Abstract
Human voltage-activated sodium (Nav) channels are adept at rapidly transmitting electrical signals across long distances in various excitable tissues. As such, they are amongst the most widely targeted ion channels by drugs and animal toxins. Of the nine isoforms, Nav1.8 and Nav1.9 are preferentially expressed in DRG neurons where they are thought to play an important role in pain signaling. Although the functional properties of Nav1.8 have been relatively well characterized, difficulties with expressing Nav1.9 in established heterologous systems limit our understanding of the gating properties and toxin pharmacology of this particular isoform. This review summarizes our current knowledge of the role of Nav1.8 and Nav1.9 in pain perception and elaborates on the approaches used to identify molecules capable of influencing their function.
Keywords: Nav1.8, Nav1.9, pain, animal toxins, voltage sensor, voltage-activated sodium channel
1. Introduction
Of the nine voltage-activated sodium (Nav) channel isoforms found in humans (Nav1.1–1.9), Nav1.9 remains the least understood [1]. More than a decade ago, the slowly activating and inactivating ionic currents generated by Nav1.9 were discovered in dorsal root ganglia (DRG) neurons [2] where it appears alongside the similarly slow currents produced by Nav1.8 [3,4,5,6]. Subsequent experiments suggest that these two Nav channel isoforms may also be expressed in tissues such as enteric neurons [7,8], heart [9], retina [10], dental pulp [11], and trigeminal ganglia [11,12]; however, the strategic presence of Nav1.8 and Nav1.9 in nociceptive DRG neurons inspired various genetic approaches geared towards exploring their role in pain signaling [6,13,14,15,16,17,18,19]. While such studies are valuable for revealing the physiological role of Nav channels, these methods are of limited use for discovering pharmacological tools that evoke specific responses from these channels. An approach that has been successfully employed to investigate the functional properties of ion channels is to express them in heterologous systems and record ionic currents using electrophysiological techniques [20,21,22,23]. Although Nav1.8 can be functionally expressed in Xenopus oocytes or mammalian cell lines [24,25] (albeit not abundantly), fundamental questions about the function and pharmacological sensitivities of Nav1.9 remain unanswered because previous attempts to express this channel in heterologous systems have been unsubstantiated [15]. In addition, studying Nav1.9-mediated currents in native DRG neurons is technically challenging because only a fraction of isolated neurons produce a measurable amount [24,25] and other Nav channel isoforms, such as Nav1.8, interfere with these measurements since they activate over a similar voltage range [26,27]. Despite the existing Nav1.9 expression difficulties, creative approaches have generated insights into its functional properties and revealed molecules that interfere with its gating mechanism. This review will highlight these approaches as well as the compounds found to influence Nav1.8 and Nav1.9.
2. The Role of Nav1.8 and Nav1.9 in Pain
Given the abundant expression of Nav1.8 and Nav1.9 in sensory neurons, multiple studies with genetically altered mice have provided important insights into the physiological roles of these Nav channel isoforms in pain perception [6,13,14,15,16,17,18]. (Nav1.3 and Nav1.7 are also thought to be involved in nociception [16] but fall beyond the scope of this review.) Knockout mice [13,17,28], as well as siRNA and antisense deoxynucleotide studies [29] suggest a contribution of Nav1.8 to inflammatory pain, neuropathic pain and response to noxious stimuli [13,28,30,31] whereas Nav1.9 knockout mice have a largely absent inflammatory hyperalgesia in response to inflammatory mediators [6,14,17,32]. In addition, behavioral assays on these mice implicate a role for Nav1.9 in the development of visceral mechanical hypersensitivity associated with acute inflammation [33]. Although Nav1.8 was reported to be critical for the perception of cold pain [18], it was recently shown that Nav1.9 also has a crucial task in the pathogenesis of neuropathic pain, and specifically in the development of cold, but not mechanical allodynia [17]. Bearing in mind the potential limitations of the various models used, contradictory results were obtained by intraplantar carrageenan injection tests which revealed a reduced inflammatory-induced mechanical hypersensitivity in Nav1.9-/- mice [34].
Detailed electrophysiological measurements on isolated sensory neurons suggest that Nav1.9 is unique in that it underlies the persistent sodium current in small diameter DRG neurons [26,27] (Figure 1a) that may drive spontaneous discharge during inflammation and that as such, unique DRG neuron properties such as subthreshold electrogenesis or oscillatory bursting discharges are absent in Nav1.9 knockout mice [3]. In addition, it was demonstrated that inflammatory mediators can dynamically regulate putative Nav1.9 currents in wild-type DRG neurons isolated from mice [3,5,6]. It is this apparent critical role in pain sensitivity that makes Nav1.8 and Nav1.9 desirable drug targets. Therefore, the discovery of molecules capable of modulating the slow currents of these particular Nav channel isoforms will be of great value to pharmacologically dissect their physiological role in wild-type DRG neurons. To this end, challenges associated with identifying and recording Nav1.8 and Nav1.9 currents must be addressed.
3. Current Approaches for Studying Nav1.8 and Nav1.9 Function
One way to investigate the underlying molecular mechanisms that govern Nav channel gating is to remove the channel from its native environment, express it in heterologous systems such as Xenopus oocytes or mammalian cells, and record its ionic current in isolation. With varying degrees of success, this approach has been effectively employed for almost all Nav channel isoforms, yet functional expression of Nav1.9 remains a challenge [15,35,36]. Although successful recordings of Nav1.9 ionic currents in a mammalian cell line have been reported [37], the results have yet to be substantiated. Challenges of a different sort arise when attempting to measure Nav1.9-mediated currents in native tissues [4]. For example, Nav1.9 expression varies greatly between different types of DRG neurons [14,38], with most successful recordings originating from small-diameter (≤30 µm) capsaicin-sensitive neurons [39] (Figure 1a). Furthermore, the presence of ionic currents generated by Nav1.8 (Figure 1a) interferes with the identification of those produced by Nav1.9 as both isoforms are active over a similar membrane voltage range and selective inhibition of Nav1.8 with a pharmacological agent is difficult to accomplish [2,4,25,27]. To sidestep these technical limitations, various groups have come up with inventive solutions. For instance, the majority of Nav1.9 gating data was obtained by recordings from Nav1.8 knockout mice DRG neurons in which only the Nav1.9-mediated persistent current is present (in combination with exposure to tetrodotoxin to inhibit other Nav channel subtypes—see next section). Alternatively, researchers interested in exploring the functional properties of Nav1.9 in wild-type DRG neurons may add fluoride to the intracellular solution, thereby shifting the Nav1.9 gating characteristics to more hyperpolarized voltages [3,4,14,40]. Because Nav1.8 gating is essentially unaltered upon fluoride application, this method results in a narrow voltage window in which Nav1.9-generated sodium currents can be examined (Figure 1a). Although clever, this approach does require holding the membrane at voltages that are physiologically irrelevant. It is also possible to transfect the human (h)Nav1.9 clone into DRG neurons of a Nav1.9 knockout mouse, resulting in the restoration of certain pain sensations in addition to spontaneous firing patterns attributed to Nav1.9 function [5]. While promising, this approach suffers from the large volume of work required to produce, identify, and measure hNav1.9-expressing neurons.
However, as a result of all these efforts a body of work is now available that highlights the unique functional characteristics of Nav1.9. Compared to other Nav channel isoforms, Nav1.9-generated currents in DRG neurons reveal ultraslow activation and inactivation kinetics (Figure 1a) which is likely the product of a substantially different amino acid sequence, particularly in the voltage-sensing regions [25,41]. In addition, activation and steady-state inactivation relationships display significant overlap, suggesting a role for Nav1.9 in establishing the membrane voltage which may in turn influence gating of other Nav channel isoforms present in DRG neurons and thus, overall neuronal excitability [2]. As a result of its slow gating characteristics, it is generally agreed that Nav1.9 is not directly responsible for action potential generation. A potential mechanism contributing to the unique Nav1.9 gating process was reported recently when an established protein engineering approach was adapted to circumvent heterologous expression obstacles [25]. By identifying and transplanting structural motifs from the putative voltage sensor in each of the four Nav1.9 domains (I–IV) into the corresponding position in four-fold symmetric voltage-activated potassium (Kv) channels (Figure 1b), it became possible to investigate the individual contribution of these motifs to channel gating. One of the most intriguing results of this study is that Kv channels containing defined Nav1.9 voltage-sensing regions from domains II, III, and IV exhibit slow kinetics comparable to those observed for domain IV from Nav1.2 and Nav1.4. This suggests that the distinct gating characteristics of Nav1.9 observed in DRG neurons may result from slow activation of its voltage sensors. Notwithstanding the importance of identifying structural motifs within Nav1.9 voltage sensors that modulate overall channel function, other regions undoubtedly influence channel function as well. Therefore, heterologous expression of the full-length channel remains highly desirable. Nonetheless, a chimeric approach may be a valuable tool to discover molecules such as animal toxins that can influence Nav1.9 gating through an interaction with one or more voltage sensors.
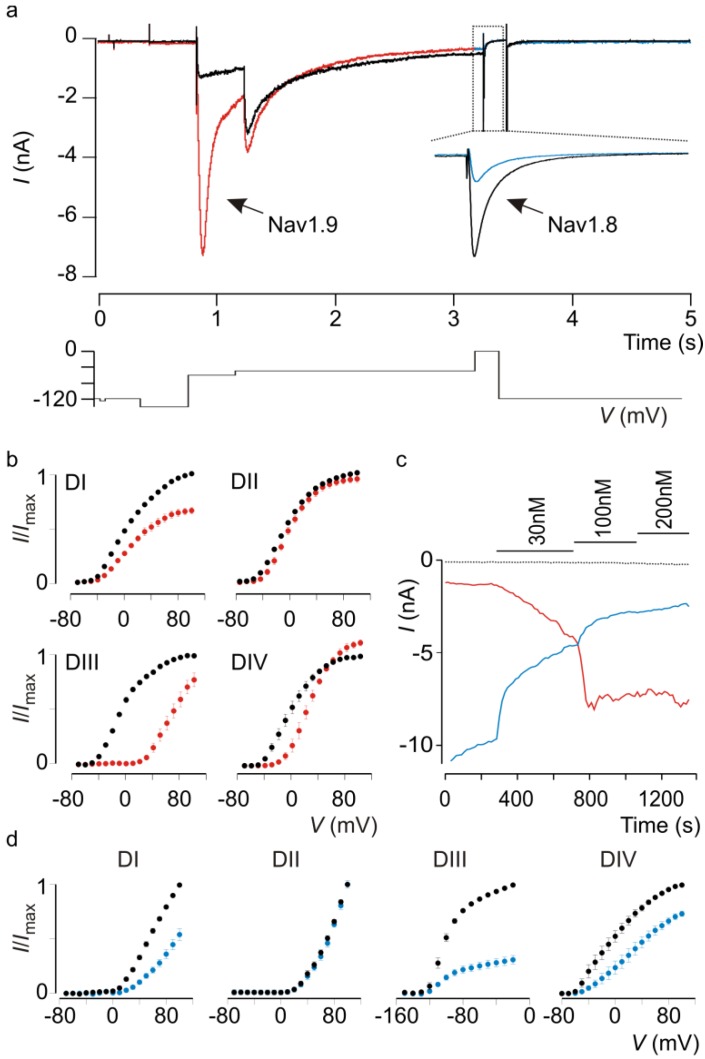
Figure 1.
The tarantula toxin ProTx-I influences Nav1.8 and Nav1.9 currents in rat DRG neurons.
Effect of ProTx-I on Nav1.9 and Nav1.8 currents in rat DRG neurons and chimeric ratNav1.9/Kv2.1 constructs. (a) Evoked currents in control conditions (black) and after addition of 100 nM ProTx-I (red for Nav1.9, blue for Nav1.8). Nav1.9 currents are greatly potentiated whereas Nav1.8 currents are strongly inhibited. Time scale for Nav1.9 currents is indicated in the X-axis. Voltage protocol used to elicit both Nav1.9 (first depolarization to −55 mV) and Nav1.8 currents (second depolarization to 0 mV) in a rat DRG neuron is shown below the trace. For clarity purposes, insert showing Nav1.8 inhibition by ProTx-I was obtained at 0mV from a DRG neuron held at −50 mV until Nav1.9 currents were fully inactivated. (Experiments performed by Michelino Puopolo, Stony Brook Medicine). (b) Effect of ProTx-I on chimeric constructs where paddle motifs were transferred from ratNav1.9 into ratKv2.1. Normalized tail current voltage-activation relationships are shown where tail current amplitude is plotted against test voltage before (black) and in the presence of toxin (red). Concentration used is 100 nM. The holding voltage was −90 mV, the test pulse duration was 300 ms, and the tail voltage was −60 mV. n = 3, and error bars represent SEM. (c) Concentration-dependent effect of ProTx-I on Nav1.9 currents (red) at −55 mV and Nav1.8 currents (blue) at 0 mV recorded in DRG neurons. Dotted trace represents leak current measured at −120 mV. Time scale is indicated on the X-axis. (d) Effect of ProTx-I on chimeric constructs where paddle motifs were transferred from ratNav1.8 into ratKv2.1. Normalized tail current voltage-activation relationships are shown where tail current amplitude is plotted against test voltage before (black) and in the presence of toxin (blue). Concentration used is 100 nM. n = 4, and error bars represent SEM.
4. Pharmacological Properties of Nav1.8 and Nav1.9
Nav1.8 channels interact with various commercially available compounds such as menthol [42], lidocaine [43], tetracaine [44], vinpocetine [45], ambroxol [46], lamotrigine [44], mexilitine [47], veratridine [48], and A-803467 [49], whereas very few animal toxins have been shown to be capable of reshaping Nav1.8 currents. ProTx-I and ProTx-II from the Thrixopelma pruriens tarantula were among the first spider toxins to be characterized as potent inhibitors of Nav1.8 opening [50], possibly by stabilizing one or more of its voltage sensors in the closed state [25,51,52] (Figure 1a,c,d). In 2006, CcoTx1, CcoTx2, and CcoTx3 from the Ceratogyrus cornuatus tarantula and PaurTx3 from the Phrixotrichus auratus tarantula were reported to inhibit rat Nav1.8 currents, albeit at high concentrations [53]. Although other spider toxins such as JZTX-V [54] and JZTX-IX [55] from the Chinese spider Chilobrachys jingzhao may also inhibit Nav1.8, their potential inhibitory effects have yet to be confirmed in heterologous systems. The µO-conotoxin MrVIB is a well-characterized Nav1.8 blocker isolated from the venom of the cone snail Conus marmoreus and is capable of drastically reducing Nav1.8-generated currents at nanomolar concentrations [56,57]. Although the toxin does not selectively target Nav1.8, intrathecal infusion of MrVIB in rat inflammatory pain models reduces allodynia and hyperalgesia [57]. Mutagenesis experiments on various Nav channel isoforms suggest that MrVIB exerts its effect by associating with pore loops in domain II and domain III within Nav1.8 and Nav1.4, respectively [58,59]. Finally, Nav1.8 function is profoundly modulated by batrachotoxin, an extremely potent toxin found in the skin secretions of poison dart frogs and certain birds endemic to New Guinea [60]. Mechanistic studies on several Nav channel variants suggest that batrachotoxin may interact with residues within the pore or at the channel-lipid interface to cause persistent channel activation [61,62]. Although most of the aforementioned toxins could prove to be promising lead compounds for drug design, one caveat is that their efficacy on the hNav1.8 variant remains to be determined.
Compared to other Nav channel isoforms, the discovery of ligands capable of influencing Nav1.9 function has been hindered by the absence of a reliable heterologous expression system. However, studies on wild-type Nav1.9 channels expressed in neuronal tissues from various animals have revealed a variety of endogenous molecules that can influence channel function. Pertinent to its role in pain perception, inflammatory mediators such as PGE2 and serotonin [6,63] as well as secreted proteins that augment neuronal survival (e.g., brain-derived neurotrophic factor (BDNF) [35] and glial-derived neurotrophic factor (GDNF) [64,65]) have been reported to increase Nav1.9 currents in DRG neurons. While these compounds may individually influence Nav1.9 expression or through a G-protein coupled mechanism [6], a “soup” consisting of multiple inflammatory mediators such as bradykinin, histamine, PGE3 and norepinephrine can also act together to achieve a similar increase in Nav1.9-mediated currents [3]. Finally, the neurokinin-3 receptor agonist, senktide, was shown to potentiate Nav1.9 currents in guinea pig enteric afferent neurons [66], yet the mechanism underlying this observation or its translation to human DRG neurons has not been investigated.
Although peptides isolated from venomous organisms have proven their value in probing the role of ion channels in neuronal excitability and to gain insight into their intricate gating machinery [67], the exploration of Nav1.9 toxin sensitivity is still in its infancy. Tetrodotoxin (TTX) and saxitoxin (STX) are naturally occurring guanidinium toxins that interact potently with the Nav channel pore region and occlude the sodium ion permeation pathway [68]. TTX is widely used to divide the Nav channel family into two groups based on their sensitivity towards the toxin; TTX-sensitive channels (Nav1.1–Nav1.4, Nav1.6–Nav1.7) are inhibited by nanomolar concentrations whereas Nav1.8 and Nav1.9 require millimolar amounts to be blocked completely (Nav1.5 requires micromolar concentrations to be blocked). Interestingly, it was shown on one occasion that nanomolar concentrations of STX can reversibly block BDNF-evoked TTX-resistant sodium currents in CA1 pyramidal neurons and SH-SY5Y neuroblastoma cells [35], suggesting that Nav1.9 may be sensitive to this toxin. However, similar STX concentrations did not inhibit TTX-resistant currents in nociceptive neurons [6]. µ-conotoxins, a subset of conotoxins isolated from cone snail venoms, have been shown to compete with TTX and STX and inhibit ion flow through Nav channels by interacting with the pore region [69]. Interestingly, two groups simultaneously reported that the µ-conotoxin µ-SIIIA from Conus striatus selectively inhibits TTX-resistant but not TTX-sensitive currents in rat DRG neurons [70,71]. Although the authors suggest that this toxin and relatives such as µ-KIIIA [70] and µ-SmIIIAs [72] may primarily inhibit Nav1.8, a potential inhibitory effect of these peptides on Nav1.9 opening should not be ruled out.
In recent work, chimeric constructs between Nav1.9 and various Kv channels were exploited to investigate the contribution of specific regions within Nav1.9 voltage sensors, the S3b-S4 paddle motifs, to forming animal toxin receptors [25]. These paddle motifs were originally identified in Kv channels in which their role in driving voltage sensor activation and subsequent opening of the pore makes them a prime target for tarantula toxins [73,74,75,76,77,78,79], possibly through a lipid-mediated mechanism [80,81,82]. Later, it was shown that paddle motifs are also an integral part of the voltage sensors in Nav1.2 and Nav1.4 where they are extensively targeted by a variety of toxin families [51]. Since the pharmacological sensitivities of Nav1.9 remain largely unexplored, chimeric constructs were employed to search for toxins that might interact with Nav1.9 channels (Figure 1b). To this end, eighteen toxins from tarantula, scorpion and sea anemone venom were screened against four Nav1.9/Kv2.1 paddle constructs. The two most interesting toxins that emerged were the scorpion toxin TsVII from Tityus serrulatus and the tarantula toxin ProTx-I, both of which interact strongly with Nav1.9 paddle motifs (Figure 1b) and potently facilitate the slowly activating and inactivating Nav1.9-mediated current in rat DRG neurons (Figure 1a). In addition to targeting Nav1.9, TsVII and ProTx-I have very different actions on Nav1.8; TsVII produces a concentration-dependent facilitation of Nav1.9 currents while only marginally inhibiting Nav1.8, whereas ProTx-I causes both a pronounced potentiation of Nav1.9 currents and a robust concentration-dependent inhibition of Nav1.8, thereby indicating that this toxin may be a formidable tool to discriminate between sodium currents generated by these two channel isoforms in DRG neurons (Figure 1a,c). Of interest is that both toxins possess a different paddle target profile when comparing the rat and human isoform. In addition, ProTx-I targets different voltage sensors in Nav1.2, suggesting that Nav1.9 has its own unique pharmacological profile that may be advantageous for drug design.
5. Future Challenges
Although scientists have made astonishing progress in understanding the mechanisms and various types of pain, many gaps still remain and developing more effective pain relievers continues to be a major challenge. In particular, the enigmatic Nav channel Nav1.9 constitutes one of the most elusive pain targets, mainly due to various complications associated with identifying its sodium current in native neurons and the lack of reliable heterologous expression systems. In contrast, Nav1.8 can be expressed in Xenopus oocytes and mammalian cell lines; however, it can be challenging to obtain sodium current densities that are practical for conducting experiments. Moreover, Nav1.8 seems to be quite insensitive to animal toxins capable of inhibiting other Nav channel isoforms at low concentrations, possibly resulting from its unique amino acid composition of the paddle region. As a result, the search for and characterization of pharmacological agents that influence Nav1.8 and Nav1.9 action is severely lagging when compared to other Nav channel isoforms. However, various creative approaches have led to the discovery of endogenous molecules and animal toxins capable of altering the function of Nav1.8 and Nav1.9. With the advent of novel approaches to heterologous expression problems and high-throughput screening, it may be a matter of time before these intriguing Nav channels become readily available for detailed mechanistic studies of their unique gating properties and for screening animal venoms from which toxins that reshape channel activity can be isolated.
Acknowledgments
We thank Bruce Bean (Harvard Medical School, USA) and Michelino Puopulo (Stony Brook Medicine, USA) for making the DRG data available and Julie Kaae Klint for helpful discussions. This work was supported by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R00NS073797 to F.B. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Catterall W.A., Goldin A.L., Waxman S.G. International union of pharmacology. XlVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 2.Cummins T.R., Dib-Hajj S.D., Black J.A., Akopian A.N., Wood J.N., Waxman S.G. A novel persistent tetrodotoxin-resistant sodium current in sns-null and wild-type small primary sensory neurons. J. Neurosci. 1999;19:RC43. doi: 10.1523/JNEUROSCI.19-24-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maingret F., Coste B., Padilla F., Clerc N., Crest M., Korogod S.M., Delmas P. Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism. J. Gen. Physiol. 2008;131:211–225. doi: 10.1085/jgp.200709935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maruyama H., Yamamoto M., Matsutomi T., Zheng T., Nakata Y., Wood J.N., Ogata N. Electrophysiological characterization of the tetrodotoxin-resistant Na+ channel, Na(v)1.9, in mouse dorsal root ganglion neurons. Pflugers Arch. Eur. J. Physiol. 2004;449:76–87. doi: 10.1007/s00424-004-1315-0. [DOI] [PubMed] [Google Scholar]
- 5.Ostman J.A., Nassar M.A., Wood J.N., Baker M.D. Gtp up-regulated persistent Na+ current and enhanced nociceptor excitability require Nav1.9. J. Physiol. 2008;586:1077–1087. doi: 10.1113/jphysiol.2007.147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priest B.T., Murphy B.A., Lindia J.A., Diaz C., Abbadie C., Ritter A.M., Liberator P., Iyer L.M., Kash S.F., Kohler M.G., et al. Contribution of the tetrodotoxin-resistant voltage-gated sodium channel Nav1.9 to sensory transmission and nociceptive behavior. Proc. Natl. Acad. Sci. USA. 2005;102:9382–9387. doi: 10.1073/pnas.0501549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rugiero F., Mistry M., Sage D., Black J.A., Waxman S.G., Crest M., Clerc N., Delmas P., Gola M. Selective expression of a persistent tetrodotoxin-resistant Na+ current and Nav1.9 subunit in myenteric sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:2715–2725. doi: 10.1523/JNEUROSCI.23-07-02715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillsley K., Lin J.H., Stanisz A., Grundy D., Aerssens J., Peeters P.J., Moechars D., Coulie B., Stead R.H. Dissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null mice. J. Physiol. 2006;576:257–267. doi: 10.1113/jphysiol.2006.113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verkerk A.O., Remme C.A., Schumacher C.A., Scicluna B.P., Wolswinkel R., de Jonge B., Bezzina C.R., Veldkamp M.W. Functional Nav1.8 channels in intracardiac neurons: The link between scn10a and cardiac electrophysiology. Circ. Res. 2012;111:333–343. doi: 10.1161/CIRCRESAHA.112.274035. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien B.J., Caldwell J.H., Ehring G.R., Bumsted O’Brien K.M., Luo S., Levinson S.R. Tetrodotoxin-resistant voltage-gated sodium channels Na(v)1.8 and Na(v)1.9 are expressed in the retina. J. Comp. Neurol. 2008;508:940–951. doi: 10.1002/cne.21701. [DOI] [PubMed] [Google Scholar]
- 11.Wells J.E., Bingham V., Rowland K.C., Hatton J. Expression of Nav1.9 channels in human dental pulp and trigeminal ganglion. J. Endod. 2007;33:1172–1176. doi: 10.1016/j.joen.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Padilla F., Couble M.L., Coste B., Maingret F., Clerc N., Crest M., Ritter A.M., Magloire H., Delmas P. Expression and localization of the Nav1.9 sodium channel in enteric neurons and in trigeminal sensory endings: Implication for intestinal reflex function and orofacial pain. Mol. Cell. Neurosci. 2007;35:138–152. doi: 10.1016/j.mcn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Akopian A.N., Souslova V., England S., Okuse K., Ogata N., Ure J., Smith A., Kerr B.J., McMahon S.B., Boyce S., et al. The tetrodotoxin-resistant sodium channel sns has a specialized function in pain pathways. Nat. Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- 14.Amaya F., Wang H., Costigan M., Allchorne A.J., Hatcher J.P., Egerton J., Stean T., Morisset V., Grose D., Gunthorpe M.J., et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J. Neurosci. 2006;26:12852–12860. doi: 10.1523/JNEUROSCI.4015-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dib-Hajj S., Black J.A., Cummins T.R., Waxman S.G. NaN/Nav1.9: A sodium channel with unique properties. Trends Neurosci. 2002;25:253–259. doi: 10.1016/s0166-2236(02)02150-1. [DOI] [PubMed] [Google Scholar]
- 16.Dib-Hajj S.D., Cummins T.R., Black J.A., Waxman S.G. Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 2010;33:325–347. doi: 10.1146/annurev-neuro-060909-153234. [DOI] [PubMed] [Google Scholar]
- 17.Leo S., D’Hooge R., Meert T. Exploring the role of nociceptor-specific sodium channels in pain transmission using Nav1.8 and Nav1.9 knockout mice. Behav. Brain Res. 2010;208:149–157. doi: 10.1016/j.bbr.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann K., Leffler A., Babes A., Cendan C.M., Carr R.W., Kobayashi J., Nau C., Wood J.N., Reeh P.W. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- 19.Baker M.D., Wood J.N. Involvement of Na+ channels in pain pathways. Trends Pharmacol. Sci. 2001;22:27–31. doi: 10.1016/S0165-6147(00)01585-6. [DOI] [PubMed] [Google Scholar]
- 20.Gurdon J.B., Lane C.D., Woodland H.R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971;233:177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- 21.Neher E., Sakmann B., Steinbach J.H. The extracellular patch clamp: A method for resolving currents through individual open channels in biological membranes. Pflugers Arch. Eur. J. Physiol. 1978;375:219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- 22.Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. Eur. J. Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 23.Barnard E.A., Miledi R., Sumikawa K. Translation of exogenous messenger rna coding for nicotinic acetylcholine receptors produces functional receptors in xenopus oocytes. Proc. R. Soc. Lond. B Biol. Sci. 1982;215:241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- 24.Akopian A.N., Sivilotti L., Wood J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 25.Bosmans F., Puopolo M., Martin-Eauclaire M.F., Bean B.P., Swartz K.J. Functional properties and toxin pharmacology of a dorsal root ganglion sodium channel viewed through its voltage sensors. J. Gen. Physiol. 2011;138:59–72. doi: 10.1085/jgp.201110614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blair N.T., Bean B.P. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J. Neurosci. 2002;22:10277–10290. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coste B., Crest M., Delmas P. Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. J. Gen. Physiol. 2007;129:57–77. doi: 10.1085/jgp.200609665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr B.J., Souslova V., McMahon S.B., Wood J.N. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport . 2001;12:3077–3080. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- 29.Dong X.W., Goregoaker S., Engler H., Zhou X., Mark L., Crona J., Terry R., Hunter J., Priestley T. Small interfering RNA-mediated selective knockdown of Na(v)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience . 2007;146:812–821. doi: 10.1016/j.neuroscience.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 30.Joshi S.K., Mikusa J.P., Hernandez G., Baker S., Shieh C.C., Neelands T., Zhang X.F., Niforatos W., Kage K., Han P., et al. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. Pain. 2006;123:75–82. doi: 10.1016/j.pain.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Porreca F., Lai J., Bian D., Wegert S., Ossipov M.H., Eglen R.M., Kassotakis L., Novakovic S., Rabert D.K., Sangameswaran L., et al. A ccomparison of the potential role of the tetrodotoxin-insensitive sodium channels, PN3/SNS and NaN/SNS2, in rat models of chronic pain. Proc. Natl. Acad. Sci. USA. 1999;96:7640–7644. doi: 10.1073/pnas.96.14.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritter A.M., Martin W.J., Thorneloe K.S. The voltage-gated sodium channel Nav1.9 is required for inflammation-based urinary bladder dysfunction. Neurosci. Lett. 2009;452:28–32. doi: 10.1016/j.neulet.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 33.Martinez V., Melgar S. Lack of colonic-inflammation-induced acute visceral hypersensitivity to colorectal distension in Na(v)1.9 knockout mice. Eur. J. Pain. 2008;12:934–944. doi: 10.1016/j.ejpain.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Lolignier S., Amsalem M., Maingret F., Padilla F., Gabriac M., Chapuy E., Eschalier A., Delmas P., Busserolles J. Nav1.9 channel contributes to mechanical and heat pain hypersensitivity induced by subacute and chronic inflammation. PLoS One. 2011;6:e23083. doi: 10.1371/journal.pone.0023083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blum R., Kafitz K.W., Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Na(v)1.9. Nature. 2002;419:687–693. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- 36.Tate S., Benn S., Hick C., Trezise D., John V., Mannion R.J., Costigan M., Plumpton C., Grose D., Gladwell Z., et al. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat. Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- 37.Vanoye C.G., Ehring G.R., George A.L. Functional analysis of stably expressed human Nav1.9. Biophys. J. 2012;102:527. [Google Scholar]
- 38.Fang X., Djouhri L., Black J.A., Dib-Hajj S.D., Waxman S.G., Lawson S.N. The presence and role of the tetrodotoxin-resistant sodium channel Na(v)1.9 (NaN) in nociceptive primary afferent neurons. J. Neurosci. 2002;22:7425–7433. doi: 10.1523/JNEUROSCI.22-17-07425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Binshtok A.M., Bean B.P., Woolf C.J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 40.Coste B., Osorio N., Padilla F., Crest M., Delmas P. Gating and modulation of presumptive Nav1.9 channels in enteric and spinal sensory neurons. Mol. Cell. Neurosci. 2004;26:123–134. doi: 10.1016/j.mcn.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Herzog R.I., Cummins T.R., Waxman S.G. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J. Neurophysiol. 2001;86:1351–1364. doi: 10.1152/jn.2001.86.3.1351. [DOI] [PubMed] [Google Scholar]
- 42.Gaudioso C., Hao J., Martin-Eauclaire M.F., Gabriac M., Delmas P. Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain. 2012;153:473–484. doi: 10.1016/j.pain.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Leffler A., Reiprich A., Mohapatra D.P., Nau C. Use-dependent block by lidocaine but not amitriptyline is more pronounced in tetrodotoxin (TTX)-resistant Nav1.8 than in TTX-sensitive Na+ channels. J. Pharmacol. Exp. Ther. 2007;320:354–364. doi: 10.1124/jpet.106.109025. [DOI] [PubMed] [Google Scholar]
- 44.John V.H., Main M.J., Powell A.J., Gladwell Z.M., Hick C., Sidhu H.S., Clare J.J., Tate S., Trezise D.J. Heterologous expression and functional analysis of rat Nav1.8 (SNS) voltage-gated sodium channels in the dorsal root ganglion neuroblastoma cell line ND7-23. Neuropharmacology . 2004;46:425–438. doi: 10.1016/j.neuropharm.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X., Dong X.W., Crona J., Maguire M., Priestley T. Vinpocetine is a potent blocker of rat Nav1.8 tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp. Ther. 2003;306:498–504. doi: 10.1124/jpet.103.051086. [DOI] [PubMed] [Google Scholar]
- 46.Gaida W., Klinder K., Arndt K., Weiser T. Ambroxol, a Nav1.8-preferring Na(+) channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory pain. Neuropharmacology . 2005;49:1220–1227. doi: 10.1016/j.neuropharm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Dekker L.V., Daniels Z., Hick C., Elsegood K., Bowden S., Szestak T., Burley J.R., Southan A., Cronk D., James I.F. Analysis of human Nav1.8 expressed in SH-SY5Y neuroblastoma cells. Eur. J. Pharmacol. 2005;528:52–58. doi: 10.1016/j.ejphar.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 48.Farrag K.J., Bhattacharjee A., Docherty R.J. A comparison of the effects of veratridine on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in isolated rat dorsal root ganglion neurons. Pflugers Arch. Eur. J. Physiol. 2008;455:929–938. doi: 10.1007/s00424-007-0365-5. [DOI] [PubMed] [Google Scholar]
- 49.Jarvis M.F., Honore P., Shieh C.C., Chapman M., Joshi S., Zhang X.F., Kort M., Carroll W., Marron B., Atkinson R., et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc. Natl. Acad. Sci. USA. 2007;104:8520–8525. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Middleton R.E., Warren V.A., Kraus R.L., Hwang J.C., Liu C.J., Dai G., Brochu R.M., Kohler M.G., Gao Y.D., Garsky V.M., et al. Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry. 2002;41:14734–14747. doi: 10.1021/bi026546a. [DOI] [PubMed] [Google Scholar]
- 51.Bosmans F., Martin-Eauclaire M.F., Swartz K.J. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature. 2008;456:202–208. doi: 10.1038/nature07473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosmans F., Swartz K.J. Targeting voltage sensors in sodium channels with spider toxins. Trends Pharmacol. Sci. 2010;31:175–182. doi: 10.1016/j.tips.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bosmans F., Rash L., Zhu S., Diochot S., Lazdunski M., Escoubas P., Tytgat J. Four novel tarantula toxins as selective modulators of voltage-gated sodium channel subtypes. Mol. Pharmacol. 2006;69:419–429. doi: 10.1124/mol.105.015941. [DOI] [PubMed] [Google Scholar]
- 54.Zeng X., Deng M., Lin Y., Yuan C., Pi J., Liang S. Isolation and characterization of Jingzhaotoxin-V, a novel neurotoxin from the venom of the spider Chilobrachys jingzhao. Toxicon. 2007;49:388–399. doi: 10.1016/j.toxicon.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Deng M., Kuang F., Sun Z., Tao H., Cai T., Zhong L., Chen Z., Xiao Y., Liang S. Jingzhaotoxin-IX, a novel gating modifier of both sodium and potassium channels from Chinese tarantula Chilobrachys jingzhao. Neuropharmacology. 2009;57:77–87. doi: 10.1016/j.neuropharm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Bulaj G., Zhang M.M., Green B.R., Fiedler B., Layer R.T., Wei S., Nielsen J.S., Low S.J., Klein B.D., Wagstaff J.D., et al. Synthetic muO-conotoxin MrVIB blocks TTX-resistant sodium channel Nav1.8 and has a long-lasting analgesic activity. Biochemistry . 2006;45:7404–7414. doi: 10.1021/bi060159+. [DOI] [PubMed] [Google Scholar]
- 57.Ekberg J., Jayamanne A., Vaughan C.W., Aslan S., Thomas L., Mould J., Drinkwater R., Baker M.D., Abrahamsen B., Wood J.N., et al. MuO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc. Natl. Acad. Sci. USA. 2006;103:17030–17035. doi: 10.1073/pnas.0601819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knapp O., Nevin S.T., Yasuda T., Lawrence N., Lewis R.J., Adams D.J. Biophysical properties of Na(v) 1.8/Na(v) 1.2 chimeras and inhibition by microO-conotoxin MrVIB. Br. J. Pharmacol. 2012;166:2148–2160. doi: 10.1111/j.1476-5381.2012.01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leipold E., DeBie H., Zorn S., Borges A., Olivera B.M., Terlau H., Heinemann S.H. MuO conotoxins inhibit NaV channels by interfering with their voltage sensors in domain-2. Channels (Austin) 2007;1:253–262. doi: 10.4161/chan.4847. [DOI] [PubMed] [Google Scholar]
- 60.Bosmans F., Maertens C., Verdonck F., Tytgat J. The poison dart frog’s batrachotoxin modulates Nav1.8. FEBS Lett. 2004;577:245–248. doi: 10.1016/j.febslet.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 61.Du Y., Garden D.P., Wang L., Zhorov B.S., Dong K. Identification of new batrachotoxin-sensing residues in segment iiis6 of the sodium channel. J. Biol. Chem. 2011;286:13151–13160. doi: 10.1074/jbc.M110.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S.Y., Wang G.K. Batrachotoxin-resistant Na+ channels derived from point mutations in transmembrane segment d4-s6. Biophys. J. 1999;76:3141–3149. doi: 10.1016/S0006-3495(99)77465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rush A.M., Waxman S.G. PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res. 2004;1023:264–271. doi: 10.1016/j.brainres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 64.Cummins T.R., Black J.A., Dib-Hajj S.D., Waxman S.G. Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J. Neurosci. 2000;20:8754–8761. doi: 10.1523/JNEUROSCI.20-23-08754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fjell J., Cummins T.R., Dib-Hajj S.D., Fried K., Black J.A., Waxman S.G. Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain Res. Mol. Brain Res. 1999;67:267–282. doi: 10.1016/s0169-328x(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 66.Copel C., Osorio N., Crest M., Gola M., Delmas P., Clerc N. Activation of neurokinin 3 receptor increases Na(v)1.9 current in enteric neurons. J. Physiol. 2009;587:1461–1479. doi: 10.1113/jphysiol.2009.169409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bosmans F., Escoubas P., Nicholson G.M. Animal Toxins: State of the Art – Perspectives in Health and Biotechnology. Editora UFMG; Belo Horizonte, Brazil: 2009. [Google Scholar]
- 68.Fozzard H.A., Lipkind G.M. The tetrodotoxin binding site is within the outer vestibule of the sodium channel. Mar. Drugs. 2010;8:219–234. doi: 10.3390/md8020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang M.M., Gruszczynski P., Walewska A., Bulaj G., Olivera B.M., Yoshikami D. Cooccupancy of the outer vestibule of voltage-gated sodium channels by micro-conotoxin KIIIA and saxitoxin or tetrodotoxin. J. Neurophysiol. 2010;104:88–97. doi: 10.1152/jn.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bulaj G., West P.J., Garrett J.E., Watkins M., Zhang M.M., Norton R.S., Smith B.J., Yoshikami D., Olivera B.M. Novel conotoxins from Conus striatus and Conus kinoshitai selectively block TTX-resistant sodium channels. Biochemistry. 2005;44:7259–7265. doi: 10.1021/bi0473408. [DOI] [PubMed] [Google Scholar]
- 71.Wang C.Z., Zhang H., Jiang H., Lu W., Zhao Z.Q., Chi C.W. A novel conotoxin from Conus striatus, mu-SIIIA, selectively blocking rat tetrodotoxin-resistant sodium channels. Toxicon. 2006;47:122–132. doi: 10.1016/j.toxicon.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 72.West P.J., Bulaj G., Garrett J.E., Olivera B.M., Yoshikami D. Mu-conotoxin SMIIIA, a potent inhibitor of tetrodotoxin-resistant sodium channels in amphibian sympathetic and sensory neurons. Biochemistry. 2002;41:15388–15393. doi: 10.1021/bi0265628. [DOI] [PubMed] [Google Scholar]
- 73.Swartz K.J. Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon. 2007;49:213–230. doi: 10.1016/j.toxicon.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alabi A.A., Bahamonde M.I., Jung H.J., Kim J.I., Swartz K.J. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450:370–375. doi: 10.1038/nature06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chakrapani S., Cuello L.G., Cortes D.M., Perozo E. Structural dynamics of an isolated voltage-sensor domain in a lipid bilayer. Structure. 2008;16:398–409. doi: 10.1016/j.str.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang Y., Lee A., Chen J., Ruta V., Cadene M., Chait B.T., MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 77.Jiang Y., Ruta V., Chen J., Lee A., MacKinnon R. The principle of gating charge movement in a voltage-dependent K+ channel. Nature. 2003;423:42–48. doi: 10.1038/nature01581. [DOI] [PubMed] [Google Scholar]
- 78.Long S.B., Tao X., Campbell E.B., MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 79.Phillips L.R., Milescu M., Li-Smerin Y., Mindell J.A., Kim J.I., Swartz K.J. Voltage-sensor activation with a tarantula toxin as cargo. Nature. 2005;436:857–860. doi: 10.1038/nature03873. [DOI] [PubMed] [Google Scholar]
- 80.Milescu M., Bosmans F., Lee S., Alabi A.A., Kim J.I., Swartz K.J. Interactions between lipids and voltage sensor paddles detected with tarantula toxins. Nat. Struct. Mol. Biol. 2009;16:1080–1085. doi: 10.1038/nsmb.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Milescu M., Vobecky J., Roh S.H., Kim S.H., Jung H.J., Kim J.I., Swartz K.J. Tarantula toxins interact with voltage sensors within lipid membranes. J. Gen. Physiol. 2007;130:497–511. doi: 10.1085/jgp.200709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee S.Y., MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430:232–235. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]