Abstract
For the first time, a sensitive reversed-phase HPLC electrochemical array method has been developed for the quantitative analysis of eight major ginger components ([6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione) in eleven ginger-containing commercial products. This method was valid with unrivaled sensitivity as low as 7.3 – 20.2 pg of limit of detection and a range of 14.5 to 40.4 pg of limit of quantification. Using this method, we quantified the levels of eight ginger components in eleven different commercial products. Our results found that both levels and ratios among the eight compounds vary greatly in commercial products.
Keywords: Ginger, Gingerols, Shogaols, Electrochemical array detection, Quantification
INTRODUCTION
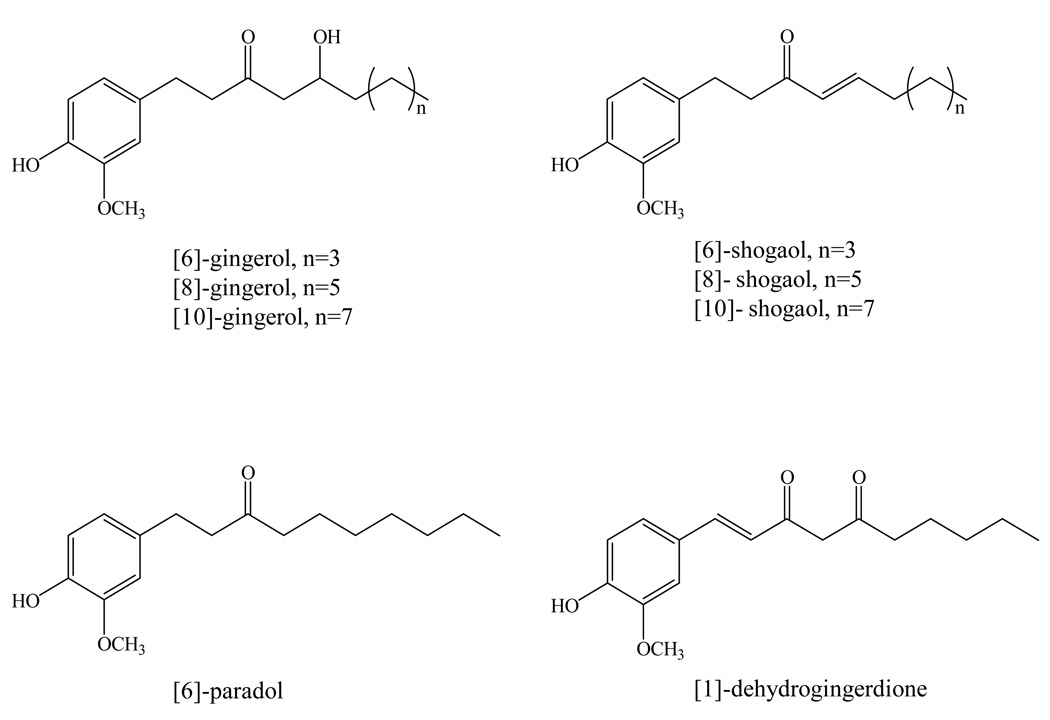
Ginger (Zingiber officinale Roscoe), a tropical and sub-tropical cultivated plant, is derived from Zingiberaceae and has been used worldwide as spice, dietary supplement and traditional medicine for centuries (1). Moreover, ginger has been suggested for the treatment of headaches, nausea and colds in Asian, Indian, Arabic, and African area since ancient times. In Western alternative medicine practice, ginger is primarily defined as a plant useful for the prevention of nausea and motion sickness (2). Recently, ginger has drawn much more attention for anti-inflammation, antioxidative, anti-carinogenic, and anti-mutagenic properties due to the long history of medicinal use as well as being rich in bioactive constituents (3–6). A series of bioactive compounds such as gingerols, paradols and shogaols have been identified in ginger and suggested to play significant roles both for flavoring and healthy contribution. Gingerols, a series of chemical homologues differentiated by the length of their unbranched alkyl chains, were identified as the major pungent components in the ginger oleoresin from fresh rhizome, with [6]-gingerol (Figure 1) being the most abundant. Gingerols are not stable during storage or thermal processing as they generate the dehydration products, shogaols, which are predominant pungent constituents in the ginger oleoresin from dried ginger (7, 8). It has been reported that shogaols were minor components in fresh ginger and the ratio of [6]-shogaol to [6]-gingerol was about 1:1 in dried ginger (7, 8). Other gingerol- or shogaol-related compounds have also been reported in ginger rhizome, such as [6]-paradol, [1]-dehydrogingerdione, [6]- and [10]-gingerdione, [4]-, [6]-, [8]-, and [10]-gingerdiol, and diarylheptanoids (7, 9). However, these minor compounds only account for about 1 to 10% of the overall amount of gingerols and shogaols (7, 9).
Figure 1.
Chemical structures of [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione.
Although most animal studies with ginger extract showed antioxidative, anti-inflammatory, and anti-tumor activities, no report has considered that the instability of gingerols during thermal processing and long-term storage will affect the chemical profile of the ginger extract used in their animal studies (10, 11). They either did not quantify the levels of the active components in their raw material or simply used the total levels of gingerols as the standard. Therefore, fast and accurate analytical methods are needed for the determination and quantification of the active components in ginger and its related products.
A high-performance liquid chromatography (HPLC) method with single channel electrochemical detector has been developed to analyze the pungent principles of ginger 20 years ago (12). However, a single channel electrochemical detector usually could not provide sufficient profile of analyte’s responses under different potential in the same run. A gas chromatographic-mass spectrometric (GC-MS) has also been used to analyze gingerols in the ginger extracts (13). However, the instability of gingerols under high temperature limits the application of this method. An HPLC-UV-ESI (Electrospray Ionization)-MS method has been developed by He et al. for identification of major components in ginger extracts (14). But the high-price instrumentation and tedious sample preparation apparently limit the application of this method for the samples with complex matrices, such as biofluids and ginger-containing commercial products. An HPLC photodiode array (PDA) method was also reported to analyze gingerols and shogaols in commercial products recently (15). Although, the PDA detector provides a “third dimension” to HPLC-absorbance techniques by examining an analyte’s spectrum and identifying the compounds both by retention time and by spectral behavior, the low sensitivity and resolution limit the application of PDA detector to a more complex sample matrix; co-elution, misidentification and quantification errors can still occur due to the inadequate sensitivity (16).
The HPLC combined with an electrochemical array detector (ECD) offers several advantages over UV and MS detectors, especially the extraordinary sensitivity for redox sensitive compounds. Besides much higher sensitivity, a CoulArray ECD also offers on-line generation of qualitative data and the ability to resolve peaks due to different voltammetric characteristics. For gingerols and shogaols, the length of unbranched alkyl chains provides the polarity differences and the hydroxyl groups offer differently sensitive voltammetric responses. Generally, frequent column maintenances and careful sample preparation are required during LC/MS analysis of biological substances. The clean cell activity using high electro potential in ECD cells after each run is another advantage of ECD to compare to LC/MS, indicating the potential of applying this instrument to the analysis of biofluids and tissue samples. Clean cell activity is used to apply a high electro potential to the cells for a short period of time, to clean the electrode surfaces and prevent the contamination of later samples with previous samples. Due to this convenient function, frequent cleaning and maintenance are not needed. Several studies have proven the sensitivity, selectivity, and stability of HPLC-ECD in the analyzing of biofluid samples, such as measuring the levels of tea polyphenols in human plasma, urine and tissue samples (17–19).
In this study, we developed a very sensitive HPLC method coupled with electrochemical array detection for the quantitative analysis of [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione and quantified their levels in eleven different commercial ginger products.
MATERIALS AND METHODS
General
[6]-, [8]-, and [10]-Gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione standards were purified from ginger extract in our laboratory (20). In brief, the ginger standards were purified using different column chromatography and the structures of these eight compounds were confirmed on the basis of their 1H and 13C NMR analysis (20). Sodium phosphate, phosphoric acid, HPLC-grade methanol, acetonitrile and tetrahydrofuran were obtained from Fisher Scientific (Fair Lawn, NJ, USA). HPLC-grade water was prepared using a Millipore Milli-Q Academic purification system (Bedford, MA, USA). All the ginger-related products were purchased from local supermarkets.
Preparation of [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione Standards
HPLC-grade methanol was added to each standard to produce a stock standard of 10 mg/mL and stored at −80°C for future use. We found that all eight ginger standards are stable under −80°C. Serial dilutions of the 10 mg/mL stock standards were prepared using methanol (100%) for calibration studies. Concentration ranges of each standard were determined to ensure that the calibration curve can cover the level of each component in all commercial products.
Sample Preparation of Ginger-containing Commercial Products
The levels of [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione were determined in eleven ginger-containing commercial products from local supermarket including three crystal ginger beverages (manufactured in granule form), five ginger tea bags (tea bag with dried rhizome and tea leaves in it), and three ground ginger powders (dried ginger rhizome). Crystal ginger products were ground to fully mix, weighed and placed into a 50 mL centrifuge tube. The fine powders of products were dissolved in the deionized water to make 10 mg/ml solutions. Then the solutions were filtered by nylon syringe filter (0.45 µm) obtained from Fisher Scientific (Fair Lawn, NJ, USA) and diluted 10-fold with HPLC-grade methanol (100%) for further analysis. Three tea bags from each brand of product were mixed together. The mixed tea leaves and dried ginger rhizome in tea bags were then grounded, weighed, and placed in 50 mL centrifuge tubes. The fine powders were extracted three times with methanol, each time for 24 hours at room temperature. The final concentration was adjusted to 10 mg of ginger product powder per mL of methanol. Then the solutions were centrifuged at 17 × 1000 g (accuSpin Micro, Fisher Scientific, PA, USA) for 5 minutes at room temperature. The supernatant was further filtered by syringe filter and diluted 10-fold with HPLC-grade methanol for HPLC analysis. Ground ginger powder was directly weighed, placed in 50 mL centrifuge tube and extracted three times, 24 hours each with methanol at room temperature. The final concentration was adjusted to 10 mg of ginger product powder per mL of methanol. The solutions were centrifuged, filtered, and diluted following the same procedure as for tea bags. Triplicate samples were prepared for all commercial products.
HPLC Analysis
An HPLC-ECD/UV system (ESA, Chelmsford, MA, USA) consisting of an ESA model 584 HPLC pump, an ESA model 542 autosampler, an ESA organizer, an ESA coularray detector coupled with two ESA model 6210 four sensor cells, and an ESA 526 UV detector was used in our study. Chromatographic analysis was performed on a 150 mm × 4.6 mm, 5 µm, Supelcosil™ LC-18 column. The mobile phases were consisted of solvent A (30 mM sodium phosphate buffer, pH 3.35) and Solvent B (15 mM sodium phosphate buffer containing 58.5% acetonitrile and 12.5% tetrahydrofuran, pH 3.45). The gradient elution had the following profile: 50% – 55% B from 0 min to 10 min; 55% – 60% B from 10 min to 14 min; 60% – 65% B from 14 min to 15 min; 65% – 100% B from 15 min to 40 min; then 50% B from 40.1 min to 53 min with a flow rate of 1.0 mL/min. The cells were then cleaned at a potential of 1000 mV for 1 min. The injection volume of the sample was 10 µL. The peak identifications of all eight ginger components were based on the retention time of the standards and further confirmed by comparing their peak patterns under different voltammetric potentials to those of the individual standards.
Method Validation
This analytical method was validated by determining the linearity, limit of quantification (LOQ), limit of detection (LOD), recovery rate, and precision. The calibration curves were established individually for the eight ginger compounds using five different concentrations and response linearity was assessed with standards diluted in 100% methanol using a least squares regression analysis of peak area response vs. concentration of the standards. The calibration curves of [6]-gingerol and [6]-shogaol were prepared in a range of 1.0 – 20.0 µg/mL and 1.0 – 5.0 µg/mL, respectively, in triplicate. The calibration curves for [8]-gingerol, [10]-gingerol and [10]-shogaol were determined using five dilutions by methanol in the range of 0.1 – 3.0 µg/mL, in triplicate. The calibration curves for [8]-shogaol, [6]-paradol and [1]-dehydrogingerdione were established using five dilutions by methanol in the range of 0.1 – 1.0 µg/mL, in triplicate. The linearity was evaluated in the most sensitive channel for each standard: the peak areas of [6]-gingerol was determined on channel 4 with 300 mV of cell potential and the peak areas of [8]- and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione were obtained on channel 5 with 350 mV of cell potential. The intraday variations of [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione with a concentration of 1.0 µg/mL were measured using their dominant channels. The interday variations were determined by comparing the results obtained on three different days. Triplicate injections were performed to test both intraday and interday variation of each sample. Limit of detection (LOD) and limit of quantification (LOQ) were estimated using a signal to noise ration of 3:1 and 10:1, respectively. Recovery for each individual compounds was calculated by the amounts obtained from the first two extractions and then divided by the total amounts obtained from all three extractions.
RESULTS AND DISCUSSION
Optimization of the Chromatographic Conditions
Mobile phase used for HPLC with an EC detector should follow several important criteria. The EC detector requires a stable level of electrolyte within a suitable range in the mobile phases. The level of electroactive impurities and noise may be promoted if the level of electrolyte is too high. However, a drifting baseline and poor signal for the analyte can occur if the mobile phase electrolyte concentration is too low. Under these circumstances, any pH changes due to the sample can then influence the signal from the analyte. To prevent those influences caused by pH changes and to provide greater stability for both the chromatographic and electrochemical reactions, buffered systems were usually used. In our study, 30 mM and 15 mM, respectively, of sodium phosphate were added to the mobile phases A and B to serve these purposes. The concentrations of sodium phosphate for both mobile phases A and B used in this study were directly adopted from previous laboratory protocol for analyzing tea polyphenols in biological samples (21). Phosphate salt was chosen due to the low background current at high electrode potentials.
Once the chromatographic conditions have been optimized, a potential is usually chosen based upon experience or literature. For unknown compounds, a hydrodynamic voltammogram (HDV) consisting of applied potential (mV) vs. normalized response was used to determine the applied potential: it is usually best to start at −100 mV and then increment by +100 mV. This “rough” HDV can then be refined using smaller incremental potentials (typically 25 or 50 mV increments). Most HDVs generated by ESA application chemists are at the 50 mV resolution; this is an excellent compromise between time taken to generate the HDV and EC resolution.
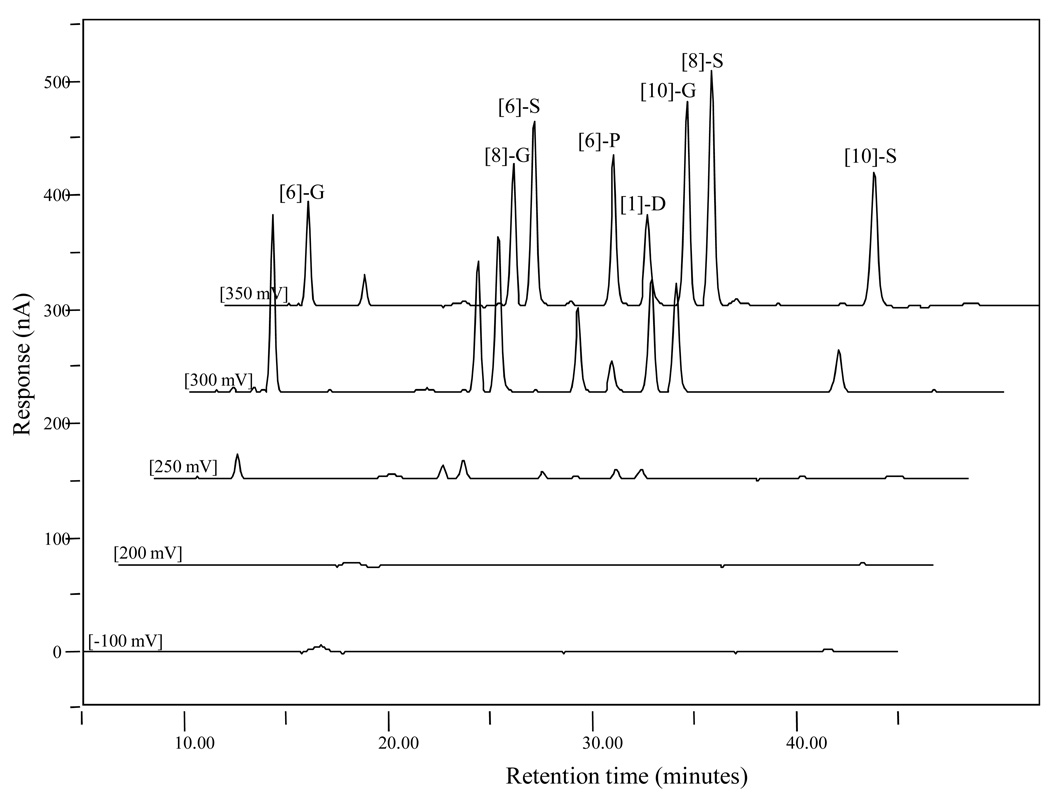
The choice of the potential in this study was established by plotting the current values measured at different applied potentials after the injection of 10 µL aliquots of 1 µg/mL of eight ginger standards mixture solution (Figure 2). The testing potential starts at −100 mV to reduce all the possible impurities, thereby decreasing the interference of chromatography background, with 50 mV as the incremental potential until 400 mV. Based upon the chromatogram profile shown in Figure 2, maximum currents of 300 mV for [6]-gingerol and 350 mV for [8]- and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione were observed. The responses of those eight compounds at 400 mV were very low (data not shown). The peak area ratios at 300 mV vs. 350 mV for each individual compound were calculated. By comparing the ratios of gingerols and shagaols side by side ([6]-G vs. [6]-S: 1.72 vs. 0.87; [8]-G vs. [8]-S: 0.95 vs. 0.48; and [10]-G vs. [10]-S: 0.57 vs. 0.30), we found that gingerols were much easier to be oxidized under lower potential (300 mV), which could be due to the additional hydroxyl group on the side chain compared to shogaols. Also the ratios of peak area at 300 mV and 350 mV for both gingerols and shogaols decreased when the side chain length increased, indicating that the length of side chain was related to the oxidation efficacy under potential charge.
Figure 2.
LC chromatograms of a mixed [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione standard (1 µg/mL) under different electrochemical potentials.
Method Development and Validation
Based on the developed analytical method, the calibration curves for the eight ginger standards have been established as shown in Table 1. As described in the experimental part above, all standards were prepared in a range to cover the concentration of correlated compounds in commercial samples. Regression analysis performed by the least-squares method yielded the standard curve equations and correlation coefficients (Table 1).
Table 1.
Linearity of eight ginger standards.
| Concentration range (µg/mL) | Linear regression equation | r2 | |
|---|---|---|---|
| [6]-gingerol | 1.0 – 20.0 | y = 2.7084x + 1.7942 | 0.9868 |
| [8]-gingerol | 0.1 – 3.0 | y = 3.2288x + 0.0477 | 0.9988 |
| [10]-gingerol | 0.1 – 3.0 | y = 3.3596x + 0.1895 | 0.9985 |
| [6]-shogaol | 1.0 – 5.0 | y = 3.8596x + 0.3068 | 0.9977 |
| [8]-shogaol | 0.1 – 1.0 | y = 4.1659x + 0.0166 | 0.9951 |
| [10]-shogaol | 0.1 – 3.0 | y = 1.8994x + 0.0786 | 0.9990 |
| [6]-paradol | 0.1 – 1.0 | y = 3.1967x − 0.0199 | 0.9964 |
| [1]-dehydrogingerdione | 0.1 – 1.0 | y = 1.7756x − 0.0116 | 0.9923 |
Linearity data obtained from diluted standards are shown in Table 1. The slope obtained from least squares regression analysis indicates high sensitivity (µC/µg) for all analytes. Based on a signal to noise ratio of 3:1, the limits of detection for the eight standard compounds were approximately from 7.3 to 20.2 pg (Table 2). And the limits of quantification were calculated based on a signal to noise ratio of 10:1 with a range of 14.5 to 40.4 pg for all analytes (Table 2). These results are 620 to 1725 folds lower than the previously published HPLC-UV method with 25 ng, indicating the potential of applying our method for analyzing biofluids and tissue samples (15). For replicate injections of mixture standards, intra-day response coefficients of variation ranged from 1.23 to 11.2% for all ananlytes as shown in Table 2. The inter-day coefficients of variation were also determined comparing the results obtained on three different days and ranged from 4.32% to 9.27%.
Table 2.
Validation parameters for the HPLC-ECD method.
| Interday Variation (%RSD) (n=3) | Intraday Variation (%RSD) (n=3) | LOD (pg) | LOQ (pg) | |
|---|---|---|---|---|
| [6]-G (300mV) | 6.87 ± 2.47 | 6.52 ± 0.85 | 8.3 | 16.6 |
| [8]-G (350mV) | 5.76 ± 2.57 | 1.23 ± 0.10 | 9.6 | 19.1 |
| [10]-G (350mV) | 5.54 ± 2.04 | 5.15 ± 0.33 | 7.3 | 14.5 |
| [6]-S (350mV) | 4.68 ± 2.59 | 1.41 ± 0.19 | 11.3 | 22.6 |
| [8]-S (350mV) | 4.32 ± 2.64 | 6.61 ± 0.53 | 20.2 | 40.4 |
| [10]-S (350mV) | 4.57 ± 3.02 | 11.2 ± 0.53 | 8.6 | 17.2 |
| [6]-P (350mV) | 4.83 ± 2.84 | 5.85 ± 0.44 | 17.8 | 35.5 |
| 1-D (350mV) | 9.27 ± 0.75 | 9.61 ± 0.73 | 8.5 | 17.1 |
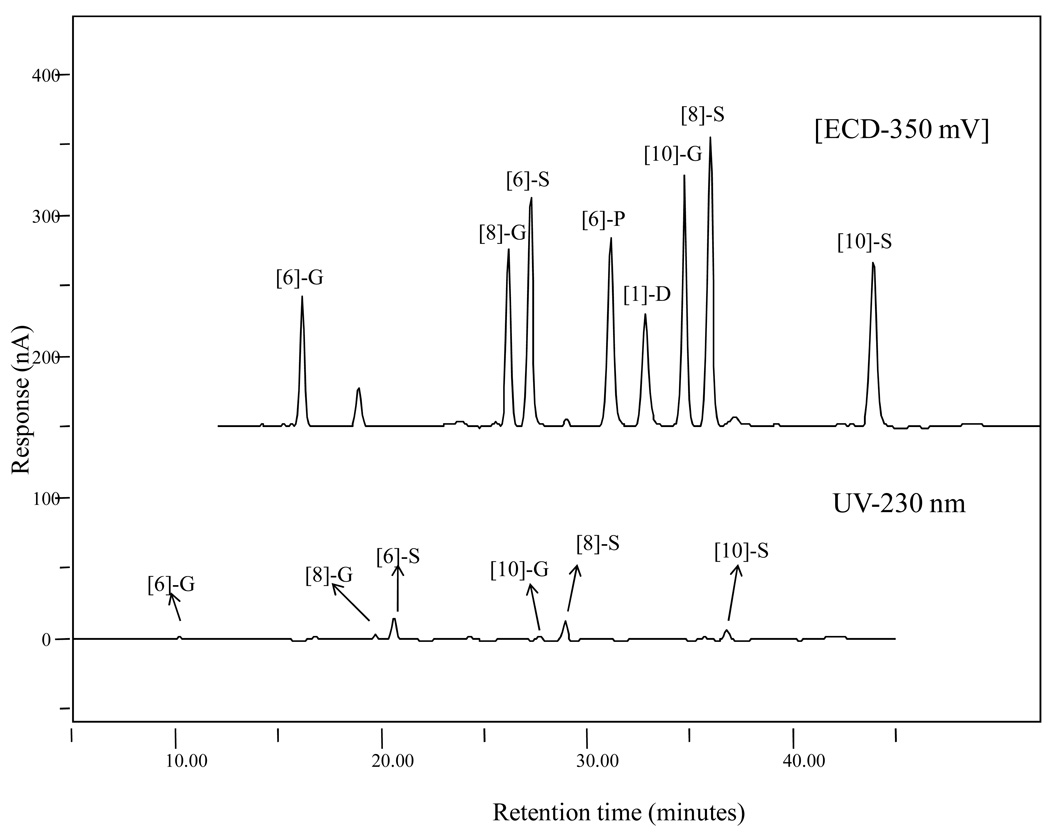
To compare the sensitivity of our method to the ordinary UV analysis, 10 µL of mixed standard solution with a concentration of 1 µg/mL was analyzed by both the UV and EC detectors in the same run. The UV channel was selected at 230 nm as a specific wavelength for detection of gingerols and shogaols based on reference data carried out by He et al. (14). As Figure 3 shows, all eight standards showed much better responses at 350 mV than at 230 nm indicating that the ECD performed with extraordinary sensitivity compared to the traditional UV detector. The peak areas of all eight ginger standards measured by ECD were 1.31, 2.12, 3.35, 3.06, 4.01, 2.67, 2.66 and 1.76 µC for [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione, respectively. The peak areas measured by UV detector for all eight ginger standards in the same order as listed above were 0.0323, 0.0418, 0.223, 0.0379, 0.0107, 0.045, 0.197 and 0.112 µC, respectively.
Figure 3.
LC chromatograms of a mixed [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione standard (1 µg/mL) obtained by electrochemical (350 mV) and UV detection (230 nm).
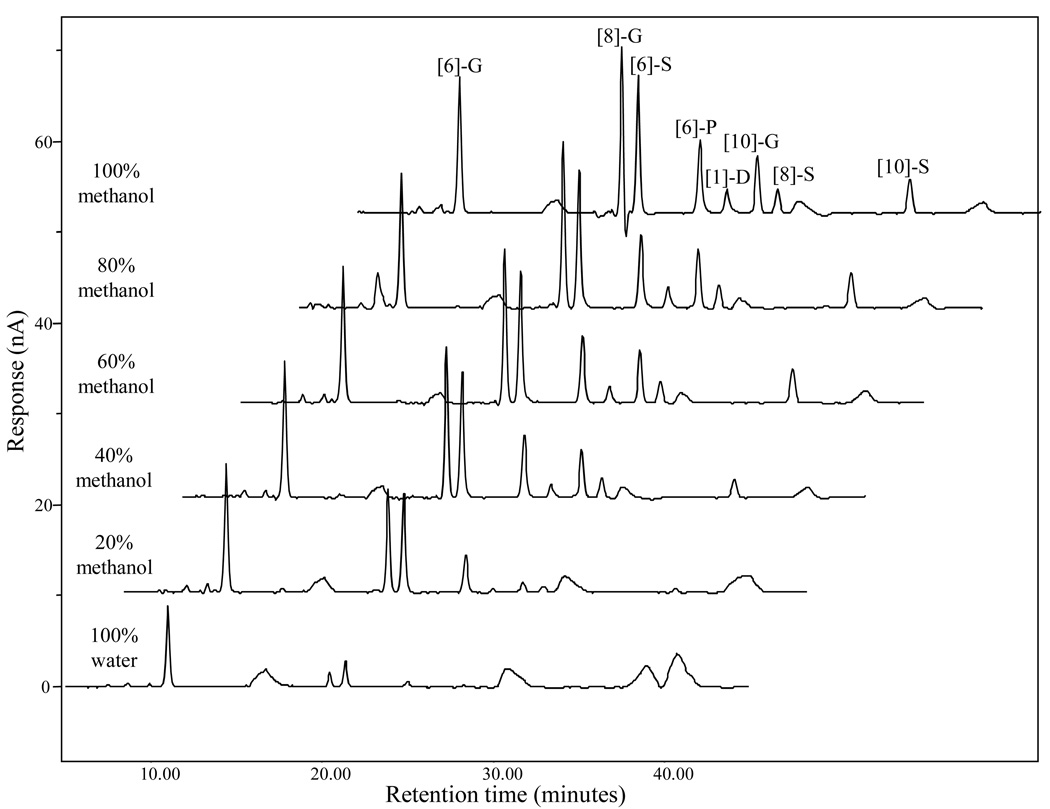
In our previous study, the detector response of the tea polyphenol peaks was found to be associated with the percentage of organic solvent in the samples (21). In this study, we compared the electrochemical response of eight ginger standards in different solvent systems. Five mixed standard were prepared by combining the eight ginger standards (100 ng/mL) in water, 20%, 40%, 60%, 80% aqueous methanol, respectively, and 100% methanol. The chromatograms shown in Figure 4 clearly demonstrate how the solvent affected the ECD’s sensitivity of our standards. By comparing peak areas of each compound at the 350 mV channel, the sensitivities of [6]-gingerol, [8]-gingerol, [6]-shogaol and [6]-paradol were discovered to be almost two, fifteen, five, and thirteen folds higher in methanol (100%) than in water (100%). Also, the detection of [1]-dehydrogingerdione, [10]-gingerol, [8]-shogaol, and [10]-shogaol were seven, twelve, five, and eleven times more sensitive in methanol (100%) than methanol/water (20/80%) (Those compounds are not detectable in water). Based on the above conclusions, we prepared all the samples using 100% methanol.
Figure 4.
LC chromatograms of a mixed [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione standard (100 ng/mL) dissolved in different solvent systems.
Analysis of Commercial Products
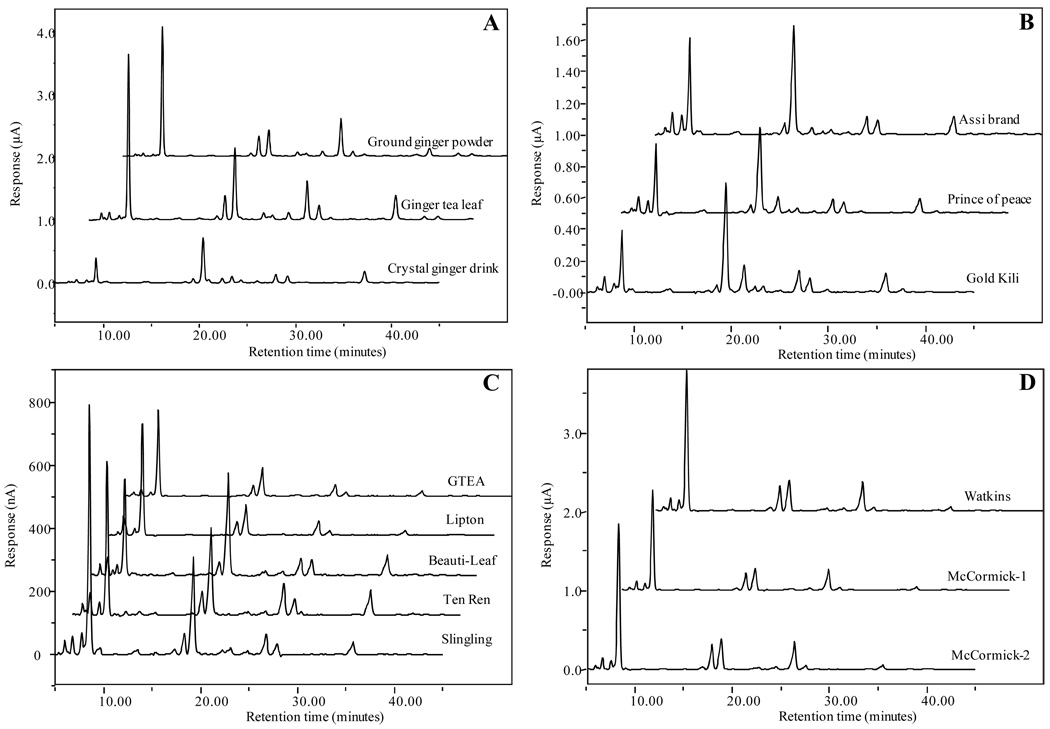
The chromatograms of ginger-containing commercial products are presented in Figure 5. Figure 5A represents commercial products in three different forms (ground ginger powder, ginger tea leaf, and ginger crystal); Figure 5B, 5C, and 5D represent three crystal products, five tea leaf products, and three ground ginger powder products, respectively. All ginger products have such high levels of [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol that they are easily detected by the CoulArray ECD. The detailed levels of the eight ginger components in the eleven commercial products are listed in Table 4. The [6]-gingerol concentration in different ginger products was found to be higher than that of [8]- or [10]- gingerol concentration, but not always higher than [6]-shogaol, especially in crystalized products. For instance, the concentrations of [6]-shogaol were around 1.25 to 2.06 times higher than [6]-gingerol in all three crystal products. Possible reasons may be granulation, drying, or the crystallization process in which generated heat caused the conversion from gingerols to shogaols (22). The concentration of all eight components in different commercial products varies greatly. For instance, the levels of [6]-gingerol in ground powder are almost 35 to 80 times higher than those in crystalized products. Meanwhile, the level of each component varies a lot even in the same type of product. For example, the concentration of [6]-gingerol in “Sample #4” is almost 4.5 times higher than the concentration in “Sample #8”. Similarly, the level of [6]-shogaol in “Sample #8” is much lower than in the “Sample #4”. The levels of both [6]-paradol and [1]-dehydrogingerdione are significantly lower than the other six components in all commercial products, even too low to be detected in certain products, mostly in ginger-tea products.
Figure 5.
LC chromatograms of ginger related commercial products: (A) selected ground ginger powder, ginger tea leaf, and crystal ginger drink; (B) three crystal ginger drink samples (Sample #1, #2, and #3); (C) five ginger tea leaf samples (Sample #4, #5, #6, #7, and #8); (D) three ground ginger powder samples (Sample #9, #10, and #11).
Table 4.
Concentrations of [6]-, [8]-, and [10]-gingerol, 6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione in 11 commercial available ginger products: ginger crystal drink (Sample #1, #2, and #3), ginger tea leaf (Sample #4, #5, #6, #7, and #8), and ground ginger powder (Sample #9, #10, and #11)
| Product Number | Concentration (mg/100 g of product) | |||||||
|---|---|---|---|---|---|---|---|---|
| 6-gingerol | 8-gingerol | 10-gingerol | 6-shogaol | 8-shogaol | 10-shogaol | 6-paradol | 1-dehydrogingerdione | |
| #1 | 11.58 ± 0.39 | 1.52 ± 0.10 | 2.78 ± 0.07 | 16.23 ± 0.07 | 1.90 ± 0.01 | 6.09 ± 0.17 | 0.80 ± 0.18 | 0.58 ± 0.03 |
| #2 | 15.70 ± 0.30 | 1.92 ± 0.11 | 3.42 ± 0.38 | 19.68 ± 1.13 | 2.50 ± 0.27 | 7.12 ± 1.08 | 0.21 ± 0.07 | 0.59 ± 0.16 |
| #3 | 9.74 ± 0.24 | 1.29 ± 0.05 | 4.66 ± 0.22 | 20.07 ± 0.34 | 2.52 ± 0.10 | 7.76 ± 0.41 | 0.90 ± 0.09 | 0.30 ± 0.01 |
| #4 | 60.87 ± 7.02 | 9.32 ± 0.30 | 10.22 ± 1.44 | 25.75 ± 2.51 | 0.74 ± 0.10 | 10.33 ± 1.70 | N/D | N/D |
| #5 | 123.07 ± 10.47 | 17.75 ± 1.92 | 17.94 ± 1.00 | 35.08 ± 2.12 | 1.70 ± 0.10 | 11.24 ± 0.93 | N/D | N/D |
| #6 | 78.59 ± 8.73 | 14.87 ± 1.04 | 18.90 ± 2.36 | 111.64 ± 12.34 | 16.21 ± 0.96 | 51.90 ± 7.34 | 0.19 ± 0.02 | 4.48 ± 0.87 |
| #7 | 148.34 ± 18.76 | 27.45 ± 3.85 | 38.13 ± 5.27 | 96.54 ± 9.58 | 16.30 ± 1.86 | 65.37 ± 8.11 | N/D | 2.90 ± 0.54 |
| #8 | 277.51 ± 25.60 | 24.15 ± 2.82 | 23.44 ± 2.14 | 111.00 ± 8.66 | 8.72 ± 0.43 | 31.13 ± 2.38 | 4.73 ± 1.27 | 6.89 ± 7.10 |
| #9 | 767.40 ± 14.78 | 131.29 ± 3.04 | 157.38 ± 7.47 | 145.62 ± 2.71 | 11.56 ± 2.36 | 47.22 ± 4.15 | 4.85 ± 0.11 | 19.42 ± 8.70 |
| #10 | 554.87 ± 49.77 | 104.37 ± 11.34 | 136.54 ± 15.29 | 115.67 ± 13.76 | 8.58 ± 1.71 | 41.02 ± 4.54 | 2.95 ± 0.67 | 15.36 ± 1.41 |
| #11 | 772.33 ± 47.74 | 140.04 ± 10.00 | 173.40 ± 11.78 | 149.54 ± 8.47. | 10.91 ± 1.52 | 45.58 ± 7.52 | 6.56 ± 2.34 | N/D |
N/D: not detected
In conclusion, a new HPLC-ECD array method has been developed allowing for the determination and quantification of eight ginger components: [6]-, [8]-, [10]-gingerol, [6]-, [8]-, [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione in eleven commercial products with higher accuracy than previously reported. The method resulted in clearly increased chromatographic separation as well as higher detection sensitivity compared to traditional UV detection. The peak areas detected by ECD were around 19 to 120 times higher than those detected by UV in the same run. The very low limits of detection and quantification at pg level were obtained enabling the detection of the eight ginger components from complex commercial samples, indicating the potential of applying this method to analyze ginger components and their metabolites in biofluids collected in future animal and human studies.
It has been reported that gingerols are the major pungent components in fresh ginger and they are not stable during storage or thermal processing as they generate the dehydration products, shogaols, which are predominant pungent constituents in the ginger oleoresin from dried ginger (7, 8). This is the potential reason that the levels of gingerols and shogaols in commercial ginger products vary significantly. Therefore, it is important to standardize ginger products used in in vivo study using both gingerols and shogaols as mark compounds.
Table 3.
Recovery percentages and relative standard deviations of eight ginger components
| % Recovery | % RSD (n=3) | |
|---|---|---|
| [6]-gingerol | 96.93 | 4.20 |
| [8]-gingerol | 97.86 | 2.52 |
| [10]-gingerol | 97.92 | 3.10 |
| [6]-shogaol | 97.52 | 2.32 |
| [8]-shogaol | 97.01 | 2.86 |
| [10]-shogaol | 97.10 | 2.77 |
| [6]-paradol | 98.82 | 1.48 |
| [1]-dehydrogingerdione | 99.40 | 0.66 |
ACKNOWLEDGEMENT
This work was supported by NIH grant CA138277 and CA138277S1 to S. Sang.
Abbreviations
- ECD
electrochemical detection
- [6]-G
[6]-gingerol
- [8]-G
[8]-gingerol
- [10]-G
[10]-gingerol
- [6]-S
[6]-shogaol
- [8]-S
[8]-shogaol
- [10]-S
[10]-shogaol
- [6]-P
[6]-paradol
- [1]-D
[1]-dehydrogingerdione
Literature Cited
- 1.Leung AY, Foster S. Encyclopedia of Common Natural Ingredients used in Food, Drugs and Cosmetics. 2nd edn. New York: Wiley; 1996. pp. 271–274. [Google Scholar]
- 2.Grant KL, Lutz RB. Alternative Therapies: Ginger. Am J Health Syst Pharm. 2000;57(10):945–947. doi: 10.1093/ajhp/57.10.945. [DOI] [PubMed] [Google Scholar]
- 3.Katiyar SK, Agarwal R, Mukhtar H. Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale rhizome. Cancer Res. 1996;56:1023–1030. [PubMed] [Google Scholar]
- 4.Lee E, Park KK, Lee JM, Chun KS, Kang JY, Lee SS, Surh YJ. Suppression of mouse skin tumor promotion and induction of apoptosis in HL-60 cells by Alpinia oxyphylla Miquel (Zingiberaceae) Carcinogenesis. 1998;19:1377–1381. doi: 10.1093/carcin/19.8.1377. [DOI] [PubMed] [Google Scholar]
- 5.Lee E, Surh YJ. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol. Cancer Lett. 1998;134:163–168. doi: 10.1016/s0304-3835(98)00253-5. [DOI] [PubMed] [Google Scholar]
- 6.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-jB and IjBa kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24:6957–6969. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 7.Govindarajan VS. Ginger--chemistry, technology, and quality evaluation: part 1. Crit Rev Food Sci Nutr. 1982;17(1):1–96. doi: 10.1080/10408398209527343. [DOI] [PubMed] [Google Scholar]
- 8.Ravindran PN, Boca Raton KNB. Ginger. The Genus Zingiber. Florida, USA: CRC Press; 2005. [Google Scholar]
- 9.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46(2):409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 10.Shobana S, Naidu KA. Antioxidant activity of selected Indian spices. Prostaglandins Leukot Essent Fatty Acids. 2000;62(2):107–110. doi: 10.1054/plef.1999.0128. [DOI] [PubMed] [Google Scholar]
- 11.Surh YJ, Park KK, Chun KS, Lee LJ, Lee E, Lee SS. Anti-tumor-promoting activities of selected pungent phenolic substances present in ginger. J Environ Pathol Toxicol Oncol. 1999;18(2):131–139. [PubMed] [Google Scholar]
- 12.Smith RM. Analysis of the pungent principles of ginger and grains of paradise by high-performance liquid chromatography using electrochemical detection. Chromatographia. 1982;16:155–157. [Google Scholar]
- 13.Harvey DJ. Gas chromatographic and mass spectrometric studies of ginger constituents : Identification of gingerdiones and new hexahydrocurcumin analogues. J. Chromatogr. 1981;212(1):75–84. [Google Scholar]
- 14.He XG, Bernart MW, Lian LZ, Lin LZ. High-performance liquid chromatography–electrospray mass spectrometric analysis of pungent constituents of ginger. J. Chromatogr A. 1998;796:327–334. [Google Scholar]
- 15.Schwertner HA, Rios DC. High-performance liquid chromatographic analysis of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol in ginger-containing dietary supplements, spices, teas, and beverages. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856(1–2):41–47. doi: 10.1016/j.jchromb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Svendsen CN. Multi-electrode array detectors in high-performance liquid chromatography: a new dimension in electrochemical analysis. Analyst. 1993;118:123–129. [Google Scholar]
- 17.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1025–1032. [PubMed] [Google Scholar]
- 18.Sun CL, Yuan JM, Lee MJ, Yang CS, Gao YT, Ross RK, Yu MC. Urinary tea polyphenols in relation to gastric and esophageal cancers: a prospective study of men in Shanghai, China. Carcinogenesis. 2002;23(9):1497–1503. doi: 10.1093/carcin/23.9.1497. [DOI] [PubMed] [Google Scholar]
- 19.Yuan JM, Gao YT, Yang CS, Yu MC. Urinary biomarkers of tea polyphenols and risk of colorectal cancer in the Shanghai Cohort Study. Int J Cancer. 2007;120(6):1344–1350. doi: 10.1002/ijc.22460. [DOI] [PubMed] [Google Scholar]
- 20.Sang S, Hong J, Wu H, Liu J, Yang CS, Pan MH, Badmaev V, Ho CT. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J Agric Food Chem. 2009;57(22):10645–10650. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MJ, Prabhu S, Meng X, Li C, Yang CS. An improved method for the determination of green and black tea polyphenols in biomatrices by high-performance liquid chromatography with coulometric array detection. Anal Biochem. 2000;279(2):164–169. doi: 10.1006/abio.2000.4487. [DOI] [PubMed] [Google Scholar]
- 22.Bhattarai S, Tran VH, Duke CC. The stability of gingerol and shogaol in aqueous solutions. J Pharm Sci. 2001;90(10):1658–1664. doi: 10.1002/jps.1116. [DOI] [PubMed] [Google Scholar]