Summary
In contrast to the well-established roles of the striatum in movement generation and value-based decisions, its contributions to perceptual decisions lack direct experimental support. Here we show that electrical microstimulation in the monkey caudate nucleus influences both choice and saccade response time on a visual motion discrimination task. Within a drift-diffusion framework, these effects consist of two components. The perceptual component biases choices toward ipsilateral targets, away from the neurons’ predominantly contralateral response fields. The choice bias is consistent with a non-zero starting value of the diffusion process, which increases and decreases decision times for contralateral and ipsilateral choices, respectively. The non-perceptual component decreases and increases non-decision times toward contralateral and ipsilateral targets, respectively, consistent with the caudate’s role in saccade generation. The results imply a causal role for the caudate in perceptual decisions used to select saccades that may be distinct from its role in executing those saccades.
Introduction
The basal ganglia have been known for more than a century to play important roles in movement control (Ferrier, 1873; Wilson, 1914). Over the last several decades, their roles in more cognitive functions, including various forms of decision making, have also become better appreciated (Brown et al., 1997; Divac et al., 1967; Middleton and Strick, 2000). For example, the basal ganglia have been causally linked to reward-modulated behavior and represent a key component in value-based decision making (Barto, 1995; Cai et al., 2011; Hikosaka et al., 2006; Hollerman et al., 2000; Kable and Glimcher, 2009; Samejima and Doya, 2007). It is unclear if and how the basal ganglia also contribute to perceptual decisions that link sensory input to oculomotor output.
Support for the basal ganglia’s role in perceptual decision making comes from several sources. The basal ganglia receive diverse anatomical inputs from almost all parts of sensory and sensory-motor cortical areas (Fig. 1A). These areas include the middle temporal (MT) and medial superior temporal (MST) areas of extrastriate cortex, lateral intraparietal cortex (LIP), and parts of prefrontal cortex including the frontal eye field (FEF) (Maunsell and van Essen, 1983; Saint-Cyr et al., 1990; Selemon and Goldman-Rakic, 1985; Selemon and Goldman-Rakic, 1988; Yeterian and Pandya, 1995), all with well-characterized activity related to a task linking a decision about visual motion to saccadic eye movements (Britten et al., 1996; Britten et al., 1992; Ding and Gold, 2012; Ditterich et al., 2003; Hanks et al., 2006; Kim and Shadlen, 1999; Newsome et al., 1989; Roitman and Shadlen, 2002; Salzman et al., 1992; Shadlen and Newsome, 1996). Theoretical studies have ascribed several decision-related computations to specific components of the basal ganglia (Berns and Sejnowski, 1995; Bogacz and Gurney, 2007; Lo and Wang, 2006; Rao, 2010). Single-unit activity in the caudate nucleus, a primary input station of the basal ganglia, can encode a number of decision-related signals in monkeys performing the visual motion saccade task (Ding and Gold, 2010). fMRI studies revealed striatal activation in human subjects performing visual motion discrimination tasks (Forstmann et al., 2008; van Veen et al., 2008). In contrast, the frequency of clinically observed perceptual impairments is much lower than that of motor deficits for diseases associated with basal ganglia dysfunction (e.g., Parkinson’s disease). This observation seems to argue against a major role of the basal ganglia in perceptual decision-making, although non-motor symptoms are often under-reported or unrecognized by clinicians (Chaudhuri et al., 2006).
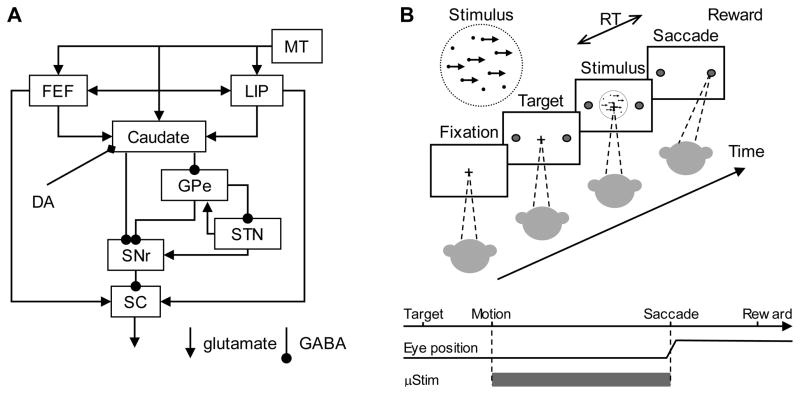
Figure 1. Simplified oculomotor system diagram (A) and the behavioral task (B).
A. A schematic drawing illustrating the major connections of the oculomotor basal ganglia for saccade control. Abbreviations: MT: middle temporal visual area; LIP: lateral intraparietal cortex; FEF: frontal eye field; GPe: external segment of the globus pallidus; STN: subthalamic nucleus; SNr: substantia nigra pars reticulata; SC: superior colliculus; DA: dopamine. B. The monkey decides the direction of random-dot motion and then responds, at a self-determined time, by making a saccade to foveate one of two choice targets. Saccades to the target in the direction of coherent motion (assigned randomly for 0% coherence) are followed by juice reward. Electrical microstimulation is delivered during motion viewing and terminated when the monkey makes a saccade.
In this study, we used electrical microstimulation in the caudate nucleus in monkeys performing a visual motion discrimination task (Fig. 1) to address three questions: 1) Is there a causal link between caudate activity and perceptual decision behavior? 2) What are the specific decision-related computations that are influenced by caudate activity? 3) How do the basal ganglia’s roles in perceptual decisions relate to their roles in movement control? The results indicate that the basal ganglia can bias perceptual decisions towards particular alternatives. These effects are distinct from their role in movement execution. Thus, the basal ganglia appear to make multiple, causal contributions to simple decisions that link sensory input to motor output.
Results
Microstimulation did not alter the monkeys’ task strategy
As described in previous reports (Ding and Gold, 2010, 2012), the performance of the two monkeys on the RT dots task depended critically on the strength (coherence) of the motion stimulus. Both monkeys achieved near-perfect accuracy and had the shortest RTs for coherences >20%, with steadily decreasing accuracy and increasing RT at lower coherences (Fig. 2). We fit choice and RT performance simultaneously with a drift-diffusion model (DDM, see Experimental Procedures and curves in Fig. 2), and we fit choice data alone using logistic functions (Fig. S2). We quantified performance using two measures estimated from the fits: choice bias, corresponding to the horizontal position of the psychometric curve (Fig. 2, top panels); and discrimination threshold, corresponding to the steepness of the psychometric curve.
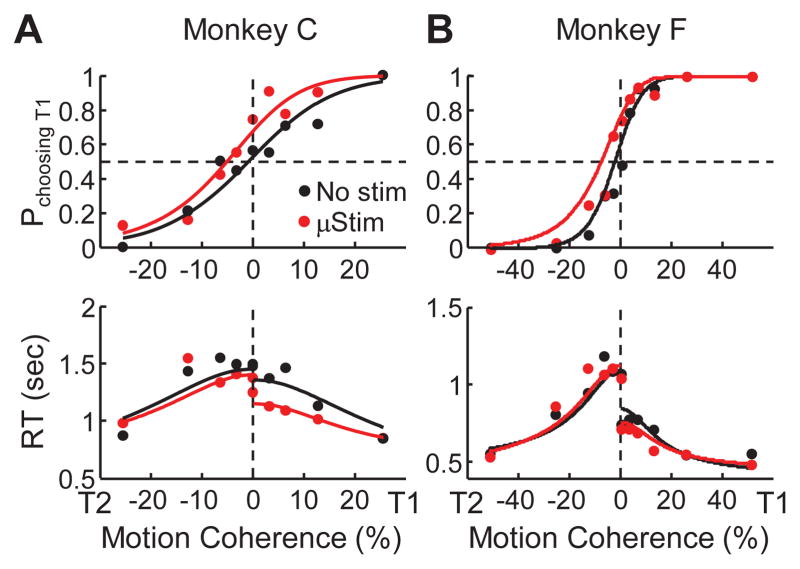
Figure 2. Example performance.
Example psychometric (top) and chronometric (bottom) functions from a single session for monkey C (A) and monkey F (B). Psychometric functions are plotted as the fraction of trials in which the monkey chose the T1 target as a function of signed coherence, where positive/negative coherence indicates motion towards T1/T2. Chronometric functions are plotted as the mean RT, measured as the time between motion stimulus onset and saccade onset, on correct trials as a function of signed coherence. Solid curves are simultaneous fits of both functions to a drift-diffusion model (DDM) with asymmetric bounds, separately for trials with (red) and without (black) electrical stimulation.
We examined the effects of electrical microstimulation on performance in 43 sessions (n=29 and 14, for monkey C and F, respectively). The microstimulation sites were within the general regions sampled in our previous recording study (Fig. S1; Ding and Gold, 2010). The motion directions used were similar to our previous recoding studies for the caudate nucleus and FEF (Table S1; Ding and Gold, 2010, 2012). The inclusion of randomly interleaved microstimulation trials did not appear to affect the monkeys’ overall strategy for solving the task. For example, when comparing performance on trials without microstimulation from this study to performance of the same monkeys on the same task in a recent study in which no microstimulation was used (Ding and Gold, 2012), choice bias and discrimination threshold were not significantly different for both monkeys and for all motion axes tested (Wilcoxon rank sum test, p > 0.05). Moreover, the DDM fit separately to trials with and without microstimulation in this study had comparable goodness-of-fits (Wilcoxon signed rank test for H0: equal log-likelihood, p=0.14).
Microstimulation influenced choice and RT
The effects of caudate microstimulation on performance are shown for two representative sessions in Fig. 2. In both cases, microstimulation caused the monkeys to favor the T1 choice (ipsilateral to the microstimulation sites), reflected in a leftward shift of the psychometric function (top panels). The T1 choices also tended to have a shorter mean RT on microstimulation trials, reflected in a downward shift in the chronometric function for positive coherence values (bottom panels). Using the DDM fit simultaneously to psychometric and chronometric data, the change in bias when comparing trials with and without microstimulation (Δbias; positive/negative values imply more T2/T1 choices on microstimulation trials) was −4.2% and −5.0% coherence for monkeys C and F, respectively, for these sessions (bootstrap methods, p<0.05 for both). In contrast, Δ threshold (positive/negative values imply higher/lower threshold on microstimulation trials) was −1.1% and 2.2% coherence, respectively (bootstrap methods, p>0.05 for both).
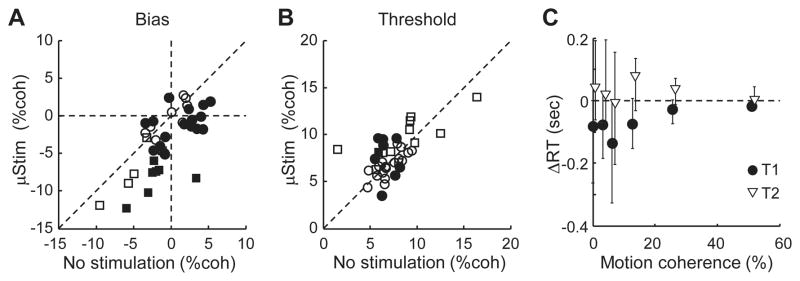
Across sessions, electrical microstimulation had a consistent effect on choice biases, inconsistent effects on thresholds, and mixed effects on RTs (Fig. 3). A significant Δbias was observed in 18 out of 29 and 7 out of 14 sessions for monkeys C and F, respectively (we defined a significant effect as a session in which the value measured on trials with microstimulation fell outside of the mean±2SD of the distribution of values measured using bootstrapping from trials without microstimulation). Moreover, Δbias tended to be negative, representing an increased preference for ipsilateral or upward choices (Fig. 3A; mean Δbias=−2.7% coherence, t-test for H0: mean=0, p<0.0001). In contrast, a significant Δthreshold was observed in only 8 and 1 sessions for monkeys C and F, respectively, with a mean value across all sessions that did not differ significantly from zero (Fig. 3B; mean=0.2% coherence; p=0.47). For sessions with significant non-zero Δbias, the mean RT for correct, microstimulation-favored choices was shorter on microstimulation trials (Wilcoxon signed rank test, p=0.0026), an effect that was larger for lower coherences (Fig. 3C, circles). The mean RT for correct, other choices was not different between microstimulation conditions (p=0.37, Fig. 3C, triangles). For other sessions, RT was not significantly affected for either choice (p=0.41 and 0.15 for T1 and T2 choices, respectively).
Figure 3. Summary of microstimulation effects (n = 43 sessions).
A and B. Scatterplots of bias (A) and threshold (B) estimates from DDM fits for trials with (ordinate) and without (abscissa) caudate microstimulation. Sessions with significant stimulation effects (see Experimental Procedures) are indicated by filled symbols. C. Median difference in mean RT between trials with and without microstimulation. Error bars indicate 25th and 75th percentiles.
The observed Δbias was robust and independent of our choice of fitting with a DDM. Using logistic-only psychometric fits, microstimulation-induced Δbias was observed in 17 out of 29 and 9 out of 14 sessions for monkeys C and F, respectively (Fig. S2). The mean Δbias was −2.5% coherence (p<0.0001). The mean Δthreshold was −0.2% coherence, which did not differ significantly from zero (p=0.57). The microstimulation effects also did not persist beyond the trial on which it was applied: there were no consistent effects on bias or threshold for the next trial (considering only such trials without microstimulation) in sessions with a significant current-trial effect (paired t-test, p=0.42 and 0.34 for bias and threshold, respectively).
Microstimulation-induced choice bias correlated with neuronal spatial tuning
Some of the session-by-session variability in Δbias reflected differences in the spatial tuning properties of nearby neurons (Fig. 4). We quantified the spatial selectivity of isolated units by computing an ROC index constructed from average firing rates recorded during motion viewing, separated by T1 and T2 choices (Fig. S3). ROC index values<0.5 represent higher firing rates for T2 choices, whereas values >0.5 represent higher firing rates for T1 choices. Sites where more than one spatially tuned neurons were recorded on the same electrode were excluded (n=3).
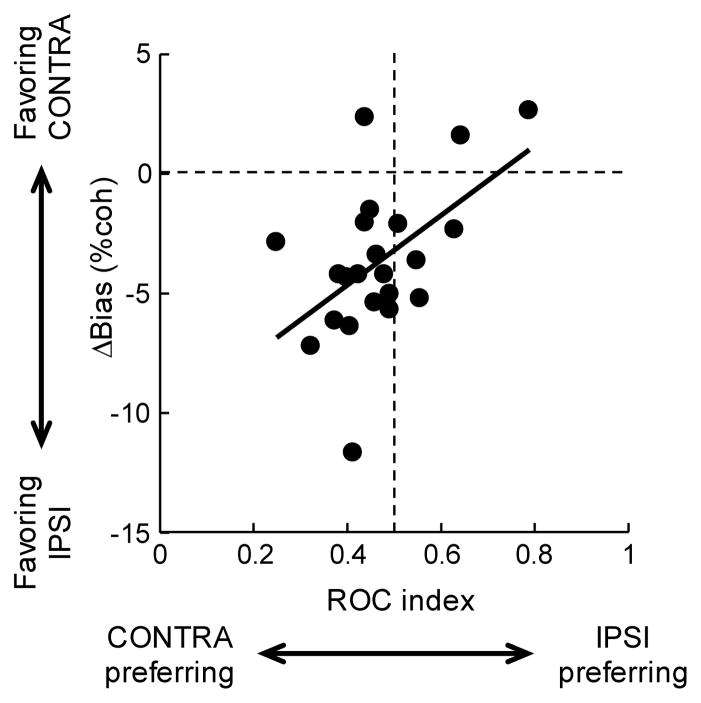
Figure 4. Microstimulation-induced choice bias correlates with the spatial tuning of neurons recorded at the site of microstimulation.
Spatial tuning was quantified using an ROC index (see also Fig. S3); values greater/less than 0.5 indicate stronger responses for T1/T2 choices. Choice bias is quantified as Δbias, the difference in estimated bias for trials with and without microstimulation; positive/negative values indicate increased preferences for T2/T1 choices. Data clustering in the top right and bottom left quadrants indicate that the microstimulation biases choice away from neurons’ preferred direction. Only sessions with a significant microstimulation-induced Δbias and single recorded neurons are included (n=22). Line is a linear fit (F-value=7.41, p=0.013).
For sites with statistically significant Δbias, the microstimulation-induced bias tended to be in the opposite direction as the spatial selectivity of nearby neurons (Fig. 4). As previously reported, caudate neurons have predominantly contralateral spatial preferences for this task (Ding and Gold, 2010), reflected as more data points to the left of the vertical dashed line in the figure (ROC indices <0.5). In contrast, the microstimulation-induced choice bias predominantly favored ipsilateral choices, reflected as more data points below the horizontal dashed line in the figure (Δbias<0).
In addition, the magnitude of Δbias varied systematically with the value of the ROC index (linear regression in Fig. 4). Microstimulation at sites with the strongest contralateral-preferring responses tended to have the strongest ipsilateral-biasing effects. In contrast, microstimulation at sites with the strongest ipsilateral-preferring responses tended to have slightly contralateral-biasing effects. For the 9 sites with statistically significant Δthreshold, we did not observe any relationship between threshold change and neuronal spatial selectivity (data not shown; linear regression, p=0.88).
Microstimulation-induced choice bias reflected a non-zero starting value in DDM
We further used the DDM to test two (not necessarily mutually exclusive) hypotheses about the source of the microstimulation-induced choice biases (Hanks et al., 2006). One possibility is that microstimulation corresponds to an asymmetry in the amount of evidence needed for each of the two choices. When the decision bounds are fixed, the starting value (SV) of the accumulating decision variable in the DDM controls the relative diffusion distance for the two choices. In this case, a positive SV indicates a decrease in the distance for T1 choices and an increase in the distance for T2 choices, thus creating a bias toward T1 choices. The second possibility is that microstimulation adds momentary evidence (ME) to the accumulating decision variable, favoring the more frequent choice. In this case, ME modulates the rate of accumulation, with a positive value indicating that extra evidence for the T1 choice is added at every time step during evidence accumulation, thus creating a bias toward T1 choices.
Based on parameter fits using revised DDMs, the microstimulation-induced bias was better characterized using non-zero values of SV than ME (Fig. 5). Using a model containing both SV and ME terms, best-fitting values of SV, but not ME, tended to be different from zero and thus account for the choice biases (Fig. 5A; sign test for zero median: p=0.004 and 0.286, respectively). Using two reduced models with either SV or ME terms, but not both, the fits yielded positive values for both terms and thus could, in principle, account for a negative Δbias (Fig. 5B, median: 12% of bound distance and 2.7% coherence; p=0.0002 and 0.0041, respectively). However, the SV-only model accounted for the observed Δbias better than the ME-only model (Fig. 5C), resulting in a larger log-likelihood (equivalent to smaller Bayesian Information Criteria, or BIC, given the same number of parameters for the two models; Wilcoxon signed-rank test, p=0.012). Similar results hold if only sessions with negative Δbias were included in the analyses. Thus, within the DDM framework, microstimulation-induced choice bias was better characterized as a change in the relative amount of evidence needed for each choice than a change in the actual evidence.
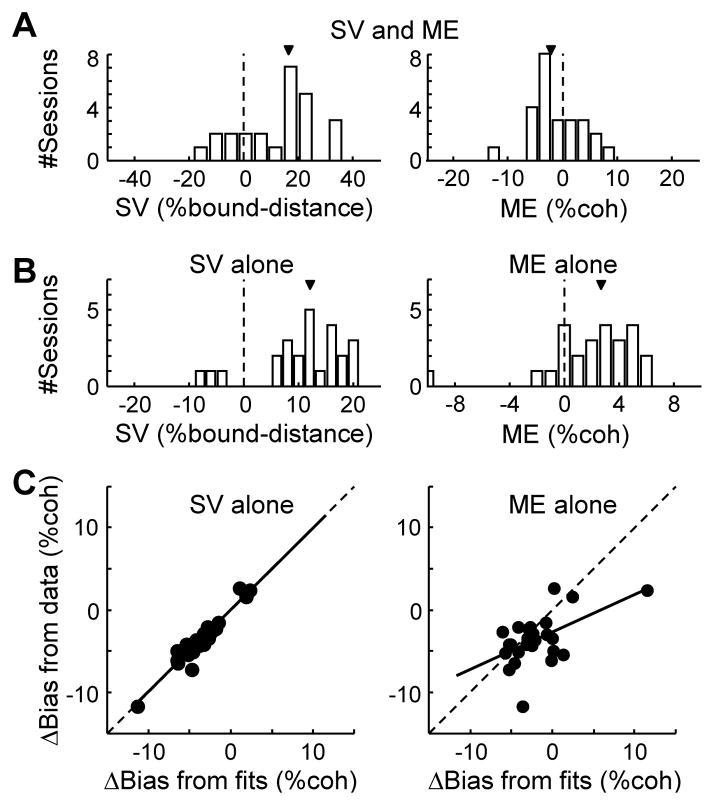
Figure 5. Microstimulation-induced Δbias is captured by a non-zero starting value in the DDM.
A. Histograms of best-fitting parameters SV (starting value, left) and ME (momentary evidence, right) in a DDM model with both terms (model 2; see also Table S2 and Experimental Procedures). Arrows indicate median values. SV is expressed as the percentage of bound distance (A+B in DDM). B. Histograms of best-fitting parameters SV (left) and ME (right) in the reduced models with each term alone (models 3 and 4, respectively). C. Scatterplots of Δbias measured from experimental data (ordinate), using independent DDM fits for trials with and without microstimulation, and Δbias predicted (abscissa) using the SV-alone model (left, model 3) or the ME-alone model (right, model 4). Solid lines are linear fits (slope: 0.99 and 0.45, F-value: 251.8 and 9.9, p: <0.0001 and 0.0045, respectively). For these analyses, sites with significant Δbias were included (n=25).
Microstimulation-induced changes in RT reflected both a non-zero starting value and changes in non-decision times in DDM
However, the SV term alone did not fully explain the microstimulation effect, especially the changes in RT. In particular, a positive starting value alone is expected to decrease and increase decision time toward T1 and T2 choices, respectively, with similar magnitudes (for example, see Fig. S4 and the shaded areas in Fig 6H). In contrast, caudate microstimulation resulted in increases in RT toward T2 that were much smaller in absolute magnitude than the decreases in RT toward T1 (Fig. 3C and Fig. 6H blue and red curves, respectively).
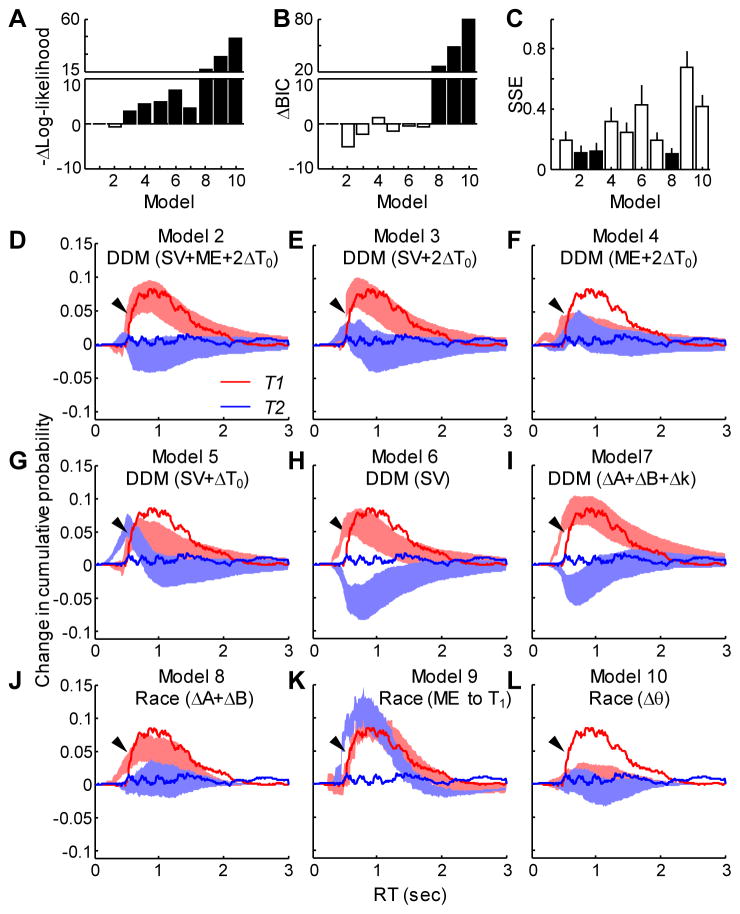
Figure 6. Comparison of models for capturing both choice and RT effects.
A. Difference in mean negative log likelihood between the given model and model 1 (two independent DDMs fit to trials with and without microstimulation). Note that the first bar is at zero by definition. The value for model 2 is close to zero. Smaller values indicate better fits. See Table S2 and panels D–L for model identities. Filled bars indicate a significant non-zero difference in means (t-test, p<0.05, with corrections for multiple comparisons). B. Difference in Bayesian Information Criterion from model 1. Same conventions as A. Smaller values indicate better fits. C. Sum of squared error (SSE, mean±SD) between the observed and simulated microstimulation-induced change in cumulative RT distributions. Filled bars indicate significantly smaller SSE in models 2, 3, and 8 than the others (p<0.05, with corrections for multiple comparison). D–L. Difference in the cumulative distribution of RT between trials with and without microstimulation (see Fig. S4) for models 2–10, as indicated. Experimental data are shown as solid curves. Simulation results are shown as shaded areas (mean ± SD). Red: T1 choice; blue: T2 choice. Arrows point to the rising portion of the T1 curve for the experimental data.
These RT effects did not result from our microstimulation protocol evoking inappropriate eye movements. For example, microstimulation did not evoke saccades or cause small eye movements: the standard deviation of eye position before saccade onset did not differ between trials with and without microstimulation (0.17°±0.06° versus 0.16°±0.04°; paired t-test, p=0.68 across sessions; Wilcoxon rank-sum test, p>0.05 for all individual sessions). Microstimulation also did not change the percentage of trials when the monkey broke fixation or made a saccade to neither choice target (paired t-test, p=0.59 and 0.99, respectively).
Instead, the microstimulation effects on RT appeared to reflect more subtle, asymmetric changes in saccade initiation. In the DDM framework, the time for decision formation is controlled by the two decision bounds (A and B), drift rate (scaled by k), and SV. The time for other aspects of the visuomotor transformation, including motor preparation and saccade initiation, is aggregated as non-decision times. RT is the sum of the decision and non-decision times. We thus hypothesized that microstimulation might also influence non-decision times. We compared six variants of the DDM and three variants of race models to the full DDM (i.e., the model used in Fig. 2) to identify the model that can best account for the microstimulation effects on psychometric and chronometric functions and also capture the microstimulation effect on cumulative RT distributions. The fitting parameters for the 10 models are listed in the second column of Table S2. Briefly, the DDM variants use combinations of parameters to capture the microstimulation effects: SV; ME; choice-dependent changes in non-decision times (ΔT01 and ΔT02); and changes in A, B, and k. The race model variants use changes in decision bounds (A and B), addition of ME to T1-accumulator alone, and changes in rectification threshold (θ). For these comparisons, we focused on sessions with negative Δbias (n=22) and used as the baseline the fitting results from the full DDM that fits trials with and without microstimulation separately (model 1).
In general, the DDM variants performed much better than the race models in fitting psychometric and chronometric functions (Fig. 6A and B). We assessed goodness-of-fit using two methods: log likelihood (Fig. 6A; Table S2), which does not take into account different numbers of fitting parameters, and BIC (Fig. 6B), which does. Both methods had smaller values (i.e., better fits) for all of the DDM variants than for all of the race models. BIC could not distinguish between the different variants of the DDM.
A more detailed analysis of the RT distributions indicated that our results were best matched by the two DDM variants that included an SV term and independent changes in non-decision times (model 2: SV+ME+2ΔT0; and model 3: SV+2ΔT0). These two DDM variants (along with the full model) and a race-model variant (model 8) produced the smallest sum-of-squares error between observed and simulated cumulative RT distributions (Fig. 6C). The better fits provided by these models can be readily appreciated with visual inspection (Fig. 6D–L). These panels depict the change in cumulative RT distributions between all trials with microstimulation versus all trials without microstimulation, computed separately for T1 (red) and T2 (blue) choices (see Fig. S4 for details). Models 2, 3, and 8 most effectively captured the asymmetry in microstimulation-induced changes in RT (larger effects for T1 than T2 choices) that we observed (Fig. 6D, E, and J, respectively; data for model 1 not shown). Models 2 and 3 also most effectively captured the dynamics of the microstimulation-induced changes in T1 RTs, including little change in short RTs but rapidly increasing effects for RTs >~500 ms (arrows). The goodness-of-fits of models 2, 3, and 8 for the changes in cumulative RT distribution, as measured by sum of squared error or R2 do not differ significantly (t-test, p>0.05). However, model 8 is worse than models 2 and 3 for fitting both psychometric and chronometric functions (Fig. 6A and B, Wilcoxon signed rank test, p<0.0001), indicating that the DDM models provided better overall fits.
Using model 3, which had the fewest parameters of models 1–3, best-fitting values of SV had a mean value of 12.2% of bound distance (sign test for non-zero median, p<0.0001), the non-decision time for choice T1 was prolonged by a median value of 41 ms (p=0.004), and the non-decision time for T2 was shortened by a median value of 62 ms (p=0.0008). Thus, caudate microstimulation seemed to have two effects: 1) a motion stimulus-dependent effect that promoted choices to T1, and 2) a motion stimulus-independent effect that delayed the execution of saccades to T1 and facilitated the execution of saccades to T2. These results were consistent with the influence of caudate microstimulation on separable decision and saccade processes, as opposed to two independent decision processes corresponding to the two alternatives in a race model.
Discussion
The caudate nucleus has been shown previously to contribute causally to saccade generation, the evaluation of expected outcomes, and mediation of reinforcement-based and associative learning (Kitama et al., 1991; Nakamura and Hikosaka, 2006a, b; Watanabe and Munoz, 2010; Williams and Eskandar, 2006). In this study we used electrical microstimulation to demonstrate for the first time that the caudate also causally contributes to perceptual decision-making. Applying microstimulation in the caudate of monkeys performing a direction-discrimination task affected both choice and RT. The effect on choice was consistent with an offset in the starting or ending value of an evidence-dependent accumulation process defined by a commonly used model of decision-making, the DDM. The effect on RT was consistent with the combined effects of the offset and concomitant facilitation and suppression of saccades toward contralateral and ipsilateral targets, respectively.
A main goal of this study was to help to position the basal ganglia pathway computationally in the overall decision process for this task. Anatomically, the caudate receives input from numerous cortical structures that contribute to the decision (Fig. 1A). These structures include area MT in extrastriate visual cortex, which contains direction-selective neurons that provide sensory evidence, and areas LIP in parietal cortex and FEF in prefrontal cortex, both of which contain neurons that encode the process of accumulating the sensory evidence over time into a decision variable that governs the saccadic response (Britten et al., 1996; Britten et al., 1992; Ding and Gold, 2012; Kim and Shadlen, 1999; Roitman and Shadlen, 2002; Shadlen and Newsome, 1996). Outputs of the oculomotor basal ganglia pathway target the superior colliculus, which also receives direct input from LIP and FEF and contains neurons that similarly encode the evidence-accumulation process (Horwitz and Newsome, 1999). We recently showed that certain task-driven neuronal activity in caudate also represents the accumulation of evidence, like in LIP, FEF, and the superior colliculus but not in MT (Ding and Gold, 2010). Our present results are consistent with these findings, indicating that caudate plays a similar, causal role in decision-making as that found previously for LIP but not MT using a comparable microstimulation protocol (Ditterich et al., 2003; Hanks et al., 2006). Together, these findings suggest that evidence accumulation used to instruct saccadic choices is implemented in a set of interconnected brain regions including LIP, FEF, the superior colliculus, and the basal ganglia pathway that indirectly links these cortical and subcortical structures.
Despite the similarities between our results and those for area LIP, we note two striking differences. The first is in the sign of choice bias, which for caudate is toward the target ipsilateral to the site of microstimulation but for LIP is toward the target contralateral to the site of microstimulation. The opposite signs are unlikely simply due to a difference in microstimulation pulse frequency, given that caudate microstimulation tends to have consistent effects on saccade behavior over a large frequency range (5–333 Hz, Watanabe and Munoz, 2010). The ipsilateral choice bias with caudate microstimulation is also unlikely an artifact from fiber-of-passage problems, given its observed relationship with the nearby neurons’ tuning properties (Fig. 4). It is conceivable that caudate microstimulation antidromically activates a distal, upstream region that has an opposite role to LIP’s in perceptual decision-making, although such a region has not yet been identified.
We thus consider an alternative explanation based on the intrinsic organization of the basal ganglia. The basal ganglia are organized into direct and indirect pathways (Fig. 1A), which are first segregated in the striatal population of projection neurons (DeLong, 1990; Graybiel and Ragsdale, 1979; Hikosaka et al., 1993; Hikosaka and Wurtz, 1983, 1985; Niijima and Yoshida, 1982). Activation of striatal projection neurons in the two pathways is assumed to have opposite effects on the basal ganglia output, resulting in net excitation or inhibition of the superior colliculus for the direct or indirect pathway, respectively (Fig. 1A). Accordingly, differential activation of these pathways has been shown to have opposite effects on motor behaviors and reinforcement learning (Kravitz et al., 2010; Kravitz et al., 2012; Nakamura and Hikosaka, 2006b) and thus, in principle, could have opposite effects on perceptual decisions. These two subpopulations of striatal projection neurons, although physically intermingled and indistinguishable with extracellular recordings, differ in their somatodendritic and synaptic properties (Ade et al., 2008; Cepeda et al., 2008; Day et al., 2008; Flores-Barrera et al., 2010; Gerfen et al., 1990; Gertler et al., 2008; Shen et al., 2007). We speculate that our electrical microstimulation preferentially activates the pathway that opposes the influence of LIP activation on downstream oculomotor structures including the superior colliculus. It will be interesting to test this hypothesis by specifically targeting the direct and indirect pathways with pharmacological manipulations or by comparing activity patterns in caudate and FEF/LIP with those found in components of the indirect pathway, such as the subthalamic nucleus or the external segment of globus pallidus.
The second difference between caudate and LIP microstimulation is their effects on RT. LIP microstimulation shortens RT for the favored choice and increases RT for the other choice with a similar magnitude (Hanks et al., 2006). In contrast, the effect of caudate microstimulation on RT is not symmetric for the two choices. Based on our modeling efforts, the best explanation for these caudate microstimulation results is a combined effect on a perceptual process favoring ipsilateral choices and a non-perceptual process (e.g., saccade execution) favoring contraversive saccades. The two effects may result from the influence of microstimulation on different neural assemblies in the caudate nucleus. This idea is consistent with the functional anatomy of the basal ganglia pathway, which is known to contain multiple parallel loops both in overall function (e.g., limbic, motor, associative) and in more microscopic domains (e.g., topographic projections throughout the pathway for body regions; Alexander and Crutcher, 1990; Alexander et al., 1986; Parent and Hazrati, 1995). A highly speculative scenario may be that activation of the direct pathway of the “motor” loop decreases and increases non-decision times for contra- and ipsilateral saccades, respectively, whereas activation of the indirect pathway of the “perception” loop biases choice towards ipsilateral targets.
The idea that caudate encodes two distinct, task-related processes – one involved in forming the perceptual decision, the other in oculomotor control – may also help to bridge seemingly conflicting results from previous studies of caudate microstimulation. Specifically, caudate microstimulation can evoke contraversive saccades and, when delivered before saccade onset and at sites with neural activity modulated on a simple visually guided saccade task, reduces RT for contraversive saccades (Kitama et al., 1991; Nakamura and Hikosaka, 2006a). These results suggest a facilitatory effect of microstimulation on contraversive saccades. In contrast, when delivered before saccade onset at “blindly” sampled sites, caudate microstimulation increases RT for contraversive saccades and, to a lesser extent, decreases RT for ipsiversive saccades on a pro-/anti-saccade task (Watanabe and Munoz, 2010, 2011). These results suggest a suppressive effect of microstimulation on contraversive saccades. In light of our observations, these previous reports may have resulted from differential activation of distinct functional groups of neurons. More specifically, microstimulation that preferentially activates neurons participating in saccade generation facilitates generation of contraversive saccades. In contrast, microstimulation that preferentially activates neurons participating in perceptual-decision formation or other cognitively demanding forms of saccade selection facilitates selection of ipsilateral saccade targets. The former effect dominates for evoked saccades and for simple saccade tasks with targeted microstimulation sites. Both effects are in place for pro-/anti-saccade tasks with blindly sampled microstimulation sites and for the dots task. The dots task enables the dissociation of perceptual decision-making and saccade effects, with manipulations of stimulus strength (Petrov et al., 2011).
In contrast to the microstimulation effects on choice bias, we did not observe a consistent effect on discrimination threshold. This result is consistent with our interpretation of caudate response properties in the context of the DDM (Ding and Gold, 2010). According to that framework, discrimination threshold is determined by the decision bounds and a constant of proportionality used to convert the evidence to a log likelihood ratio-related quantity (Gold and Shadlen, 2002; Ratcliff, 1978). The decision bounds govern the speed-accuracy trade-off and in our previous study were not encoded in caudate: unlike in LIP and FEF, evidence-accumulation activity in caudate did not converge at a DDM-like bound just prior to saccade onset on the RT dots task (Ding and Gold, 2010, 2012; Roitman and Shadlen, 2002). The constant of proportionality may already be incorporated in the inputs from MT and thus not influenced by caudate microstimulation. However, despite this consistency with our previous recording study, the lack of an effect on discrimination threshold is not consistent with previous computational modeling and fMRI studies that posit a role for the basal ganglia pathway in mediating the appropriate speed-accuracy tradeoff (Bogacz et al., 2010; Brown et al., 2004; Forstmann et al., 2008; Frank, 2006; Gurney et al., 2004; Lo and Wang, 2006; Rao, 2010; van Veen et al., 2008). This discrepancy might reflect a sampling bias in the present study favoring sites with the kind of task-modulated neural activity we described previously. More work is needed to identify other possible neural correlates of the decision bound and determine their causal role in the decision process.
In summary, caudate microstimulation influences both choice and RT in monkeys performing a demanding perceptual decision task. These effects support causal roles of the caudate nucleus - and by extension the basal ganglia - in mediating perceptual decision formation and saccade generation. In conjunction with their reported roles in valuation of different options, the basal ganglia are well positioned to play important roles in real-life, complex decisions that must take into account of multiple sources of external inputs and internal preferences.
Experimental Procedures
We used two adult male rhesus monkeys (Macaca mulatta) that were previously trained on the direction-discrimination (dots) task used in this study (Ding and Gold, 2010, 2012). Each monkey was implanted with a head holder and a recording cylinder that provided access to the right caudate. Procedures for identifying and recording from caudate neurons are described previously (Ding and Gold, 2010). Prior to the microstimulation experiment, monkey C was trained on various versions of the dots task for >5 years and used for data collection in three previous studies (Ding and Gold, 2010, 2012; Law and Gold, 2008); monkey F was trained for two years and used for data collection in two previous studies (Ding and Gold, 2010, 2012). Both monkeys showed clear sensitivity to motion strength and stimulus duration on a fixed-duration version of the task (monkey C: (Law and Gold, 2008); monkey F: Fig. S5) and speed-accuracy tradeoff on the RT task (Ding and Gold, 2010). All training, surgery, and experimental procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
The dots task requires the subject to decide the direction of random-dot motion and respond as soon as the decision is formed with a saccadic eye movement (Fig. 1A; Ding and Gold, 2010). Briefly, after the monkey maintained central fixation for an exponentially distributed duration, a random-dot motion stimulus was presented in a 5° aperture centered on the fixation point, with a fixed velocity of 6°/s in one of two opposite motion directions. Motion direction and strength (the percent of dots moving coherently in one direction) were randomly interleaved. For most sessions, the coherence values used were 0, 3.2, 6.4, 12.8, 25.6, and 51.2%. To increase the number of trials per condition for microstimulation experiments, 51.2% coherence trials were omitted in 14 sessions for monkey C, who consistently performs at 100% correct for 51.2% and nearly 100% correct at 25.6% coherence without microstimulation. After stimulus onset, the monkey was free to indicate its decision about the motion direction at any time, by making a saccade to the corresponding visual choice target. The choice targets were placed along the axis of motion, symmetric with regard to the central fixation point and at an eccentricity of 10° (Fig. 1B). The stimulus was turned off once a saccade was detected. The monkey was rewarded with juice for choosing the correct choice target (congruent with the motion direction at non-zero coherence levels; randomly picked for 0%-coherence trials). Eye position was monitored using a video-based system (ASL) sampled at 240 Hz. Reaction time (RT) was measured as the time from stimulus onset to saccade onset, the latter identified offline with respect to velocity (> 40°/s) and acceleration (> 8000°/s2).
At the beginning of a session, we identified a caudate site with single- or multi-unit activity modulated on the dots task. Neural activity was recorded using glass-coated tungsten electrodes (Alpha-Omega) or polyamide-coated tungsten electrodes (FHC, Inc.). The motion direction that elicited the largest responses was determined by online visual inspection and then used to define the axis of motion for the dots task used in the remainder of the experimental session (Table S1). Unlike cortical regions such as MT and LIP, the caudate is not topographically organized and nearby neurons do not necessarily share the same response profiles (Ding and Gold, 2010; Hikosaka et al., 1989). We thus selected microstimulation sites based on only neural activity at those sites without considering nearby neural activity. Electrical microstimulation was delivered at the same site during motion stimulus presentation (negative-leading bipolar current pulses, 300 Hz, 50–80 μA, 250 μs pulse duration). These parameters were chosen to maximize potential effect sizes while avoiding evoked saccades (Nakamura and Hikosaka, 2006a; Watanabe and Munoz, 2010, 2011). Because higher currents are needed to activate the thinner, sparsely myelinated projection axons in the caudate nucleus compared to the thicker, more myelin-dense projection axons of the cortex, the current intensity used is expected to have similar effective current spread to that of comparable microstimulation studies in cortex (Adinolfi and Pappas, 1968; Blatt et al., 1990; Felleman and Van Essen, 1991; Spatz and Tigges, 1972; Tehovnik, 1996; Tomasi et al., 2012). Trials with and without microstimulation were equally divided and randomly interleaved in a session. The neural responses were sorted offline (Plexon, Inc.). Each neuron’s spatial selectivity was quantified as a receiver operating characteristic (ROC) index, which is the area under the ROC curve constructed using average spike rate during motion viewing (from 200 ms after stimulus onset to 100 ms before saccade onset, all coherence levels were included; also see Fig. S3).
Performance was quantified with psychometric and chronometric functions (Fig. 2), which describe the relationship of motion strength (signed coherence, Coh, positive for towards T1, negative for towards T2) with choice and RT, respectively. Per laboratory convention, T1 choice targets were placed in the right hemifield (ipsilateral to the microstimulation sites) or directly above the central fixation point (for T1=90°). Performance on trials with and without microstimulation was analyzed using five methods, as follows (all model fits were accomplished using maximum-likelihood methods):
First, we fit psychometric and chronometric functions simultaneously to a drift-diffusion model (DDM), which has been used successfully to describe performance of both monkey and human subjects on the RT dots task (Hanks et al., 2006; Palmer et al., 2005). Here we used separate fits for trials with and without microstimulation, using a model with five free parameters (model 1): A, B, k, T01, and T02. According to this model, momentary motion evidence is assumed to follow a Gaussian distribution N(μ, 1), the mean of which, μ, scales with coherence: μ= k×Coh, where k governs the coherence-dependent drift. A decision variable is computed as the temporal accumulation of this momentary motion evidence. A decision (T1 or T2) is reached when the value of the decision variable reaches a decision bound (+A or −B, respectively). Decision time is defined as the interval between stimulus onset and crossing of either decision bound. RT is the sum of decision time and non-decision time (T01 for a T1 choice and T02 for a T2 choice). Within this framework, the probability of choosing T1 (i.e., the probability that the decision variable reaches bound +A first) is . The average decision time is for T1 decisions and for T2 decisions. Threshold was estimated from the choice function as one-half the difference in coherence corresponding to 25 and 75% T1 choices (Klein, 2001). Bias was defined as the signed percent coherence corresponding to 50% T1 choices. Distributions of estimated threshold and bias for trials without microstimulation were estimated by repeating fits with resampled trials. A statistically significant microstimulation effect was identified if the value from microstimulation trials fell outside the mean ±2SD of the values from resampled no-microstimulation trials. An alternative bootstrapping method, which estimates the probability of obtaining the experimentally observed Δbias/Δ threshold from all trials with shuffled microstimulation conditions, gave similar results (data not shown).
Second, to ensure that our results were not overly conditioned on the assumptions of the DDM, we also fit psychometric data alone using a logistic function, , separately for trials with and without microstimulation. Discrimination threshold was defined as one-half the difference in coherence corresponding to 25 and 75% T1 choices from the fitted function (Klein, 2001). Bias was defined as the value of α. Statistically significant microstimulation effects were detected using the bootstrap methods described above.
Third, we adopted a modified DDM to fit psychometric and chronometric functions simultaneously for trials with and without microstimulation (model 2). This model uses the same basic parameters as in the above drift-diffusion model (A, B, k, T01, and T02). In addition, we introduced two terms similar to a previous study to account for the microstimulation-induced choice biases (Hanks et al., 2006): starting value (SV) and momentary evidence (ME). SV was implemented as a change in decision bounds: +A/−B for no microstimulation trials and +A-SV/−B-SV for microstimulation trials. ME was implemented as a change in momentary motion evidence: μ= k×Coh for no microstimulation trials and μ= k×(Coh+ME) for microstimulation trials. Positive SV or ME corresponds to an increased bias towards T1. To account for possible microstimulation effects on non-decision processes, we introduced two additional non-decision times (T01′ and T02′) for trials with microstimulation.
Fourth, to further investigate effects of microstimulation on both choice and RT, we compared goodness-of-fits of six versions of the DDM (models 2–7). All of these models use the five basic parameters as in the above drift-diffusion model: A, B, k, T01 and T02. In addition, they use combinations of additional parameters to capture the microstimulation effects (see Table S2 for more details): SV; ME; choice-dependent changes in non-decision times (two sets of T01 and T02 for trials with and without microstimulation); and changes in A, B, and k (two sets of A, B, and k for trials with and without microstimulation). We also implemented race models of independent accumulators with rectified inputs (models 8–10; Smith and Vickers, 1988) to test for the possibility that caudate’s role in the decision process is inconsistent with a basic assumption of DDM, that a single decision variable governs the decision process. According to the basic race model, momentary motion evidence is assumed to follow a Gaussian distribution N(μ, 1), the mean of which, μ, scales with coherence: μ= k×Coh, where k governs the coherence-dependent drift. The motion evidence is compared to a threshold θ. One accumulator integrates the difference between the motion evidence and θ only if the difference is positive, while the other accumulator integrates the difference only if the difference is negative. If the first accumulator reaches bound +A before the other reaching bound −B, a choice toward T1 is made; if the second accumulator reaches bound −B first, a choice toward T2 is made. The steps of accumulation is converted to actual decision time by a scaling factor, α. Similar to the DDM, RT is the sum of decision and non-decision times (T01 and T02). To capture the microstimulation effects, we considered three variations of the basic race model: 1) separate changes in A and B by microstimulation, 2) a constant ME value added at each step of accumulation for the first accumulator, and 3) a change in θ. Goodness-of-fit was measured as the log-likelihood for each model and compared across models using BIC to take into account different numbers of fitting parameters.
Fifth, we examined microstimulation-induced effects on RT distributions. For each session, we collapsed trials (correct and error) across coherence levels and computed the cumulative RT distributions, separately for the two choices and microstimulation conditions (Fig. S4). For each choice, we computed the difference in cumulative RT distributions between trials with and without microstimulation. The microstimulation effect on the RT distribution was measured as the average difference across sessions, separately for the two choices. For model predictions, choice and RT data were simulated with session-specific fitting parameters and with trial numbers for the different coherence × direction conditions matched to the experimental data. Simulated data were analyzed in the same way as the experimental data. Mean and standard deviation of the simulated difference in cumulative RT distribution were estimated using bootstrap methods.
Supplementary Material
Acknowledgments
We thank Takahiro Doi, Matt Nassar, and Yin Li for helpful comments, and Jean Zweigle for animal care. This work was supported by NIH K99–EY018042 and ARRA supplement (L.D.) and R01–EY015260 (J.I.G.) from the National Eye Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. J Neurosci. 2008;28:1185–1197. doi: 10.1523/JNEUROSCI.3908-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinolfi AM, Pappas GD. The fine structure of the caudate nucleus of the cat. J Comp Neurol. 1968;133:167–184. doi: 10.1002/cne.901330203. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Barto AG. Adaptive critics and the basal ganglia. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. Cambridge, MA: MIT Press; 1995. pp. 215–232. [Google Scholar]
- Berns GS, Sejnowski TJ. How the basal ganglia make decisions. In: Damasio AR, Damasio H, Christen Y, editors. Neurobiology of Decision-Making. Berlin: Springer-Verlag; 1995. pp. 101–113. [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Gurney K. The basal ganglia and cortex implement optimal decision making between alternative actions. Neural Comput. 2007;19:442–477. doi: 10.1162/neco.2007.19.2.442. [DOI] [PubMed] [Google Scholar]
- Bogacz R, Wagenmakers EJ, Forstmann BU, Nieuwenhuis S. The neural basis of the speed-accuracy tradeoff. Trends Neurosci. 2010;33:10–16. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Visual neuroscience. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Bullock D, Grossberg S. How laminar frontal cortex and basal ganglia circuits interact to control planned and reactive saccades. Neural Netw. 2004;17:471–510. doi: 10.1016/j.neunet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:157–163. doi: 10.1016/s0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- Cai X, Kim S, Lee D. Heterogeneous coding of temporally discounted values in the dorsal and ventral striatum during intertemporal choice. Neuron. 2011;69:170–182. doi: 10.1016/j.neuron.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Ding L, Gold JI. Caudate encodes multiple computations for perceptual decisions. J Neurosci. 2010;30:15747–15759. doi: 10.1523/JNEUROSCI.2894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gold JI. Neural correlates of perceptual decision-making before, during and after decision commitment in monkey frontal eye field. Cerebral Cortex. 2012;22:1052–1067. doi: 10.1093/cercor/bhr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditterich J, Mazurek ME, Shadlen MN. Microstimulation of visual cortex affects the speed of perceptual decisions. Nat Neurosci. 2003;6:891–898. doi: 10.1038/nn1094. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Ferrier D. Experimental Researches in Cerebral Physiology and Pathology. J Anat Physiol. 1873;8:152–155. [PMC free article] [PubMed] [Google Scholar]
- Flores-Barrera E, Vizcarra-Chacon BJ, Tapia D, Bargas J, Galarraga E. Different corticostriatal integration in spiny projection neurons from direct and indirect pathways. Front Syst Neurosci. 2010;4:15. doi: 10.3389/fnsys.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmakers EJ. Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci U S A. 2008;105:17538–17542. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Banburismus and the brain: decoding the relationship between sensory stimuli, decisions, and reward. Neuron. 2002;36:299–308. doi: 10.1016/s0896-6273(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW., Jr Fiber connections of the basal ganglia. Prog Brain Res. 1979;51:237–283. [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Wickens JR, Redgrave P. Computational models of the basal ganglia: from robots to membranes. Trends Neurosci. 2004;27:453–459. doi: 10.1016/j.tins.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hanks TD, Ditterich J, Shadlen MN. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat Neurosci. 2006;9:682–689. doi: 10.1038/nn1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Miyashita N. Effects of caudate nucleus stimulation on substantia nigra cell activity in monkey. Exp Brain Res. 1993;95:457–472. doi: 10.1007/BF00227139. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J Neurophysiol. 1989;61:780–798. doi: 10.1152/jn.1989.61.4.780. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol. 1985;53:292–308. doi: 10.1152/jn.1985.53.1.292. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Prog Brain Res. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284:1158–1161. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Kitama T, Ohno T, Tanaka M, Tsubokawa H, Yoshida K. Stimulation of the caudate nucleus induces contraversive saccadic eye movements as well as head turning in the cat. Neurosci Res. 1991;12:287–292. doi: 10.1016/0168-0102(91)90118-i. [DOI] [PubMed] [Google Scholar]
- Klein SA. Measuring, estimating, and understanding the psychometric function: a commentary. Percept Psychophys. 2001;63:1421–1455. doi: 10.3758/bf03194552. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012 doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat Neurosci. 2008;11:505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, Wang XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci. 2006;9:956–963. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hikosaka O. Facilitation of saccadic eye movements by postsaccadic electrical stimulation in the primate caudate. J Neurosci. 2006a;26:12885–12895. doi: 10.1523/JNEUROSCI.3688-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J Neurosci. 2006b;26:5360–5369. doi: 10.1523/JNEUROSCI.4853-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- Niijima K, Yoshida M. Electrophysiological evidence for branching nigral projections to pontine reticular formation, superior colliculus and thalamus. Brain Res. 1982;239:279–282. doi: 10.1016/0006-8993(82)90852-6. [DOI] [PubMed] [Google Scholar]
- Palmer J, Huk AC, Shadlen MN. The effect of stimulus strength on the speed and accuracy of a perceptual decision. J Vis. 2005;5:376–404. doi: 10.1167/5.5.1. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Petrov AA, Van Horn NM, Ratcliff R. Dissociable perceptual-learning mechanisms revealed by diffusion-model analysis. Psychon Bull Rev. 2011;18:490–497. doi: 10.3758/s13423-011-0079-8. [DOI] [PubMed] [Google Scholar]
- Rao RP. Decision making under uncertainty: a neural model based on partially observable markov decision processes. Frontiers in computational neuroscience. 2010;4:146. doi: 10.3389/fncom.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R. Theory of Memory Retrieval. Psychological Review. 1978;85:59–108. [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Cyr JA, Ungerleider LG, Desimone R. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol. 1990;298:129–156. doi: 10.1002/cne.902980202. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Murasugi CM, Britten KH, Newsome WT. Microstimulation in visual area MT: effects on direction discrimination performance. J Neurosci. 1992;12:2331–2355. doi: 10.1523/JNEUROSCI.12-06-02331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K, Doya K. Multiple representations of belief states and action values in corticobasal ganglia loops. Ann N Y Acad Sci. 2007;1104:213–228. doi: 10.1196/annals.1390.024. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Motion perception: seeing and deciding. Proc Natl Acad Sci U S A. 1996;93:628–633. doi: 10.1073/pnas.93.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- Smith PL, Vickers D. The Accumulator Model of 2-Choice Discrimination. Journal of Mathematical Psychology. 1988;32:135–168. [Google Scholar]
- Spatz WB, Tigges J. Experimental-anatomical studies on the “middle temporal visual area (MT)” in primates. I. Efferent cortico-cortical connections in the marmoset Callithrix jacchus. J Comp Neurol. 1972;146:451–464. doi: 10.1002/cne.901460403. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ. Electrical stimulation of neural tissue to evoke behavioral responses. J Neurosci Methods. 1996;65:1–17. doi: 10.1016/0165-0270(95)00131-x. [DOI] [PubMed] [Google Scholar]
- Tomasi S, Caminiti R, Innocenti GM. Areal differences in diameter and length of corticofugal projections. Cereb Cortex. 2012;22:1463–1472. doi: 10.1093/cercor/bhs011. [DOI] [PubMed] [Google Scholar]
- van Veen V, Krug MK, Carter CS. The neural and computational basis of controlled speed-accuracy tradeoff during task performance. J Cogn Neurosci. 2008;20:1952–1965. doi: 10.1162/jocn.2008.20146. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Munoz DP. Saccade suppression by electrical microstimulation in monkey caudate nucleus. J Neurosci. 2010;30:2700–2709. doi: 10.1523/JNEUROSCI.5011-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Munoz DP. Saccade reaction times are influenced by caudate microstimulation following and prior to visual stimulus appearance. J Cogn Neurosci. 2011;23:1794–1807. doi: 10.1162/jocn.2010.21554. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Eskandar EN. Selective enhancement of associative learning by microstimulation of the anterior caudate. Nat Neurosci. 2006;9:562–568. doi: 10.1038/nn1662. [DOI] [PubMed] [Google Scholar]
- Wilson SAK. An experimental research into the anatomy and physiology of the corpus striatum. Brain. 1914;36:427–492. [Google Scholar]
- Yeterian EH, Pandya DN. Corticostriatal connections of extrastriate visual areas in rhesus monkeys. J Comp Neurol. 1995;352:436–457. doi: 10.1002/cne.903520309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.