Abstract
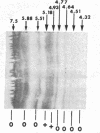
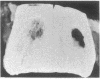
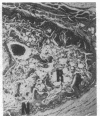
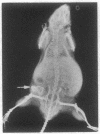
Bovine bone morphogenetic protein (bBMP) induces differentiation of mesenchymal-type cells into cartilage and bone. bBMP has an apparent Mr of 18,500 +/- 500 and represents less than 0.001% of the wet weight of bone tissue. A Mr 34,000 protein resembling osteonectin is separated by extraction with Triton X-100. A Mr 24,000 protein and about half of a Mr 22,000 protein are disassociated from bBMP by precipitation in 1.5 M guanidine hydrochloride. Aggregates of bBMP and a Mr 14,000 protein are insoluble in aqueous media; the bBMP becomes soluble when the Mr 14,000 protein is disassociated in 6 M urea and removed from the solution by ultrafiltration. Three separate molecular species with apparent Mrs 18,500, 17,500, and 17,000 are eluted at 0.10, 0.15, and 0.20 M phosphate ion concentrations, respectively, from a hydroxy-apatite column. The Mr 18,500 protein has the amino acid composition of acidic polylpeptide and includes four half-cystine residues; the pI is 4.9-5.1. The Mr 22,000 component is a chromoprotein resembling ferritin. The NH2-terminal amino acid sequence of the Mr 17,500 protein simulates histone H2B. The Mr 17,000 protein may possess calmodulin activity. Aggregates of the Mr 18,500 and other proteins induce formation of large deposits of bone; the Mr 18,500 protein alone is rapidly absorbed and induces formation of small deposits. None of the other proteins induces bone formation.
Full text
PDF
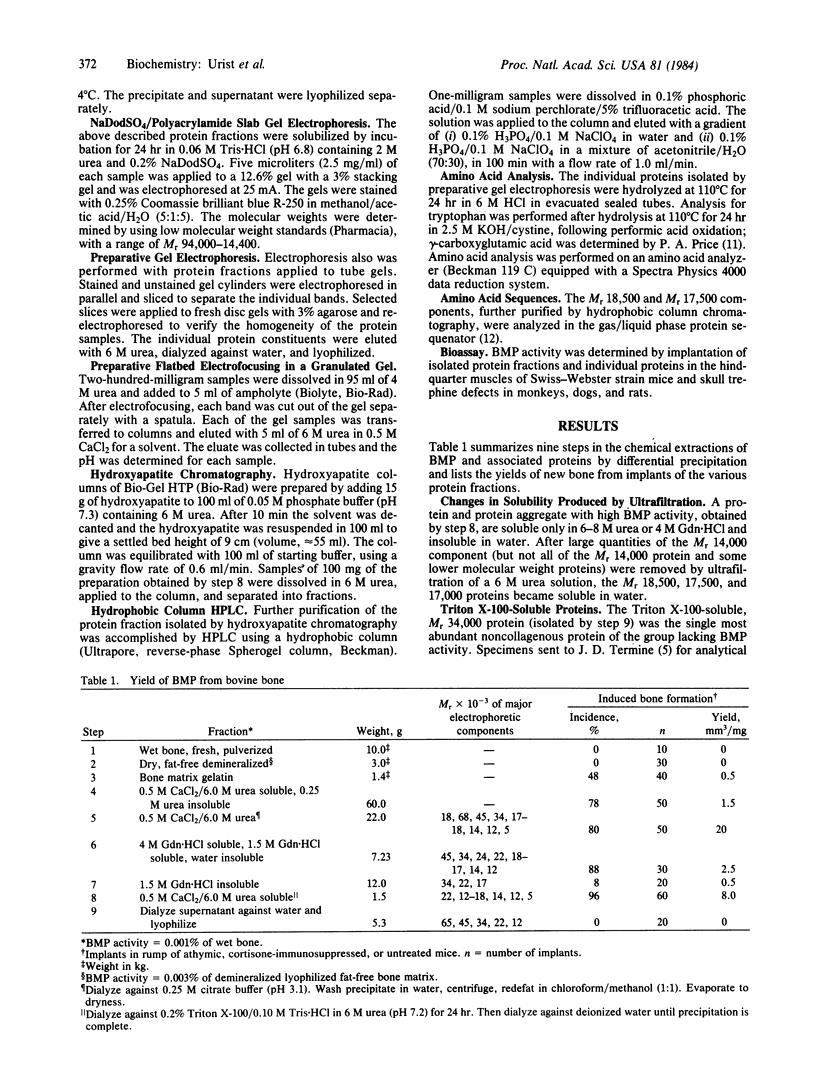
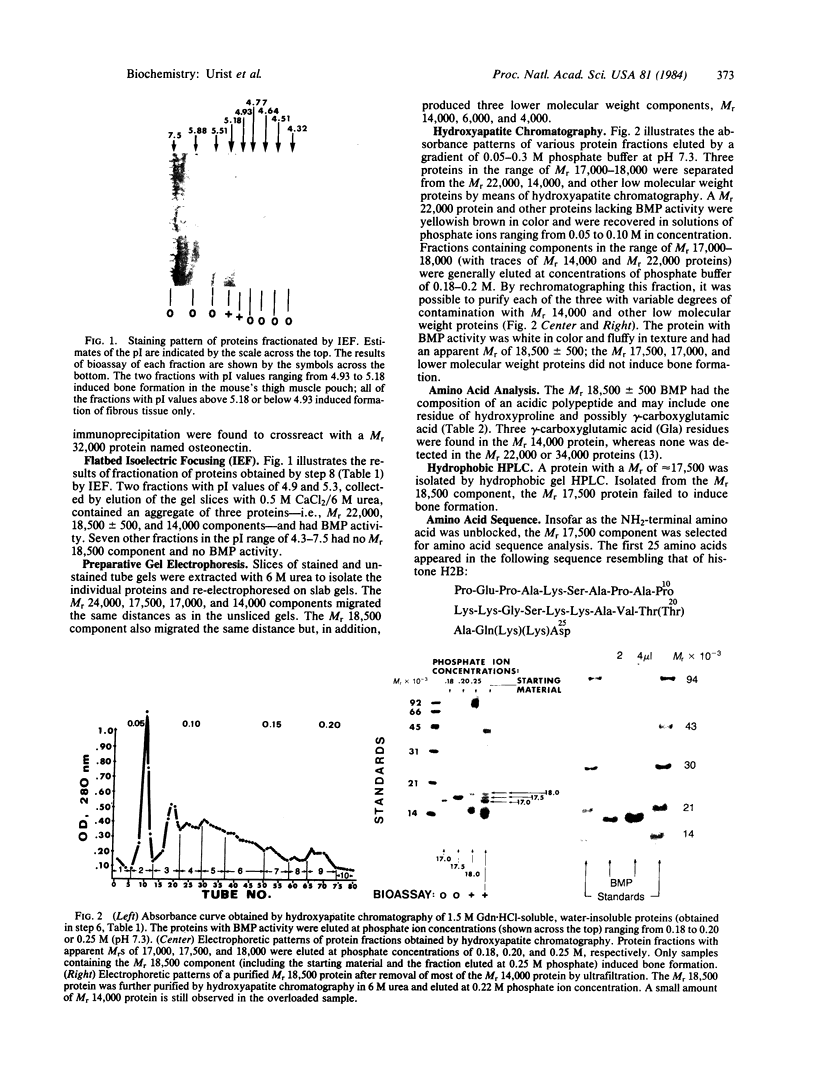


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer F. C., Urist M. R. Human osteosarcoma-derived soluble bone morphogenetic protein. Clin Orthop Relat Res. 1981 Jan-Feb;(154):291–295. [PubMed] [Google Scholar]
- Hanamura H., Higuchi Y., Nakagawa M., Iwata H., Nogami H., Urist M. R. Solubilized bone morphogenetic protein (BMP) from mouse osteosarcoma and rat demineralized bone matrix. Clin Orthop Relat Res. 1980 May;(148):281–290. [PubMed] [Google Scholar]
- Hanamura H., Higuchi Y., Nakagawa M., Iwata H., Urist M. R. Solubilization and purification of bone morphogenetic protein (BMP) from Dunn osteosarcoma. Clin Orthop Relat Res. 1980 Nov-Dec;(153):232–240. [PubMed] [Google Scholar]
- Mizutani H., Urist M. R. The nature of bone morphogenetic protein (BMP) fractions derived from bovine bone matrix gelatin. Clin Orthop Relat Res. 1982 Nov-Dec;(171):213–223. [PubMed] [Google Scholar]
- Price P. A. Analysis for gamma-carboxyglutamic acid. Methods Enzymol. 1983;91:13–17. doi: 10.1016/s0076-6879(83)91005-4. [DOI] [PubMed] [Google Scholar]
- Price P. A., Urist M. R., Otawara Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem Biophys Res Commun. 1983 Dec 28;117(3):765–771. doi: 10.1016/0006-291x(83)91663-7. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Urist M. R., DeLange R. J., Finerman G. A. Bone cell differentiation and growth factors. Science. 1983 May 13;220(4598):680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Iwata H., Ceccotti P. L., Dorfman R. L., Boyd S. D., McDowell R. M., Chien C. Bone morphogenesis in implants of insoluble bone gelatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3511–3515. doi: 10.1073/pnas.70.12.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist M. R., Lietze A., Mizutani H., Takagi K., Triffitt J. T., Amstutz J., DeLange R., Termine J., Finerman G. A. A bovine low molecular weight bone morphogenetic protein (BMP) fraction. Clin Orthop Relat Res. 1982 Jan-Feb;(162):219–232. [PubMed] [Google Scholar]
- Urist M. R., Mikulski A. J. A soluble bone morphogenetic protein extracted from bone matrix with a mixed aqueous and nonaqueous solvent. Proc Soc Exp Biol Med. 1979 Oct;162(1):48–53. doi: 10.3181/00379727-162-40616. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Mikulski A., Conteas C. N. Reversible extinction of the morphogen in bone matrix by reduction and oxidation of disulfide bonds. Calcif Tissue Res. 1975 Nov 24;19(1):73–83. doi: 10.1007/BF02563992. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Mikulski A., Lietze A. Solubilized and insolubilized bone morphogenetic protein. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1828–1832. doi: 10.1073/pnas.76.4.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist M. R., Sato K., Brownell A. G., Malinin T. I., Lietze A., Huo Y. K., Prolo D. J., Oklund S., Finerman G. A., DeLange R. J. Human bone morphogenetic protein (hBMP). Proc Soc Exp Biol Med. 1983 Jun;173(2):194–199. doi: 10.3181/00379727-173-41630. [DOI] [PubMed] [Google Scholar]