DOI] [PubMed] [Google Scholar]
- HESS H. H., POPE A. Ultramicrospectrophotometric determination of cytochrome oxidase for quantitative histochemistry. J Biol Chem. 1953 Sep;204(1):295–306. [PubMed] [Google Scholar]
- HOGEBOOM G. H., SCHNEIDER W. C. Cytochemical studies. IV. Physical state of certain respiratory enzymes of mitochondria. J Biol Chem. 1952 Feb;194(2):513–519. [PubMed] [Google Scholar]
- Hartzell C. R., Beinert H. Components of cytochrome c oxidase detectable by EPR spectroscopy. Biochim Biophys Acta. 1974 Dec 19;368(3):318–338. doi: 10.1016/0005-2728(74)90178-9. [DOI] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Effect of haematin on the oxidation of succinic acid by tissue preparations. Biochem J. 1947;41(4):503–506. doi: 10.1042/bj0410503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINNAERT K. The kinetics of cytochrome c oxidase. I. The system: cytochrome c-cytochrome oxidase-oxygen. Biochim Biophys Acta. 1961 Jun 10;50:23–34. doi: 10.1016/0006-3002(61)91055-1. [DOI] [PubMed] [Google Scholar]
- Mochan E., Nicholls P. Cytochrome c reactivity in its complexes with mammalian cytochrome c oxidase and yeast peroxidase. Biochim Biophys Acta. 1972 May 25;267(2):309–319. doi: 10.1016/0005-2728(72)90119-3. [DOI] [PubMed] [Google Scholar]
- Moreland R. B., Dockter M. E. Interaction of the "back" of yeast iso-1-cytochrome C with yeast cytochrome C oxidase. Biochem Biophys Res Commun. 1981 Mar 16;99(1):339–346. doi: 10.1016/0006-291x(81)91750-2. [DOI] [PubMed] [Google Scholar]
- Osheroff N., Brautigan D. L., Margoliash E. Definition of enzymic interaction domains on cytochrome c. Purification and activity of singly substituted carboxydinitrophenyl-lysine 7, 25, 73, 86, and 99 cytochromes c. J Biol Chem. 1980 Sep 10;255(17):8245–8251. [PubMed] [Google Scholar]
- Osheroff N., Speck S. H., Margoliash E., Veerman E. C., Wilms J., König B. W., Muijsers A. O. The reaction of primate cytochromes c with cytochrome c oxidase. Analysis of the polarographic assay. J Biol Chem. 1983 May 10;258(9):5731–5738. [PubMed] [Google Scholar]
- Rosevear P., VanAken T., Baxter J., Ferguson-Miller S. Alkyl glycoside detergents: a simpler synthesis and their effects on kinetic and physical properties of cytochrome c oxidase. Biochemistry. 1980 Aug 19;19(17):4108–4115. doi: 10.1021/bi00558a032. [DOI] [PubMed] [Google Scholar]
- Slater E. C. The measurement of the cytochrome oxidase activity of enzyme preparations. Biochem J. 1949;44(3):305–318. doi: 10.1042/bj0440305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L., Davies H. C., Nava M. E. Studies of the kinetics of oxidation of cytochrome c by cytochrome c oxidase: comparison of spectrophotometric and polarographic assays. Biochemistry. 1979 Jul 10;18(14):3140–3146. doi: 10.1021/bi00581a035. [DOI] [PubMed] [Google Scholar]
- Veerman E. C., Wilms J., Casteleijn G., Van Gelder B. F. The pre-steady state reaction of ferrocytochrome c with the cytochrome c-cytochrome aa3 complex. Biochim Biophys Acta. 1980 Mar 7;590(1):117–127. doi: 10.1016/0005-2728(80)90151-6. [DOI] [PubMed] [Google Scholar]
- Veerman E. C., Wilms J., Dekker H. L., Muijsers A. O., van Buuren K. J., van Gelder B. F., Osheroff N., Speck S. H., Margoliash E. The presteady state reaction of chemically modified cytochromes c with cytochrome oxidase. J Biol Chem. 1983 May 10;258(9):5739–5745. [PubMed] [Google Scholar]
- Vik S. B., Georgevich G., Capaldi R. A. Diphosphatidylglycerol is required for optimal activity of beef heart cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1456–1460. doi: 10.1073/pnas.78.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms J., Dekker H. L., Boelens R., van Gelder B. F. The effect of pH and ionic strength on the pre-steady-state reaction of cytochrome c and cytochrome aa3. Biochim Biophys Acta. 1981 Aug 12;637(1):168–176. doi: 10.1016/0005-2728(81)90223-1. [DOI] [PubMed] [Google Scholar]
- Wilms J., Veerman E. C., König B. W., Dekker H. L., van Gelder B. F. Ionic strength effects on cytochrome aa3 kinetics. Biochim Biophys Acta. 1981 Mar 12;635(1):13–24. doi: 10.1016/0005-2728(81)90003-7. [DOI] [PubMed] [Google Scholar]
- YONETANI T., RAY G. S. STUDIES ON CYTOCHROME OXIDASE. VI. KINETICS OF THE AEROBIC OXIDATION OF FERROCYTOCHROME C BY CYTOCHROME OXIDASE. J Biol Chem. 1965 Aug;240:3392–3398. [PubMed] [Google Scholar]


