Abstract
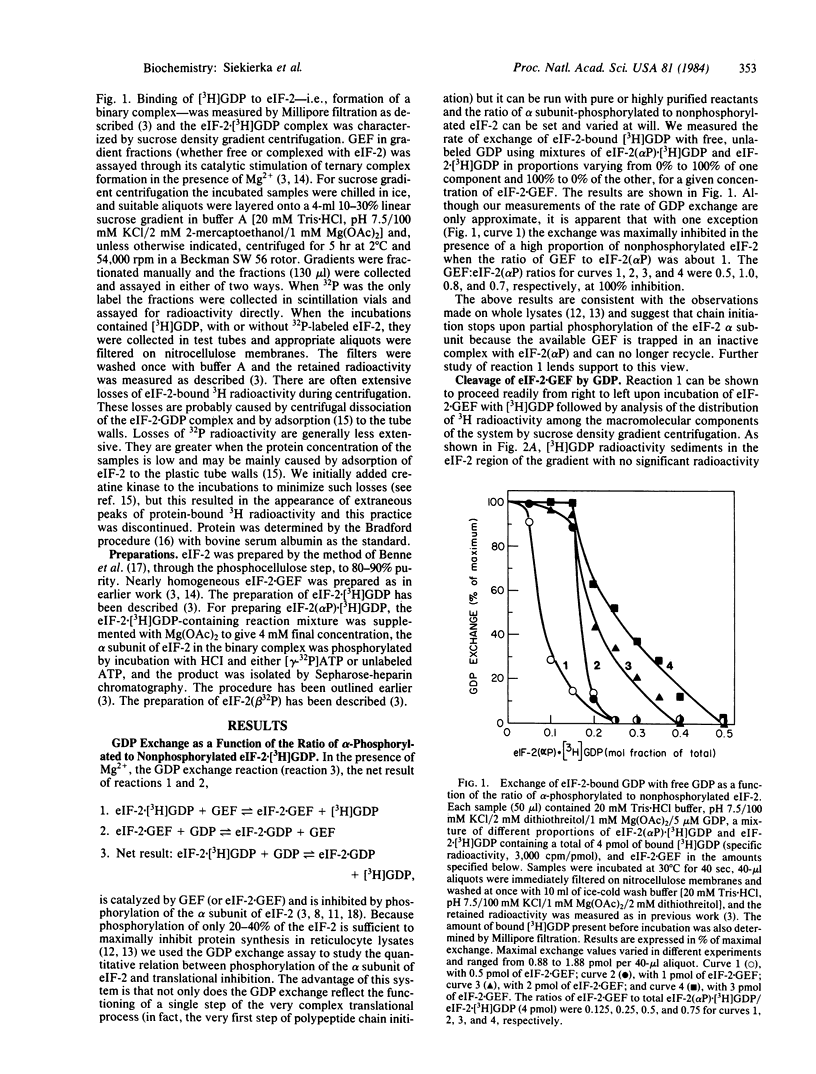
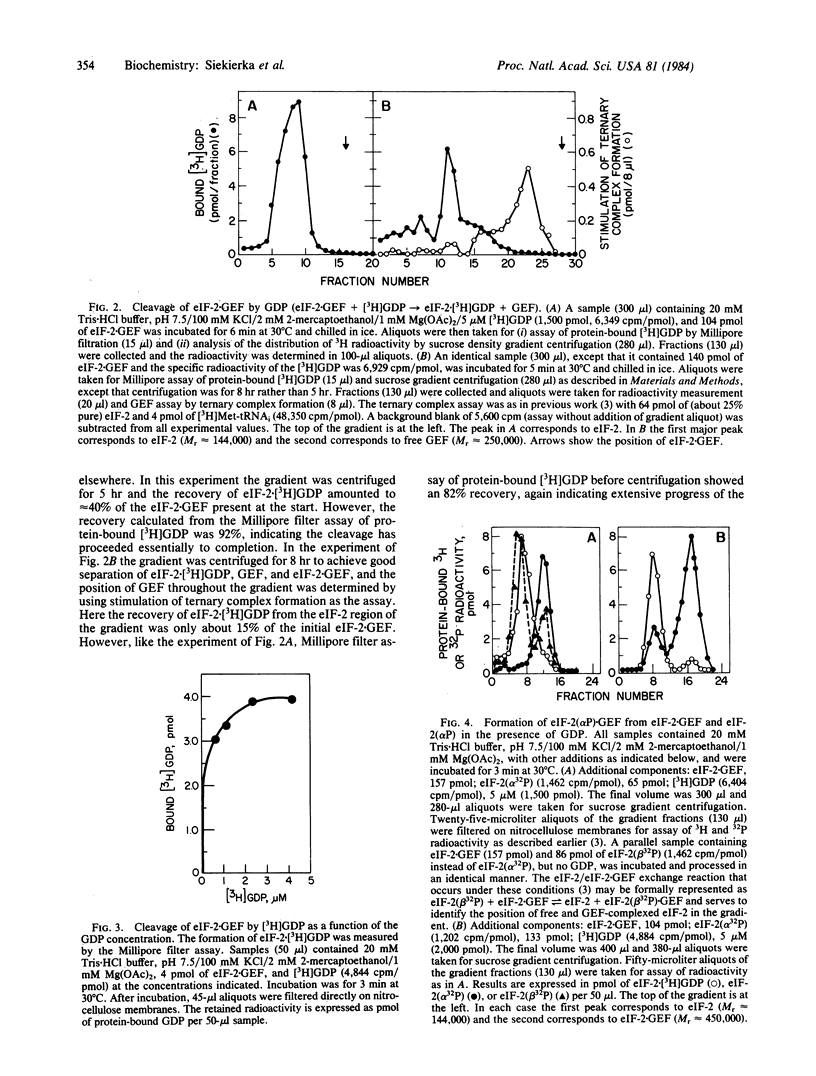
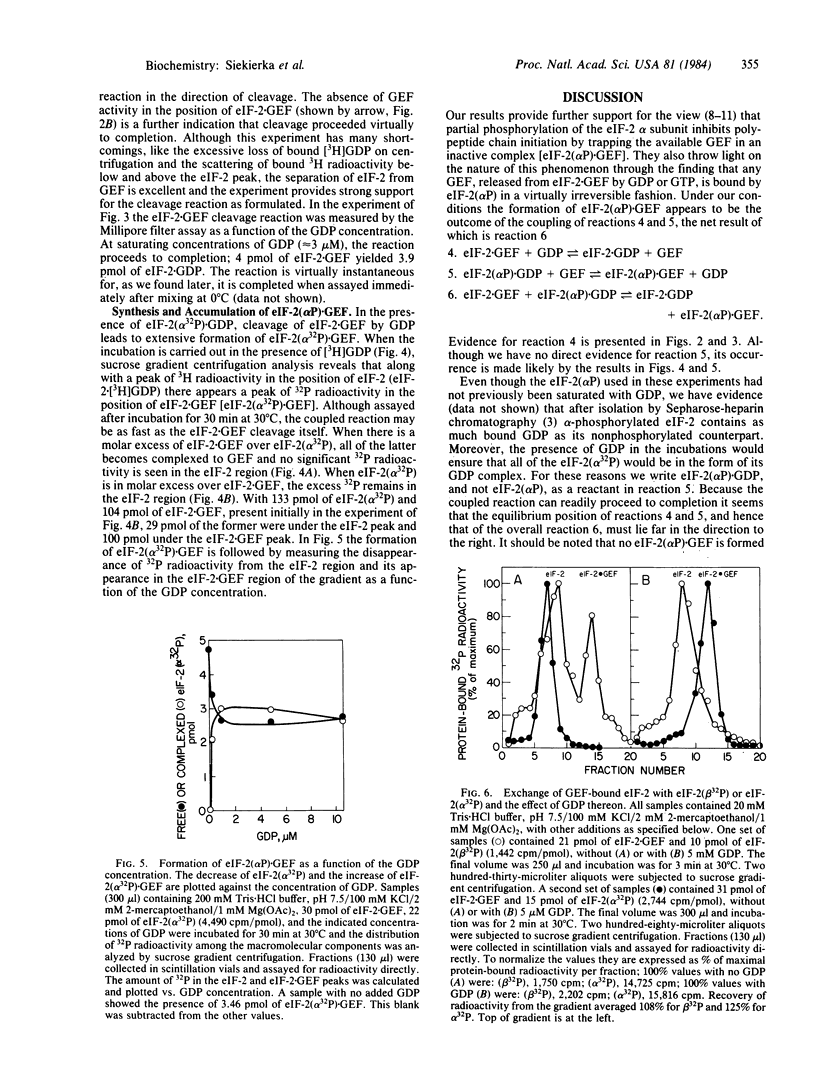
Catalysis of ternary complex formation by the GDP exchange factor (GEF), in the presence of Mg2+, is blocked by phosphorylation of the alpha subunit of the eukaryotic initiation factor 2 (eIF-2). We proposed earlier that this phosphorylation interferes with the interaction between eIF-2 and GEF (then termed ESP). If so, inhibition should be related to the extent of phosphorylation. However, work in other laboratories indicated that in fully inhibited, heme-deficient lysates only 20-40% of the eIF-2 is phosphorylated. To understand the nature of the molecular lesion in eIF-2-alpha phosphorylation we used a system of pure components in which the rate of exchange of eIF-2-bound [3H]GDP with unlabeled GDP (via the reaction eIF-2-GDP + GEF in equilibrium eIF-2-GEF + GDP) was measured by using mixtures of eIF-2(alpha P) X [eH]GDP and eIF-2 X [3H]GDP in different proportions at constant concentration of eIF-2 X GEF. If, for example, the ratio of eIF-2 X GEF to total (phosphorylated and unphosphorylated) eIF-2 X [3H]GDP was 0.25, the exchange was found to be maximally inhibited when the proportion of eIF-2(alpha P) X [3H]GDP in hte mixture reached 25%. This suggests that the reaction stops because the available GEF is trapped in an inactive complex with eIF-2(alpha P). In the absence of free GEF, eIF-2 would not be able to recycle and initiation would come to a standstill when the available eIF-2 is tied up as eIF-2 X GDP. The trapping of GEF by eIF-s(alpha P) is strongly supported by the following observation. Incubation of eIF-2 X GEF with excess [3H]GDP leads to the formation of eIF-2 X [3H] GDP and free GEF and, if eIF-2(alpha 32P) X GDP is also present, all of the GEF is converted to eIF-2(alpha 32P) X GEF. This suggests that, whereas the equilibrium of the reaction eIF-2 X GEF + GDP in equilibrium eIF-2 X GDP + GEF favors the formation of free GEF, the equilibrium of the reaction eIF-2(alpha P) X GDP + GEF in equilibrium eIF-2(alpha P) X GEF + GDP is in favor of the association of GEF to eIF-2(alpha P).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Amesz H., Hershey J. W., Voorma H. O. The activity of eukaryotic initiation factor eIF-2 in ternary complex formation with GTP and Met-tRNA. J Biol Chem. 1979 May 10;254(9):3201–3205. [PubMed] [Google Scholar]
- Benne R., Wong C., Luedi M., Hershey J. W. Purification and characterization of initiation factor IF-E2 from rabbit reticulocytes. J Biol Chem. 1976 Dec 10;251(23):7675–7681. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Pain V. M., Wong S. T., Henshaw E. C. Phosphorylation inhibits guanine nucleotide exchange on eukaryotic initiation factor 2. Nature. 1982 Mar 4;296(5852):93–95. doi: 10.1038/296093a0. [DOI] [PubMed] [Google Scholar]
- Leroux A., London I. M. Regulation of protein synthesis by phosphorylation of eukaryotic initiation factor 2 alpha in intact reticulocytes and reticulocyte lysates. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2147–2151. doi: 10.1073/pnas.79.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts R. L., Levin D. H., London I. M. Effect of phosphorylation of the alpha-subunit of eukaryotic initiation factor 2 on the function of reversing factor in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1983 May;80(9):2559–2563. doi: 10.1073/pnas.80.9.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa S. Regulation of protein synthesis initiation in eucaryotes. Arch Biochem Biophys. 1983 Jun;223(2):325–349. doi: 10.1016/0003-9861(83)90598-2. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Manne V., Mauser L., Ochoa S. Polypeptide chain initiation in eukaryotes: reversibility of the ternary complex-forming reaction. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1232–1235. doi: 10.1073/pnas.80.5.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mauser L., Ochoa S. Mechanism of polypeptide chain initiation in eukaryotes and its control by phosphorylation of the alpha subunit of initiation factor 2. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2537–2540. doi: 10.1073/pnas.79.8.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mitsui K. I., Ochoa S. Mode of action of the heme-controlled translational inhibitor: relationship of eukaryotic initiation factor 2-stimulating protein to translation restoring factor. Proc Natl Acad Sci U S A. 1981 Jan;78(1):220–223. doi: 10.1073/pnas.78.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Regulation of ternary (Met-tRNAf - GTP - eukaryotic initiation factor 2) protein synthesis initiation complex formation by the adenylate energy charge. Biochim Biophys Acta. 1976 Jan 19;418(2):195–203. doi: 10.1016/0005-2787(76)90069-1. [DOI] [PubMed] [Google Scholar]
- de Haro C., Datta A., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1978 Jan;75(1):243–247. doi: 10.1073/pnas.75.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Further studies on the mode of action of the heme-controlled translational inhibitor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1741–1745. doi: 10.1073/pnas.76.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis: studies with factors from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2713–2716. doi: 10.1073/pnas.75.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]



