Abstract
Background & Aims
We recently identified lysophosphatidic acid (LPA) as a potent antiapoptotic agent for the intestinal epithelium. The objective of the present study was to evaluate the effect of octadecenyl thiophosphate (OTP), a novel rationally designed, metabolically stabilized LPA mimic, on radiation-induced apoptosis of intestinal epithelial cells in vitro and in vivo
Methods
The receptors and signaling pathways activated by OTP were examined in IEC-6 and RH7777 cell lines and wild-type and LPA1 and LPA2 knockout mice exposed to different apoptotic stimuli
Results
OTP was more efficacious than LPA in reducing gamma irradiation–, camptothecin-, or tumor necrosis factor α/cycloheximide–induced apoptosis and caspase-3-8, and caspase-9 activity in the IEC-6 cell line. In RH7777 cells lacking LPA receptors, OTP selectively protected LPA2 but not LPA1 and LPA3 transfectants. In C57BL/6 and LPA1 knockout mice exposed to 15 Gy gamma irradiation, orally applied OTP reduced the number of apoptotic bodies and activated caspase-3–positive cells but was ineffective in LPA2 knockout mice. OTP, with higher efficacy than LPA, enhanced intestinal crypt survival in C57BL/6 mice but was without any effect in LPA2 knockout mice. Intraperitoneally administered OTP reduced death caused by lethal dose (LD)100/30 radiation by 50%.
Conclusions
Our data indicate that OTP is a highly effective antiapoptotic agent that engages similar prosurvival pathways to LPA through the LPA2 receptor subtype.
The stem cells of the intestinal mucosa represent one of the most radiation-vulnerable cell types in the mammalian body.1 Whereas free radical scavengers can ameliorate the central nervous system syndrome and the widely used bone marrow transplantation can effectively treat the hematopoietic syndrome caused by exposure to lethal doses of radiation, effectively treating the gastrointestinal syndrome due to radiation-induced apoptosis of the intestinal stem cells is more difficult.
Lysophosphatidic acid (1-radyl-2-hydroxy-sn-glycero-3-phosphate; LPA) is a growth factor–like lipid mediator with antiapoptotic actions elicited through a set of G protein–coupled receptors.2,3 At least 5 LPA G protein–coupled receptors have been identified so far.4 The LPA1, LPA2, and LPA3 receptors are encoded by the endothelial differentiation gene (EDG) family and share approximately 60% identity with each other.2,4 LPA4 and LPA5 are distantly related to the EDG family, share only 20%–24% amino acid identity with the EDG family, and functionally are less well characterized.5–7 The mouse intestine predominantly expresses the LPA1 and LPA2 receptor subtypes.8,9 Each individual LPA receptor has its distinct coupling pattern to G proteins. LPA1 couples to Gi, Gq, and G12/13; LPA2 couples to Gi, Gq, and G12/13; and LPA3 couples to only Gi and Gq, not G12/13.10 These G proteins share many downstream signaling pathways that act in a cooperative manner.11 The activation of the phosphoinositide 3-kinase (PI3K)-AKT and MEK-ERK1/2 prosurvival pathways has been well delineated in mediating LPA-initiated antiapoptotic activity.12–14 LPA activates AKT, which in turn phosphorylates BAD and procaspase 9, leading to inhibition of apoptosis.15 LPA-induced activation of mitogen-activated protein kinase/extracellular signal–regulated kinase kinase (MEK)/extracellular signal–regulated kinase (ERK) 1/2 signaling can also activate BAD phosphorylation and attenuate caspase-9.16 LPA has been shown to activate nuclear factor κB, which regulates important prosurvival genes.16,17 A specific cellular response, including the antiapoptotic effect of LPA, might be mediated through a single LPA receptor or a combination of multiple receptor subtypes, which appear to be coexpressed in most cell types.18 Because LPA receptors are ubiquitously expressed and because of the overlapping coupling patterns to G proteins, elucidating the biological responses mediated by each individual receptor requires targeted gene knockout (KO) animal models. LPA1, LPA2, and LPA1/LPA2 double KO animals have been generated and show minimal phenotypes.19–21 At the present time, it remains unknown which LPA receptor(s) is required for its antiapoptotic effect in the different organs.
LPA in the gastrointestinal tract derived from foods such as soybean,22–25 metabolism of phospholipids by phospholipases,26 or activated platelets under pathological conditions.27 High levels of phospholipids have been detected in the colonic mucosa of patients with inflammatory bowel disease, and LPA significantly reduces the degree of inflammation and necrosis in a rat model of colitis.27 LPA has been shown to stimulate restitution of intestinal epithelia via pertussis toxin (PTX)-sensitive mechanisms.28 LPA2 receptors play an important attenuating role in bacterial toxin–induced secretory diarrhea via PDZ domain–mediated protein-protein interactions, inhibiting the activation of the CFTR Cl− channel.8 Therefore, the food-derived LPA in the lumen and its receptors on the epithelium suggest a physiologic role for LPA in maintaining gastrointestinal integrity that can be explored for therapeutic intervention.
Apoptosis in the intestinal epithelium is the primary pathologic factor that leads to chemotherapy- or radiation-induced gastrointestinal damage.1,29,30 We formulated a hypothesis that LPA can be used for the protection of the mucosa from iatrogenic traumas (radiation injury in particular); if so, it could serve as a template for prosurvival drugs against radiation injury. This hypothesis is based on several lines of our work reported over the past few years. We showed that LPA protected intestinal epithelia against irradiation-induced apoptosis both in vitro and in vivo,12 also showed in vitro that LPA achieved its antiapoptotic effects through the PTX-sensitive activation of the MEK-ERK1/2 and PI3K–AKT prosurvival pathways,13 and established that the antiapoptotic effect, unlike the mitogenic and motogenic effects of LPA, did not require the transregulation of several tyrosine kinase receptors.31 However, LPA is not an optimal drug candidate. Exogenous LPA is rapidly metabolized in the gastrointestinal tract. Because complex lipids, including phospholipids, are broken down to nonpolar intermediates that traverse the plasma membrane, the action of LPA is terminated by phospholipase- and lipase-mediated deacylation or (lipid) phosphatase-mediated dephosphorylation. While this mechanism rapidly renders LPA inactive, it also limits the effect of LPA on the receptors present on the luminal surface of the epithelium. LPA consists of a glycerol backbone with a hydroxyl group, a phosphate group, and a fatty acid or fatty alcohol chain. We have developed and experimentally validated computational models of the EDG family of LPA receptors32–38 and established the absolute requirement for a negatively charged headgroup and the aliphatic tail but not for the glycerol backbone.34,39,40 Therefore, we hypothesized that long-chain fatty alcohol thiophosphates mimic LPA at the EDG family receptors and at the same time are metabolically stabilized against phospholipase cleavage, which requires a glycerol backbone. Furthermore, thiophosphates tend to be poor substrates of lipid phosphatases and render them resistant to breakdown. These observations led us to explore the pharmacologic and biologic properties of octadecenyl thiophosphate (OTP) as an orally bioavailable, metabolically stabilized, nonabsorbing LPA mimic with radioprotective action in the gut.
The present study set multiple objectives. First, we explored the pharmacologic properties of OTP by using computational and pharmacologic approaches and determined its resistance to lipase and lipid phosphatase cleavage. Next, we compared the antiapoptotic effect of OTP with that of LPA in IEC-6 cells in vitro. Third, using a receptor add-back, we examined which LPA receptor subtypes mediate survival signals to prevent radiation-induced apoptosis. Fourth, we examined whether orally applied OTP reduces radiation-induced apoptosis and caspase-3 activation and increases crypt survival in clonogenic assays conducted in C57BL/6 mice exposed to 15 Gy gamma irradiation. We also evaluated the OTP- and LPA-induced activation of those prosurvival signaling pathways in vivo that we had previously established in vitro. Finally, we tested whether intraperitoneal administration of OTP prevents death caused by LD100/30 gamma irradiation. We found that OTP mitigated radiation-induced death, protected intestinal epithelial cells from apoptosis in vitro and in vivo, and was significantly more effective compared with LPA. Both LPA and OTP reduced apoptosis and caspase-3 activation and increased crypt survival in wild-type and LPA1 KO mice; however, both were ineffective in LPA2 KO mice. Together, these data suggest that OTP is a highly effective intestinal radioprotective agent that targets LPA2 as a prosurvival receptor.
Materials and Methods
Reagents
LPA (oleoyl) was purchased from Avanti Polar Lipids (Alabaster, AL). LPA and OTP (synthesized as described by Durgam et al40) were applied to cells complexed with fatty acid–free bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO) as previously described.39 Camptothecin and cycloheximide (CHX) were purchased from Sigma Chemical Co. Recombinant rat tumor necrosis factor (TNF)-α was purchased from BD PharMingen (San Diego, CA). PD98059 was purchased from Calbiochem (San Diego, CA). PTX and Ac-DEVD-pNA and Ac-LEHD-pNA colorimetric and Ac-IETD-AFC fluorescent caspase substrates were from Biomol Laboratories, Inc (Plymouth Meeting, PA). The following antibodies and sources were used: rabbit anti–caspase-3 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), rabbit anti-active caspase-3 (Abcam, Inc, Cambridge, MA), mouse monoclonal anti-JNK1 (BD PharMingen), mouse monoclonal anti-phospho (Thr183/Tyr185)-JNK, rabbit anti-ERK1/2, rabbit anti-phospho-(Tyr202/Tyr204) ERK1/2, rabbit anti-AKT, rabbit anti-phospho-(Ser473)-AKT, rabbit anti–Bcl-2, monoclonal mouse anti–Bcl-XL (Cell Signaling, Inc, Beverly, MA), monoclonal mouse anti–phospho-(Thr180/Tyr182)-P38 (Promega, Madison, WI), and mouse monoclonal anti-actin (Calbiochem). Horseradish peroxidase–conjugated anti-rabbit and anti-mouse secondary antibody used for Western blotting was purchased from Sigma Chemical Co. Fluorescein isothiocyanate–labeled goat anti-rabbit immunoglobulin G was purchased from Molecular Probes (Eugene, OR). Normal goat serum and Vectashield Mounting Medium with DAPI were purchased from Vector Laboratories, Inc (Burlingame, CA).
Computational Modeling
The detailed methods used to develop computational models of LPA1, LPA2, and LPA3 have been reported previously.33,34,36 Briefly, our validated model of the S1P1 receptor32,33,37 was used as a template for generating LPA receptor models. Homology model development was performed using the automated algorithm implemented in the MOE software program.41 The best model was geometry optimized using the MMFF9442 force field to a root mean square gradient of 0.1 kcal/mol Å. The individual receptor models were used in docking studies with OTP bearing a total charge of −2. Docking calculations were performed using Autodock 3.0 software43 with default values for all parameters except the number of runs (10), energy evaluations (9.0 × 1010), generations (60,000), and local search iterations (3000). The complex chosen as the best geometry from each docking calculation was that with the lowest final docked energy value. Results are described using a numbering system that facilitates comparisons among homologous positions in G protein–coupled receptors as described by Ballesteros and Weinstein.44 In this system, each amino acid in the transmembrane domain is given a number in the format X.YY where X indicates the number of the transmembrane helix where the amino acid is found and YY indicates the position relative to the most conserved amino acid in that helix at reference position 50.
Radiolabeling of OTP
Tritiated OTP was synthesized in our laboratory as shown in Figure 1D. Pyridinium chlorochromate–mediated oxidation of oleyl alcohol gave oleyl aldehyde (2), which was subjected to reduction with NaB3H4 to form the tritiated oleyl alcohol (3). Alcohol 3 was phosphorylated as reported earlier40 to give protected oleyl thiophosphate ester (4). Treatment of 4 with methanolic KOH, followed by acidification, yielded [3H]-OTP. The specific activity of the product was 10.8 mCi/mmol.
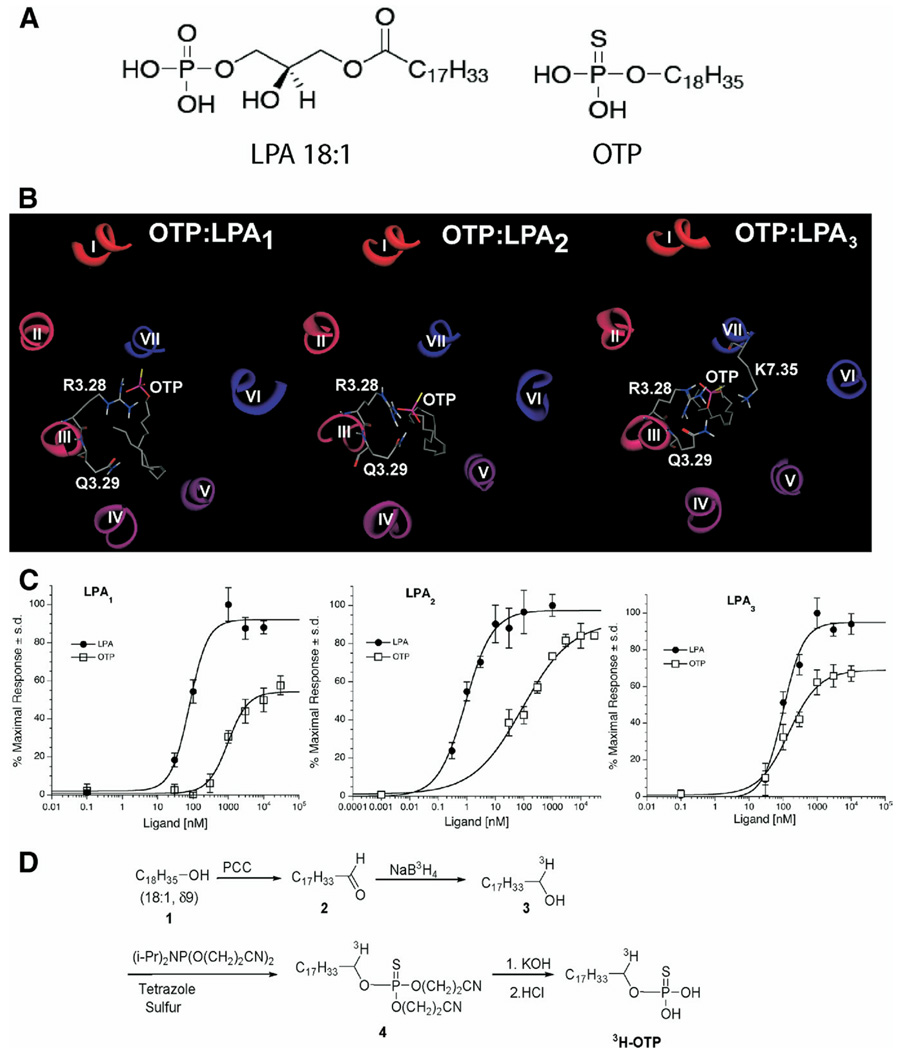
Figure 1.
(A) Chemical structures of LPA and OTP. (B) Molecular models of OTP docked into the ligand-binding pocket of LPA receptors. (C) Ca2+ transients elicited by OTP and LPA in RH7777 cells stably expressing the individual EDG family LPA receptors. Wild-type RH7777 cells show no Ca2+ transients in response to LPA up to concentrations as high as 30 µmol/L (data not shown). (D) Synthesis of 3H-labeled OTP (see Materials and Methods for details).
Determining OTP Absorption From the Gut
To determine oral absorption of OTP, female 8- to 10-week-old C57BL/6 mice (average body wt, 20 g) were used. Mice were maintained on a 12-hour light/12-hour dark cycle and fed standard laboratory mouse chow and water ad libitum. Conscious mice were administered through oral gavage 1.5 mg/kg OTP in 1 mmol/L BSA in phosphate-buffered saline (PBS) including 106 dpm OTP. Groups of 4 mice were killed after 60, 90, and 180 minutes, and blood samples were collected through cardiac puncture using 0.2% EDTA anticoagulant. Ten-microliter whole-blood samples from each mouse were mixed with Ecolume (Packard, Boston, MA) liquid scintillation cocktail and counted after 24 hours of equilibration in a liquid scintillation counter.
Assaying OTP Metabolism by Pancreatic Lipase and Lipid Phosphate Phosphatase 1
To determine the enzymatic stability of OTP by phospholipases, [3H]-OTP (5.2 × 106 dpm) mixed with 0.03 mmol/L cold OTP was subjected to enzymatic hydrolysis for 24 hours by using bovine pancreatic lipase (Sigma Chemical Co) followed by thin-layer chromatography (TLC) separation of the products using a previously established protocol.45 Lipid phosphate phosphatase (LPP) was obtained from mouse embryonic fibroblasts derived from mice with transgenic overexpression of the enzyme.46 Membrane fractions (300 µg/reaction) prepared by centrifugation at 104 × g from transgenic LPP1 fibroblasts were added to [3H]-OTP (1.5 × 106 dpm) mixed with 8 µmole cold OTP and subjected to LPP1 hydrolysis for 8 hours using a previously established protocol.46 The reaction mixture was dried in vacuo, and the residue was acidified with 100 µL 1N HCl and extracted 4 times with 0.5 mL ethyl acetate. The extracts were combined, and the solvent was evaporated. The extracted reaction products were taken up in 2 mL ethyl acetate, and a 20-µL aliquot was applied to TLC using methanol/ether solvent (2:98 vol/vol) as described.45
Cell Culture and Induction of Apoptosis In Vitro
IEC-6 and the rat hepatoma RH7777 cells were obtained from the American Type Culture Collection (Manassas, VA). IEC-6 cells were grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, insulin (10 µg/mL), and gentamicin sulfate (50 µg/mL) at 37°C in a humidified 90% air/10% CO2 atmosphere. RH7777 cells, stably expressing LPA2 receptors, were provided by Dr Fumikazu Okajima (Gunma University, Maebashi City, Japan). RH7777 cells stably expressing LPA1 or LPA3 receptors were generated by our group and characterized elsewhere.47 Wild-type and stably transfected RH7777 cells were grown in Dulbecco’s modified Eagle medium with 10% fetal bovine serum and 2 mmol/L glutamine containing 250 µg/mL G418 for the stable transfectants. Apoptosis in IEC-6 cells was induced by exposing them to 20 µmol/L camptothecin or a 25-Gy Cs137 source gamma irradiation (Mark I model 25 Gamma Irradiator; J. L. Shepherd & Associates, San Fernando, CA) at a rate of 4.80 Gy/min. DNA fragmentation and caspase-3 activity were measured 6 hours after camptothecin treatment or 18 hours postirradiation. Apoptosis in RH7777 cells was induced by 20 ng/mL TNF-α plus 10 µg/mL CHX and evaluated 6 hours later.
Pharmacologic Characterization of OTP
The ligand properties of OTP were evaluated using RH7777 cells stably transfected with each LPA receptor of the EDG family exactly as described in our previous report.40 RH7777 cells lack endogenous Ca2+ responses to LPA applied as high as 30 µmol/L, the highest concentration tested, but acquire these responses upon transfection of any of the LPA receptors.39,40,47 Briefly, RH7777 cells stably expressing human LPA1, LPA2, or LPA3 were loaded with Fura-2 AM (Invitrogen, Carlsbad, CA). Changes in intracellular Ca2+ concentration were monitored by measuring the ratio of emitted light intensity at 520 nm in response to excitation by 340-nm and 380-nm wavelength lights, respectively, using a FlexStation II robotic fluorescence plate reader (Molecular Devices, Sunnyvale, CA). Responses were monitored for 80–120 seconds. Ca2+ transients were quantified automatically by calculating the difference between maximum and baseline ratio values for each sample run in triplicate.
Reverse-Transcription Polymerase Chain Reaction
RNA was extracted using TRIzol (Invitrogen) from wild-type and KO mice using four 0.5-cm segments spaced equally along the jejunum. The following gene-specific primers were used: LPA1: forward 5′-TCTGAAGACTGTG GTCATTGTGC-3′, reverse 5′-GCCATTAGGGTTCTCGT-TGC-3′; LPA2: forward 5′-CACTCCTGGCACT GCCTCT-GTG-3′, reverse 5′-TAC GGC GCA TCT CAG CGT CTC G-3′; LPA3: forward 5′-ACACATGTCAATCATGAGGAT-3′, reverse 5′-GAA GACGGTGACTGTCTTAGG-3′; LPA4: forward 5′-GAAGGCTTCTCCAAACGT GTCTG-3′, reverse 5′-C CTTG TGCCTTGCAACTCTGAA-3′; LPA5: forward 5′-CTGATGCTC ATCAACGTGGACC-3′, reverse 5′-TAGGGCACGAAGCACAGCAG-3′; β-actin: forward 5′-GACAACG GCTCCGGCATGTG-3′, reverse 5′-TTGAGACCT TCAACACCCCAGCA-3′. Reverse-transcription polymerase chain reaction was performed using the Superscript III kit (Invitrogen), and a total of 31 cycles were performed; the products were applied in full to agarose gels and stained with ethidium bromide.
Evaluating Apoptosis by DNA Fragmentation and Caspase Activity
DNA fragmentation was measured by enzyme-linked immunosorbent assay following the procedure provided with the Cell Death Detection Kit from Roche (Indianapolis, IN) as described previously12,13 and was expressed as absorbance units (at 405 nm) per microgram protein per minute. Caspase-3 and caspase-9 activity in IEC-6 cells were measured by enzyme-linked immunosorbent assay by using the specific Ac-DEVD-pNA chromogenic substrate for caspase-3 and Ac-LEHD-pNA for caspase-9 as described previously.12,13 Small intestine samples were washed thoroughly with PBS, scraped off the muscle layer, mixed with lysis buffer, homogenized, and centrifuged. The supernatants were collected for evaluating caspase-3 activity as described previously. Caspase-3 and caspase-9 activity were expressed as picomole pNA cleaved per minute per microgram protein. Caspase-8 activity was measured by using the specific Ac-IETD-AFC fluorescent substrate. Each reaction contained 20 µL cytosolic proteins, 70 µL assay buffer (50 mmol/L HEPES [pH 7.4], 100 mmol/L NaCl, 10 mmol/L dithiothreitol, 1 mmol/L EDTA, and 0.1% [vol/vol] 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate detergent), and 10 µL of 2 mmol/L Ac-IETD-AFC dissolved in assay buffer. The enzymatic reaction was performed in 96-well plates at 37°C and monitored using a FlexStation II microplate reader (Molecular Devices) at 37°C with fluorescence excitation at 405 nm and emission at 505 nm. Protein concentration was measured by using the Bradford protein assay kit (Bio-Rad, Hercules, CA), and caspase-8 activity was expressed as arbitrary fluorescence units per milligram per minute.
Western Blotting
Caspase-3 was measured in IEC-6 cells or intestinal epithelial lysates prepared for caspase-3 activity as described in our previous reports.12,13 ERK1/2 and AKT were measured in total cellular protein lysates by using a previously described procedure.13 Briefly, IEC-6 cells were lysed in 62.5 mmol/L Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 25% glycerol, 1 mmol/L NaF, 1 mmol/L orthovanadate, and protease inhibitor cocktail (Sigma Chemical Co). The lysates were cleared by centrifugation (104 × g) for 15 minutes at 4°C, and supernatants were collected. Protein concentrations were determined using the BCA Reagent Kit (Pierce Biotechnology, Inc, Rockford, IL). For ERK1/2, AKT, p38, and JNK, 10 µg, 40 µg, 20 µg, and 50 µg, respectively, of the cell lysate was fractionated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes, blocked with 5% nonfat milk, and incubated with various primary antibodies. Blots reacted with the appropriate horseradish peroxidase–conjugated secondary antibodies and developed using the SuperSignal chemiluminescence reagent (Pierce Biotechnology, Inc).
For Western blotting of Bcl-XL and Bcl-2, IEC-6 cells were harvested and washed with PBS twice and then resuspended with 100–200 µL ice-cold lysis buffer (20 mmol/L Tris [pH 7.5], 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1% Triton X-100, protease inhibitor, and phosphatase inhibitor cocktails [Sigma Chemical Co]). Cells were sonicated 3 times for 3 seconds each, and the lysate was centrifuged at 16,100g for 20 minutes at 4°C. The supernatants were collected. Protein concentration was measured by using the BCA Protein Assay Kit (Pierce Biotechnology, Inc).
Animal Treatment and Irradiation
The whole-body irradiation protocol was reviewed and approved by the University of Tennessee Health Science Center Animal Care and Use Committee. Eight-to 10-week-old C57BL/6 mice were purchased from Harlan (Indianapolis, IN) and maintained on a 12-hour light/12-hour dark cycle and fed standard laboratory mouse chow and water ad libitum. LPA1 and LPA2 KO mice generously provided by Dr Jerold Chun (Scripps Research Institute, La Jolla, CA) were bred in house and used between the ages of 8 and 12 weeks. Mice were fasted overnight before whole-body gamma irradiation (Cs137 source at a rate of 4.80 Gy/min). LPA or OTP was administered by oral gavage 2 hours before irradiation. Mice were killed either 4 hours after irradiation by isoflurane inhalation for analysis of apoptosis or 4 days later for the clonogenic assay. Four segments of the jejunum and the ileum were fixed in 10% neutralized formaldehyde (pH 7.4) buffer and processed for histologic evaluation. Mice used in the clonogenic regeneration assay received bromodeoxyuridine (BrdU; 120 mg/kg) and 5-fluoro-2′-deoxyuridine (5FdU; 12 mg/kg) intraperitoneally 2 hours before death to label the S-phase regenerating cells in intestine.
Testing the Acute Toxicology of OTP
Female 8-week-old C57BL/6 mice (average body wt, 20 g) were maintained on a 12-hour light/12-hour dark cycle and fed standard laboratory mouse chow and water ad libitum. Mice were randomly grouped into groups of 5 and weighed daily before oral gavage of 3, 10, and 30 mg/kg OTP dissolved in 1 mmol/L BSA in PBS. Mice were observed for 1 hour after OTP treatment, and any abnormal behavior and change in regular motor activity was recorded. Weights were monitored for 5 consecutive days after OTP treatment.
Assessing Apoptosis and Crypt Survival in Intestine
Paraffin cross sections were cut perpendicular to the long axis of the small intestine and stained with H&E or immunostained with a monoclonal anti-BrdU antibody. Epithelial cell apoptosis and crypt survival were analyzed in 2 sections per segment as previously described.12 To detect apoptosis, a minimum of 100 half crypt-villus units from each experimental group were scored and the number of apoptotic bodies per intestinal circumference was counted. For the clonogenic assay, the number of surviving crypts per jejunal circumference was counted in the sections from the different segments. A surviving crypt was defined as a regenerative crypt that contained a cluster of 10 or more H&E-stained cells. The viability of surviving crypts was confirmed by positive immunostaining for BrdU or incorporation of 5FdU into 5 or more crypt cells.
Immunohistologic Staining
To identify the BrdU-labeled S-phase cells in the crypts, we used a staining protocol described in our previous report.48 For activated caspase-3 staining, formalin-fixed, paraffin-embedded tissue sections were used with heat-mediated antigen retrieval for 15 minutes. After three 5-minute washes in Tris-buffered saline (25 mmol/L Tris, 150 mmol/L NaCl, pH 7.6), slides were blocked with 10% normal goat serum in Tris-buffered saline/0.1% Tween-20 for 2 hours at room temperature. Rabbit anti-active caspase-3 antibody was diluted 1:50 in 1% BSA/Tris-buffered saline/0.1% Tween-20 and incubated at 4°C overnight. Fluorescein isothiocyanate–labeled goat anti-rabbit immunoglobulin G was applied for 2 hours after washes, and the slides were mounted with Vectashield Mounting Medium with DAPI (Vector Laboratories) and visualized by a Nikon Eclipse 80i fluorescence microscope (Dallas, TX). At least 100 crypt-villus units per animal were counted for the presence of activated caspase-3–positive cells.
Immunohistochemical staining for Bcl-XL and Bcl-2 was performed using 5-µm-thick sections cut from formalin-fixed, paraffin-embedded blocks of jejunum and ileum taken 4 hours after irradiation from a total of 6 mice. Upon antigen unmasking, immunostaining with primary antibody (1:25 dilution) was performed using the rabbit Vectastain ABC Elite Kit (Vector Laboratories) following the manufacturer’s instructions. Vector 3,3′-diaminobenzidine peroxidase substrate (Vector Laboratories) was used for color development. Stained sections were then dehydrated and counterstained with Vector Hematoxylin QS (Vector Laboratories), and immunoreactivity was assessed by at least 2 investigators.
Monitoring the Radiation-Mitigating Effect of OTP on Survival
Conscious 8-week-old female mice were injected with 0.5 mg/kg OTP or vehicle (2% dimethyl sulfoxide/ PBS) 30 minutes before irradiation with 9 Gy (LD100/30) of gamma irradiation. Each group consisted of 18 mice, which provided 90% statistical power based on a Fisher exact test with a one-sided 5% significance level. The primary end point was survival to day 30. The secondary end point was the mean survival time of decedents.
Statistical Analysis
Data are expressed as means ± SD or SEM. Each in vitro experiment was repeated at least 3 times. For animal studies, each experimental group consisted of at least 6 mice; a minimum of 8 KO mice was used per group. Student t test was used for comparing control and treatment groups. A P value of <.05 was considered significant.
Results
Molecular Modeling and Pharmacologic Characterization of OTP
Complexes of OTP with the LPA receptor models are shown in Figure 1B. The LPA1 complex showed a strong ionic interaction between the thiophosphate group and R3.28 (2.0 Å P-O:H-N distance). No interaction occurred involving Q3.29, a residue required for LPA binding and LPA-induced LPA1 receptor activation.33 Similar to the LPA1 complex, the LPA3 complex failed to show a strong hydrogen bonding interaction with Q3.29 due to the unfavorably nonlinear angle49 formed by the amide N-H and the hydrogen bond acceptor (112°). However, a greater number of strong ionic interactions were observed in the LPA3 complex involving not only R3.28 but also K7.35 (2.2 Å P-O:H-N and 2.4 Å P-S:H-N distances, respectively). In contrast to our previous studies demonstrating a role for R5.38 in LPA-induced LPA3 activation,36 OTP failed to interact with R5.38. The failure of OTP to interact with residues known to be required for LPA-induced activation of LPA1 and LPA3 is consistent with the observation of only partial agonism at these receptors (Figure 1C). OTP showed both strong ionic interaction with R3.28 (1.6 Å P-O:H-N distance) and moderate hydrogen bonding interaction with Q3.29 (142°). This hydrogen bonding angle was within the most highly populated cluster of angles observed in crystal structures.49 The LPA2 receptor lacks cationic amino acid residues at the top of TM7, so these 2 interactions comprise the required headgroup interactions for full agonism.
LPA and OTP activate LPA receptors stably expressed in RH7777 cells, providing a simple assay platform to study the pharmacologic properties of these receptors as described in many previous reports.39,40,47,50 OTP was compared in wild-type, LPA1, LPA2, and LPA3 transfected RH7777 cells using Ca2+ mobilization as a measure of receptor activation (Figure 1C). OTP was inactive in wild-type RH7777 cells up to 30 µmol/L, the highest concentration tested (data not shown). However, it activated all 3 EDG family LPA receptors with varying potency and efficacy. OTP was always less potent than oleoyl LPA at all 3 receptors and was less efficacious at LPA1 and LPA3 receptors. At the LPA2 receptor subtype, OTP was a full agonist and showed an apparent median effective concentration of 90 nmol/L compared with 1 nmol/L for oleoyl LPA. Thus, OTP is a less efficacious full agonist of the LPA2 receptor subtype.
Metabolic Resistance of OTP to Pancreatic Lipase and LPP1
The 2 major pathways of LPA breakdown include (phospho)lipase-mediated deacylation and lipid phosphate phosphatase–mediated dephosphorylation.51 To determine the enzymatic stability of OTP by pancreatic lipase and LPP1, we synthesized [3H]-OTP for monitoring the enzymatic hydrolysis followed by TLC separation of the products using previously established protocols.45,46 TLC analysis of the pancreatic lipase–mediated hydrolysis of [3H]-OTP for up to 24 hours showed neither the breakdown nor formation of the expected [3H]-oleyl alcohol (data not shown). In contrast, LPA was fully metabolized under these conditions. The lack of cleavage is consistent with the requirement for the glycerol backbone that is absent in the OTP structure. LPP1 cleavage of [3H]-OTP for up to 8 hours showed no detectable dephosphorylation, indicating that the thiophosphate moiety is considerably more resistant to cleavage by this enzyme than the phosphate moiety present in LPA.
Absorption of Orally Applied OTP Into the Blood
The absorption of orally administered OTP into the bloodstream was determined by injecting 106 dpm [3H]-OTP tracer mixed with cold OTP to yield 1.5 mg/kg into the stomach. Blood samples collected 60, 90, and 180 minutes later showed that as little as 0.67% ± 0.15%, 0.61% ± 0.2%, and 0.45% ± 0.15% of the applied radioactivity appeared in the blood compartment. Assuming that all of it was nonmetabolized OTP, this would yield a maximal blood concentration of 180 nmol/L, which could activate LPA receptors. However, TLC analysis of the radioactively labeled compounds extracted from blood revealed no more than 0.01% of the injected material at the position of the authentic [3H]-OTP standard, indicating that the little amount of radioactivity represents metabolites of the compound. The very low amount of [3H]-OTP found in the blood after oral administration is reinforced by the lack of metabolism by pancreatic lipase and LPP1 present in the intestinal lumen, because these enzymes would produce the neutral oleyl alcohol, which could more effectively be taken up than a charged compound like OTP.
Orally Applied OTP Is Nontoxic in the Effective Dose Range
We tested OTP applied through oral gavage into the stomach of mice at 3, 10, and 30 mg/kg complexed in 1 mmol/L BSA in PBS. Observation of the animals for 1 hour after administration did not show any noticeable change in behavior and motor activity. Monitoring the weight gain curves of the animals showed only a 24-hour transient decrease in weight amounting to a mean of 3 g and only in the 30 mg/kg treatment group, but these animals rebounded by the second day and the median weight of the group was not significantly different from the vehicle-treated controls beyond that up to 5 days, the time the weight of the animals was followed daily. Thus, OTP showed no serious side effects even when administered at a 60-fold concentration (the highest dose tested so far) higher than the 0.5-mg/kg dose, which was highly effective in protecting the life of animals from lethal doses of irradiation (see following text).
OTP Surpasses the Antiapoptotic Activity of LPA in IEC-6 Cells
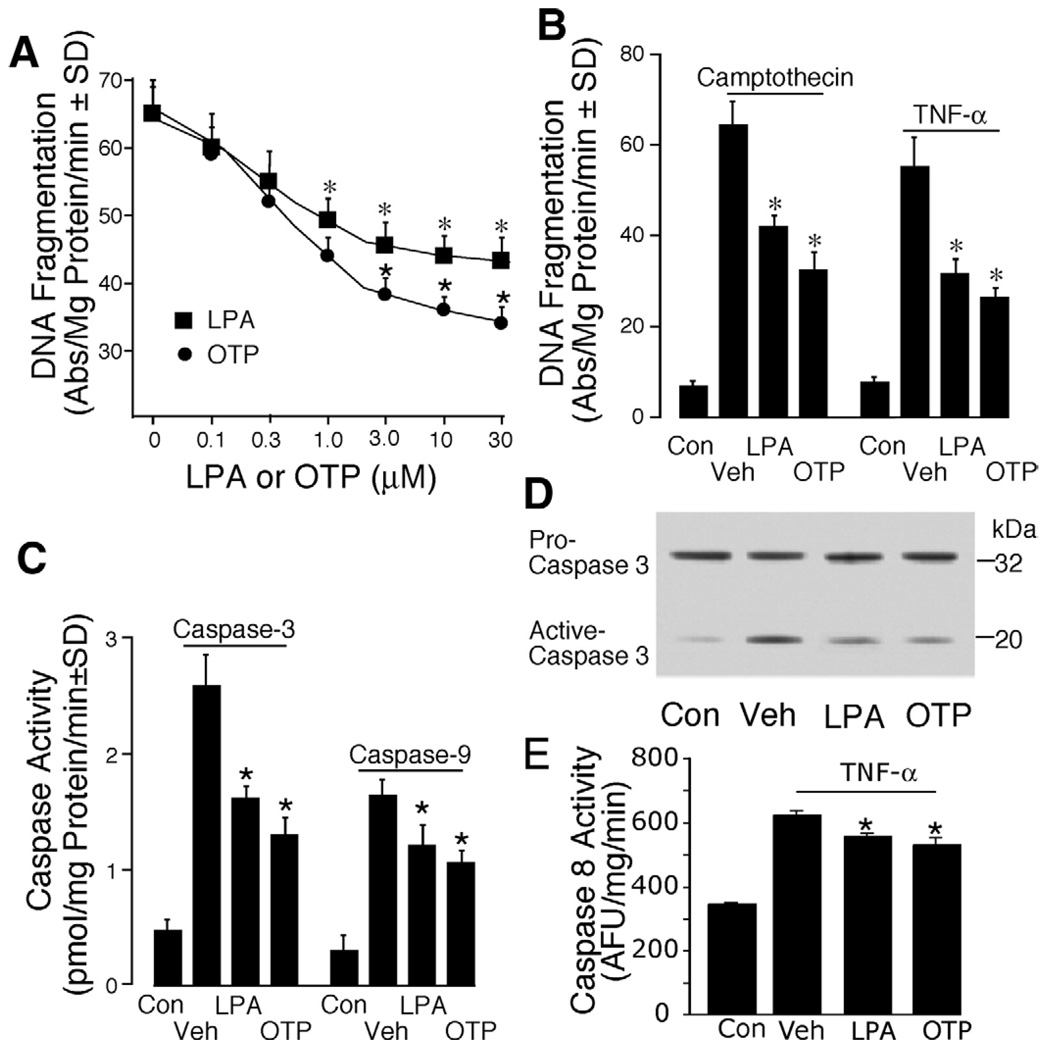
We previously showed that LPA protects intestinal epithelial cells from apoptosis induced by 4 different mechanisms.12,13,31 We compared the antiapoptotic effect of OTP with that of LPA. Although both OTP and LPA dose-dependently protected IEC-6 cells from gamma irradiation–induced DNA damage, OTP at concentrations greater than 1 µmol/L conferred significantly higher protection (Figure 2A). Both OTP and LPA applied at 10 µmol/L significantly protected IEC-6 cells from either topoisomerase inhibitor camptothecin- or the proinflammatory cytokine TNF-α–induced DNA damage as compared with control (P < .001), and at this concentration OTP showed stronger antiapoptotic activity than did LPA (P < .05; Figure 2B). OTP and LPA significantly inhibited caspase-3 and caspase-9 activity induced with gamma irradiation; again, the OTP-induced inhibition was significantly higher than that of LPA (P < .05, Figure 2C). Furthermore, analysis of caspase-3 activation by Western blotting showed that both OTP and LPA significantly inhibited the conversion of the 32-kilodalton pro-caspase into its active form, and OTP showed significantly higher inhibition compared with LPA (Figure 2D; P < .05). Lastly, a brief 15-minute OTP treatment inhibited the TNF-α–induced activation of caspase-8 (Figure 2E), which provides further support for its effect in reducing apoptosis elicited via the extrinsic pathway. These results together indicate that OTP mimics the antiapoptotic action of LPA in IEC-6 cells that express LPA1 and LPA2 receptors.13
Figure 2.
OTP is more effective in reducing apoptosis than is LPA in IEC-6 cells. (A) IEC-6 cells were washed twice and starved in serum-free Dulbecco’s modified Eagle medium overnight. OTP (circles) or LPA (squares), ranging from 0 to 30 µmol/L, was applied 15 minutes before 25 Gy gamma irradiation. DNA fragmentation was evaluated 18 hours postirradiation as described in Materials and Methods. (B) IEC-6 cells were treated with either 10 µmol/L OTP or LPA before exposure to 20 µmol/L camptothecin or 20 ng/mL TNF-α plus 10 µg/mL cycloheximide. DNA fragmentation was evaluated 6 hours later. Con, nontreated control cells; Veh, cells treated with vehicle (10 µmol/L BSA). (C) Vehicle, 10 µmol/L OTP or LPA, was applied to IEC-6 cells 15 minutes before 25 Gy gamma irradiation. Caspase-3 and caspase-9 activities were measured 18 hours postirradiation by enzyme-linked immunosorbent assay. (D) Western blot analysis of caspase-3 activation in LPA- and OTP-treated (10 µmol/L each) EC-6 cells 15 minutes before 25 Gy gamma irradiation. A total of 20 µg cytosolic protein was loaded for each lane. (E) A 15-minute OTP or LPA pretreatment inhibits 20 ng/mL TNF-α plus 10 µg/mL cycloheximide-induced caspase-8 activity. *P< .01 compared with the appropriate control group. Data shown are means ± SD of at least 3 experiments.
The Antiapoptotic Activity of OTP Requires PTX-Sensitive G Protein, Mitogen-Activated Protein Kinase, and PI3K/AKT Signaling
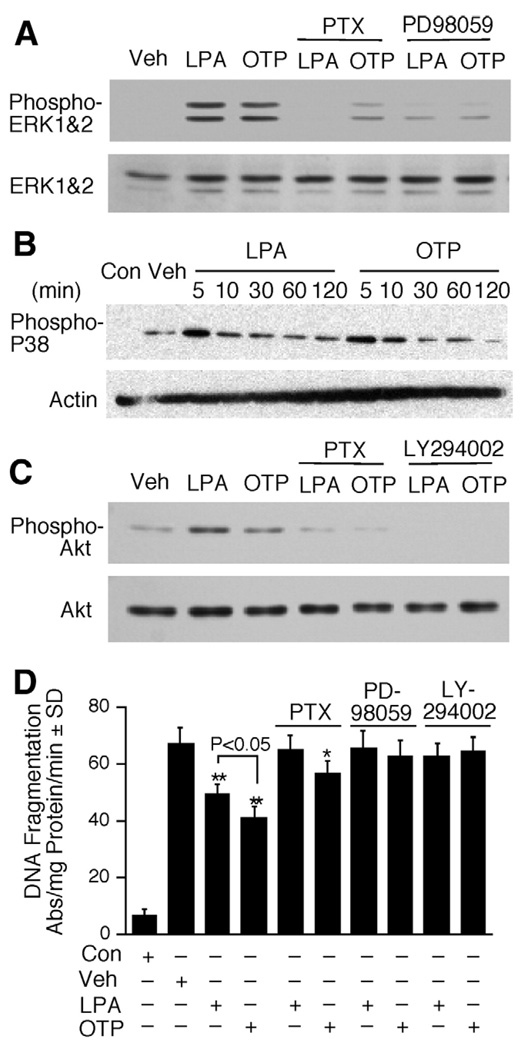
We have previously shown that in IEC-6 cells the antiapoptotic action of LPA requires PTX-sensitive G protein, MEK, and PI3K signaling.13 We found that OTP, just like LPA, activated ERK1/2 and p38 mitogen-activated protein kinase and AKT phosphorylation (Figure 3A–C). We did not detect c-Jun-N-terminal kinase phosphorylation under identical conditions (data not shown). To evaluate whether these same signaling mechanisms are required for OTP-elicited antiapoptotic activity, we examined these pathways by using pharmacologic inhibitors and exposing these cells to OTP or LPA with or without 25 Gy gamma irradiation. In nonirradiated cells, just like LPA, OTP treatment for 5 minutes induced a significant increase in ERK1/2, p38, and AKT phosphorylation (Figure 3). Blocking of ERK1/2 and AKT activation upstream by the MEK inhibitor PD98059 and PI3K inhibitor LY294002 completely abolished both OTP- and LPA-elicited activation, as well as protection (Figure 3A, C, and D), indicating that the antiapoptotic activity of OTP required the activation of the ERK1/2 and AKT pathways. Pretreatment of IEC-6 cells with PTX completely abolished LPA-elicited ERK1/2 and AKT activation (Figure 3A and C). In agreement with the requirement for a PTX-sensitive G protein in the antiapoptotic mechanism, PTX cancelled LPA-elicited protection from gamma irradiation–induced DNA damage. In contrast, although PTX abolished OTP-induced AKT phosphorylation, it only partially attenuated ERK1/2 activation, indicating that OTP-stimulated ERK1/2 activation was mediated through not only PTX-sensitive G proteins. In support of this result, PTX pretreatment only partially reduced OTP-initiated protection, which remained statistically significant following treatment with this toxin (P < .05; Figure 3D). These results indicate some similarities, as well as differences, between the signaling pathways used by OTP and LPA. Because LPA1 and LPA2 are the 2 main EDG family receptors expressed in IEC-6 cells13 and also in the mouse intestine,8,12 we next compared the role of the individual LPA receptors in the LPA-and OTP-elicited antiapoptotic response.
Figure 3.
Activation of prosurvival signaling pathways by LPA and OTP in IEC-6 cells. IEC-6 cells were pretreated with PTX (50 ng/mL) overnight, PD98059 (20 µmol/L) for 1 hour, or LY294002 (10 µmol/L) for 30 minutes, followed by the addition of 10 µmol/L OTP or LPA. Activation of (A) ERK1/2, (B) P38 mitogen-activated protein kinase, and (C) PKB/ AKT was evaluated by Western blot after treatment with 10 µmol/L OTP or LPA. A total of 20 µg lysate protein was loaded for each lane. (D) IEC-6 cells were exposed to 25 Gy gamma irradiation 15 minutes after OTP or LPA treatment (10 µmol/L). DNA fragmentation was evaluated 18 hours postirradiation. *P< .05 compared with irradiation alone. **P< .01 compared with irradiation alone. Data shown are means ± SD of at least 3 experiments.
OTP Selectively Protects LPA2 Transfectants From TNF-α–Induced Apoptosis
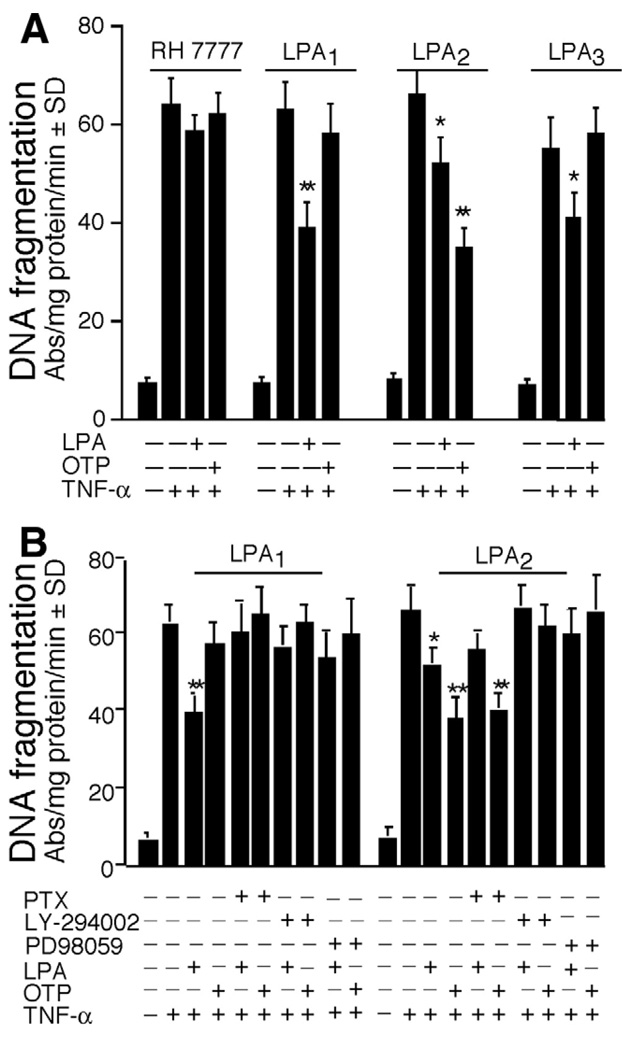
RH7777 cells do not endogenously express the EDG family LPA receptor but express low amounts of LPA5 transcripts.7 When LPA or OTP was applied to wild-type RH7777 cells exposed to TNF-α plus cycloheximide to induce apoptosis, neither compound applied at 10 µmol/L attenuated DNA fragmentation (Figure 4A). In contrast, in RH7777 cells individually transfected with LPA1, LPA2, or LPA3, DNA fragmentation was significantly reduced by LPA pretreatment (Figure 4A). OTP applied at 10 µmol/L showed no significant antiapoptotic activity in LPA1 or LPA3 stable transfectants but evoked highly significant protection in the LPA2 transfectants, surpassing the effect of that of 10 µmol/L LPA (Figure 3A; P < .001). It is important to note that OTP and LPA both activate Ca2+ transients in LPA1, LPA2, or LPA3 transfectants (Figure 1C) but not in wild-type RH7777 cells. Thus, the antiapoptotic effect of OTP is not simply linked to activation of receptor-elicited Ca2+ mobilization but to the activation of more complex signals originating from a specific receptor subtype, which is LPA2. The protective effect of LPA in LPA1 and LPA2 transfected RH7777 cells was abolished by PTX and was cancelled by pharmacologic blockade of MEK/ERK1/2 and PI3K/AKT activation, which mirrors our findings in IEC-6 cells (compare Figure 4B and Figure 3D). Just as in IEC-6 cells, the protective effect of OTP in RH7777 LPA2 transfectants was only partially PTX sensitive and was completely abolished only by blocking ERK1/2 and PI3K/AKT pathways (Figure 4B). These results identify LPA2 in this model system as the main target of OTP and pinpoint a mechanism that involves MEK/ERK1/2 and PI3K/AKT activation mediated through multiple PTX-insensitive and -sensitive G proteins. LPA2 is known to be coupled to Gq and it mediates ERK1/2 activation in part,10 a finding consistent with the partial inhibitory effect of PTX on the apoptotic effect found in IEC-6 cells and in LPA2 transfected RH7777 cells. However, this finding does not explain why OTP, unlike LPA, failed to attenuate apoptosis in LPA1 or LPA3 transfected RH7777 cells even though it activated these receptor subtypes indicated by the Ca2+ responses (Figure 1C).
Figure 4.
OTP selectively protects LPA2 transfectants against TNF-α/CHX–induced apoptosis. (A) RH7777 cells were transfected with empty pCDNA3.1 vector or LPA1, LPA2, or LPA3 and preincubated with 10 µmol/LOTP or LPA for 15 minutes, followed by TNF-α (20 ng/mL) plus CHX (10 µg/mL) exposure to induce apoptosis. Note that whereas LPA reduced TNF-α/CHX–induced DNA fragmentation in all 3 transfectants, OTP was effective only in LPA2 cells. Both ligands elicited Ca2+ transients in these same cell lines (see Figure 1C). (B) LPA1 and LPA2 transfectants were pretreated with PTX overnight (50 ng/mL), the MEK inhibitor PD98059 (20 µmol/L) for 1 hour, or the PI3K inhibitor LY294002 (10 µmol/L) for 30 minutes, followed by the addition of 10 µmol/L OTP or LPA for 15 minutes and challenged with TNF-α (20 ng/mL) plus CHX (10 µg/mL). DNA fragmentation was evaluated 6 hours after addition of TNF-α/CHX. The reduction in DNA fragmentation was partially sensitive to PTX in LPA2 cells and was abolished in LPA1 cells. *P< .05, **P< .001 compared with TNF-α/CHX alone. Data shown are means ± SD of 3 experiments.
OTP Attenuates Radiation-Induced Apoptosis in the Intestine
We have previously shown that LPA given orally prevents apoptosis induced by gamma irradiation in the stem cell region of the intestinal crypt.12 Here, we sought to test whether OTP exerted a similar effect and compared its antiapoptotic efficacy with that of LPA by quantifying apoptotic bodies in the jejunal epithelium 4 hours after 15 Gy gamma irradiation (~LD100/10). In agreement with our previous report12 using ICR mice, the number of apoptotic bodies in the jejunum of control nonirradiated animals was less than 0.5 per crypt-villus unit (Figure 5A). In vehicle-treated (100 µL of 200 µmol/L BSA in PBS) wild-type C57BL/6 mice, the number of apoptotic bodies per crypt-villus unit increased 6-fold 4 hours following gamma irradiation. Oral pretreatment with LPA (2 mg/kg into the stomach 2 hours before irradiation) significantly reduced the number of apoptotic bodies per crypt-villus unit (P < .01; Figure 5A). Oral OTP pretreatment (same timing and dose as LPA) reduced the number of apoptotic bodies by 50%, which was significantly higher than that elicited by LPA (P < .05; Figure 5A). Analysis of the distribution of the apoptotic bodies along the crypt-villus unit showed that gamma irradiation resulted in a high frequency of apoptotic cells over the stem cell zone of the crypt, 4–7 cell positions from the base (Figure 5B). Both LPA and OTP significantly reduced the number of apoptotic cells over the stem cell zone in the irradiated animals.
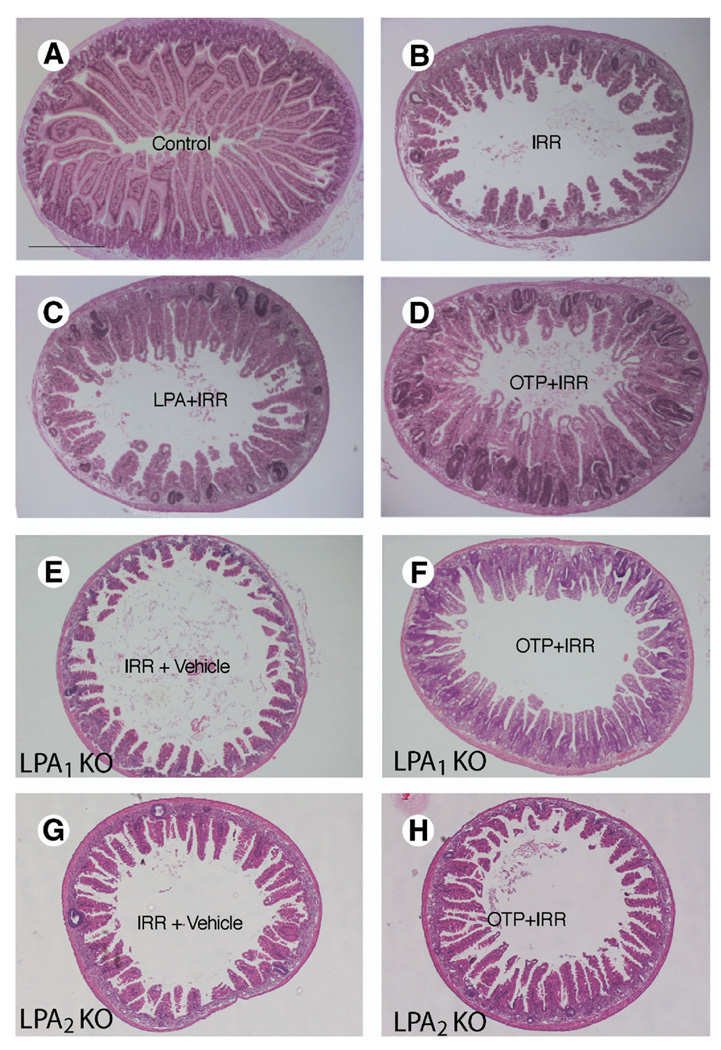
Figure 5.
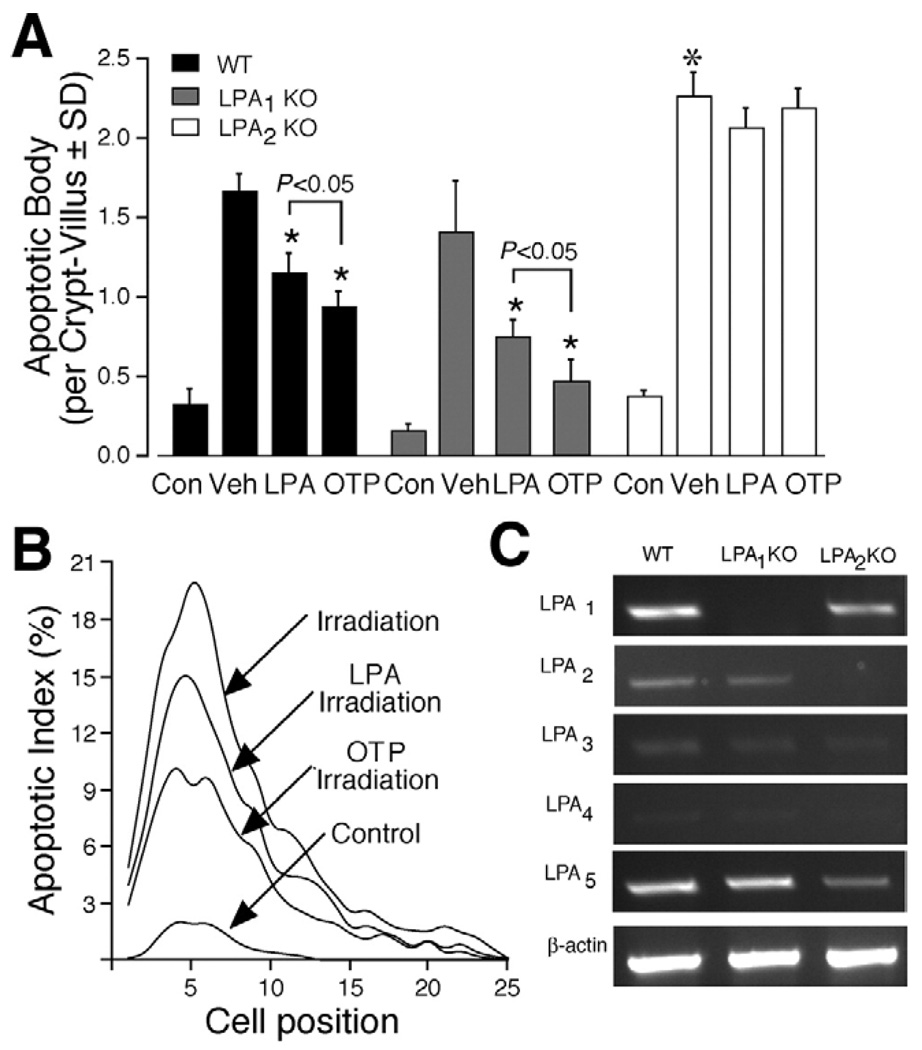
OTP inhibits apoptosis in small intestinal epithelium of mice following gamma irradiation. Wild-type C57BL/6 (closedbars) and LPA1 (gray bars) or LPA2 KO mice (open bars) were given vehicle orally (100 µL of 200 µmol/L BSA control), LPA (2 mg/kg), or OTP (2 mg/kg) 2 hours before subjecting them to 15 Gy whole body gamma irradiation. Animals were killed 4 hours after irradiation to evaluate apoptosis in the small intestine by H&E staining. (A) The mean number of apoptotic cells per crypt-villus unit. Both LPA and OTP caused a statistically significant decrease in the number of apoptotic bodies compared with the vehicle-treated animal group (P< .01). Furthermore, OTP was significantly more effective than LPA (*P< .05). The number of apoptotic bodies was significantly higher in the LPA2 KO mice compared with wild-type or LPA1 mice (*P< .01). (B) Correlation between apoptotic cell index (percentage of apoptotic cells at a given cell position) and their position along the crypt-villus axis. Cells 1–2 are Paneth cells in the graph. n = 6 animals in each group, and a minimum of 100 crypt-villus units was scored in each group. (C) Reverse-transcription polymerase chain reaction analysis of LPA G protein–coupled receptors in jejunum isolated from wild-type, LPA1−/−, and LPA2−/− mice.
Our in vitro data using the RH7777 heterologous overexpression model indicated that OTP elicited its antiapoptotic action through the LPA2 receptor subtype (Figure 4). To evaluate which LPA receptor mediates the protective effect of OTP in vivo, we performed the same treatments in LPA1 and LPA2 KO mice. We detected no difference in the basal count of apoptotic bodies between wild-type C57BL/6 mice and LPA1 or LPA2 KO mice on this same genetic background. Surprisingly, gamma irradiation induced a significantly higher rate of apoptosis at the base of the crypt, in LPA2 KO mice, compared with that in wild-type mice, whereas there was no difference between wild-type and LPA1 mice (Figure 5A). LPA and OTP had the same protective effect in wild-type and LPA1 mice. In contrast, LPA and OTP failed to attenuate gamma irradiation–induced apoptosis in LPA2 KO mice. In wild-type animals, the biggest radiation-induced increase in apoptotic bodies was found in the stem cell region of the crypt (Figure 5B). LPA and OTP caused the biggest decrease in the number of apoptotic cells in this region of the crypt, suggesting that the cellular targets of LPA and OTP include the stem cells. Reverse-transcription polymerase chain reaction analysis of the LPA receptors showed that all but LPA4 transcripts were expressed in the tissue (Figure 5C). The LPA1 and LPA2 KO animals showed no compensatory change in the expression of the other LPA receptors. Thus, the in vivo data are consistent with our in vitro findings that LPA2 is required for the antiapoptotic effect of OTP. Nonetheless, these data differ from the in vitro findings in the case of LPA and point to the essential role of LPA2 in mediating the in vivo antiapoptotic effect of LPA. Furthermore, the increased rate of apoptosis in LPA2 KO mice suggests that this receptor may play a physiologic role in the radiation sensitivity of the small intestine. These results also point to the fundamentally different role of these 2 LPA receptor subtypes in radiation sensitivity and apoptotic protection.
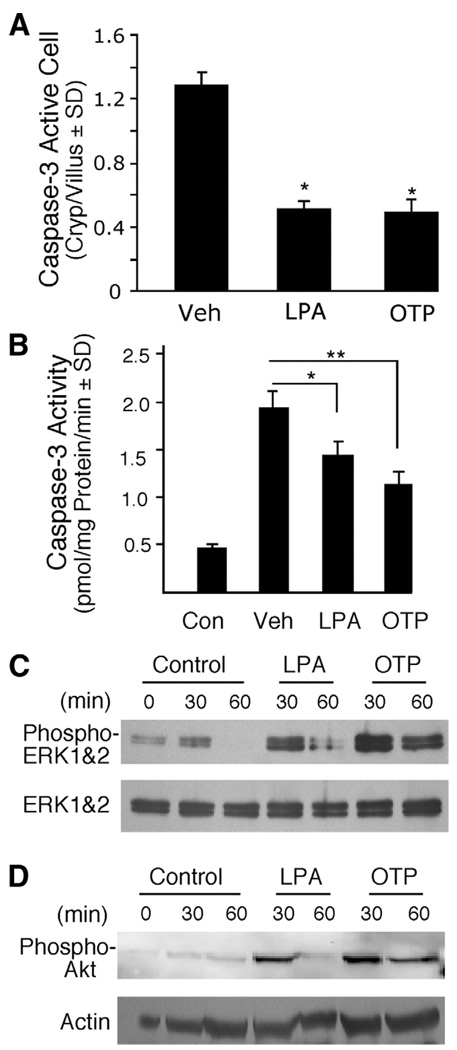
Next, we sought to obtain direct evidence that LPA and OTP treatments activate the same signal transduction mechanisms we have identified in vitro. First, we examined whether LPA and OTP attenuate the activation of the main executioner, caspase-3, as we have seen in IEC-6 cells in vitro (Figure 1C). Jejunum and ileum sections were immunostained with an antibody specific for the activated form of caspase-3, and the number of positive cells was counted in a minimum of 100 crypt-villus units in slides prepared from groups of 4 animals. In the nonirradiated vehicle-treated group, we could not detect caspase-3–positive cells, possibly due to the low amount of antigen present in cells naturally undergoing apoptosis combined with their low incidence in the healthy small intestine. In contrast, in the vehicle-treated irradiated group, caspase-3–positive cells were readily detected. Both LPA and OTP treatments significantly reduced the number of activated caspase-3–positive cells by ~60% (Figure 6A*; P < .05). The inhibition of caspase-3 activation by LPA and OTP was also confirmed in intestinal epithelial lysates by measuring enzymatic activity (Figure 6B). These experiments revealed that, in control nonirradiated animals, a low level of caspase-3 substrate cleaving activity is present, which increased 4-fold after 15 Gy gamma irradiation. LPA and OTP reduced caspase-3 activity significantly, although the inhibitory effect of OTP was greater.
Figure 6.
OTP and LPA reduced caspase-3 activation and activated prosurvival pathways in vivo. C57BL/6 mice were pretreated with 2 mg/kg LPA or OTP for 2 hours and subjected to 15 Gy radiation exposure. Mice were killed 4 hours after radiation. (A) Quantification of active caspase-3 immunoreactive cells. Paraffin-embedded jejunum sections from animals treated with radiation and pretreated with either LPA or OTP were stained with a rabbit polyclonal active caspase-3 antibody and fluorescein-labeled secondary antibody using indirect immunofluorescence as described in Materials and Methods, and the sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Active caspase-3 immunoreactive cells were counted in a minimum of 100 crypt-villus units in groups of 4 animals in the groups. Both agents significantly reduced the number of activated caspase-3–positive cells (*P< .05) when compared with vehicle-treated controls. (B) Caspase-3 activity was determined in epithelial cell lysates prepared from the same animals whose jejunum segments were used for activated caspase-3 immunostaining in A. LPA and OTP both significantly reduced caspase-3 activity in the tissue lysates, a finding in agreement with the reduced number of active caspase-3 cells. (*P< .05, **P< .01) (C and D) Jejunum tissue from mice orally treated with vehicle, 2 mg/kg LPA, or OTP for the indicated times was homogenized and lysates were analyzed using Western blotting with (C) anti–phospho-ERK1/2 or (D) anti–phospho-AKT antibodies and appropriate antibodies to the non-phosphorylated forms of the kinases to monitor equal loading. Note that the activation of both kinases was more robust and longer lasting for OTP compared with LPA.
Our previous and present in vitro studies using IEC-6 cells and other types of cells13,31 have established the requirement of ERK1/2 and AKT activation in the anti-apoptotic response to LPA and OTP. We next determined whether these pathways were activated in intestinal cell homogenates prepared from animals that have been treated with the 2 ligands by oral gavage. As shown in Figure 6C and D, both agents activated ERK1/2 and AKT phosphorylation as early as 30 minutes after administration in nonirradiated animals. However, the effect of OTP was considerably longer lasting in the tissue compared with the effect of LPA, which was absent in the samples collected 60 minutes after administration.
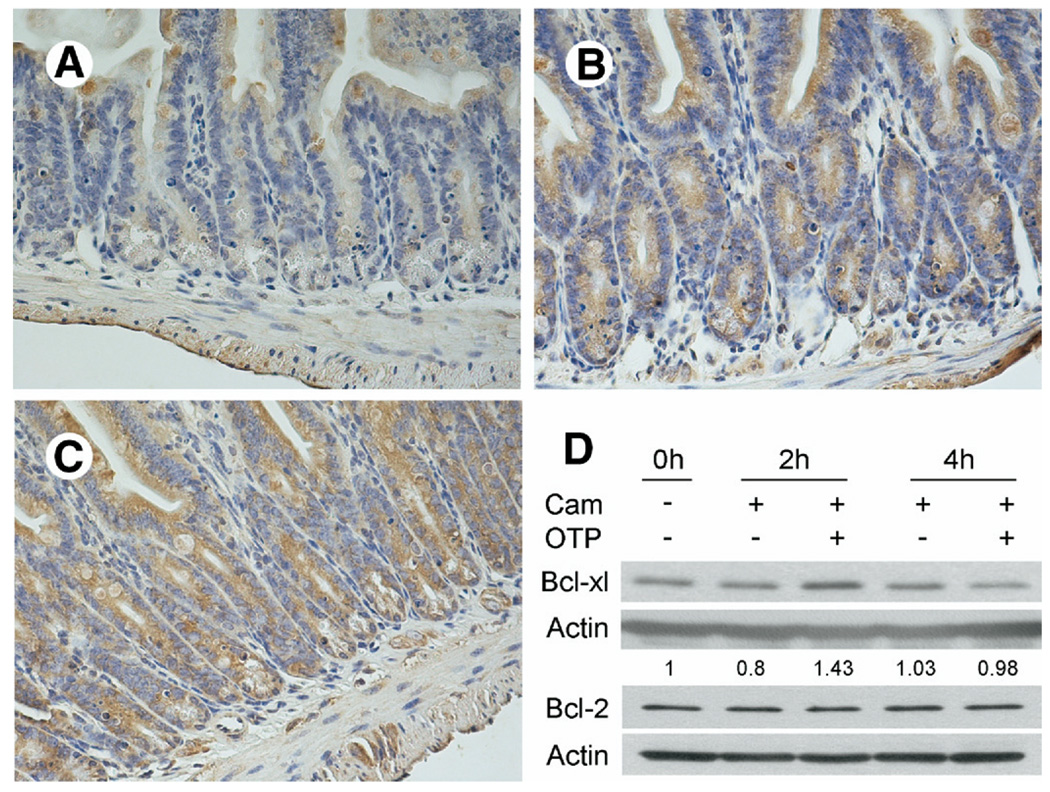
In vitro studies using IEC-6 cells showed that LPA up-regulated the expression of the antiapoptotic Bcl-2 messenger RNA and had no effect on Bcl-XL, BAD, and Bak messenger RNA abundance determined by real-time polymerase chain reaction.13 In jejunum homogenates prepared from LPA- and OTP-treated mice 4 hours after irradiation (6 hours after treatment), we could not detect a significant change in Bcl-XL or Bcl-2 expression (data not shown). However, immunostaining with a Bcl-XL specific antibody revealed marked increases in LPA- and OTP-treated animals (Figure 7) compared with jejunum and ileum (not shown) from the vehicle-treated animals. OTP treatment caused the most intense immunostaining (Figure 7C), although LPA also elicited a substantial increase in the immunoreactivity (Figure 7B). When we tested the levels of Bcl-XL or Bcl-2 protein in OTP-treated camptothecin-challenged IEC-6 cells, a transient increase in Bcl-XL but not in Bcl-2 levels was detected (Figure 7D). These results taken together establish strong similarities, as well as some differences, between the prosurvival signals elicited by these 2 agents in cultured cells in vitro and the small intestinal tissue in vivo.
Figure 7.
Immunohistochemical localization of antiapoptotic Bcl-XL protein in vehicle- (200 µmol/L BSA), LPA-, and OTP-pretreated (2 mg/kg each) irradiated small intestinal sections of C57BL/6 mice. Sections of (A) vehicle-treated mice showed less Bcl-XL expression compared with sections obtained from mice treated with (B) LPA or (C) OTP. Calibration bar = 200 µm. The patterns shown are representative to sections obtained from all mice in the corresponding group of 4 mice per treatment. (D) OTP increases the cytoplasmic level of Bcl-XL but not Bcl-2 in camptothecin-treated EC-6 cells. After overnight serum starvation, IEC-6 cells were pretreated with 10 µmol/L OTP or vehicle for 1 hour before challenge with 20 µmol/L camptothecin (Cam). A 20 µg cytoplasmic protein was loaded for each lane and blotted with anti–Bcl-XL or anti–Bcl-2 antibodies as described in Materials and Methods. Note that OTP increased the amount of Bcl-XL but not of Bcl-2 that peaked at 2 hours after camptothecin and decreased to control level by 4 hours.
OTP and LPA Enhance Intestinal Crypt Survival After Radiation Injury
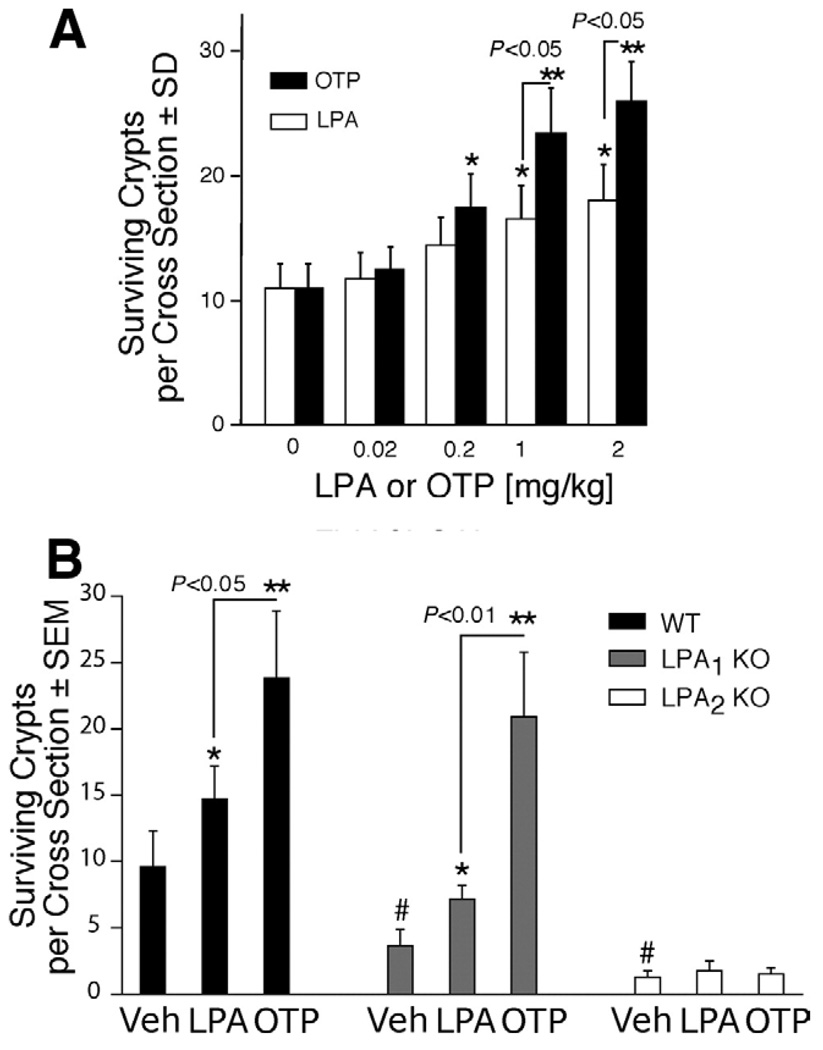
Gamma irradiation induces apoptosis in crypt cells, which in turn reduces the regenerative or clonogenic potential leading to the disruption of the barrier and absorptive function in the injured gut. We examined the effect of LPA and OTP on crypt survival in irradiated mice using H&E staining 5FdU and BrdU incorporation to monitor regenerating S-phase enterocytes. Irradiation with a dose of 15 Gy caused a 90% reduction in the number of crypts in jejunum in wild-type mice within 4 days (Figure 8 and Figure 9). Oral LPA or OTP pretreatment enhanced intestinal crypt survival in a dose-dependent manner (Figure 8A). OTP treatment of mice with doses >0.2 mg/kg significantly enhanced crypt survival, while LPA treatment required a minimum dose of 1.0 mg/kg to cause a significant protection in crypt survival (P < .05; Figure 8A). OTP at doses >1 mg/kg maintained a significantly greater number of crypts than did LPA (P < .05; Figure 8A). Following 15 Gy gamma irradiation, oral OTP applied at 2 mg/kg increased the number of surviving crypts in the jejunum from 10 crypts to an average of 27 crypts (Figure 8A).
Figure 8.
Clonogenic regeneration assays reveal increased intestinal crypt survival in OTP- and LPA-treated mice following irradiation. (A) Wild-type C57BL/6 mice were given either vehicle (BSA 200 µmol/ L), LPA, or OTP (0–2 mg/kg) orally 2 hours before being subjected to 15 Gy whole body gamma irradiation. (B) Wild-type C57BL/6 (closedbars), LPA1 KO (gray bars), or LPA2 KO mice (open bars) were orally given vehicle, LPA, or OTP, 2 mg/kg, 2 hours before 15 Gy gamma irradiation, and the animals were killed 4 days after irradiation. Crypt survival was evaluated by H&E staining combined with BrdU immunostaining. n = 6 in each group. Data are expressed as means ± SD of surviving crypts per cross section. The level of significance based on Student t test was *P< .05 or **P< .01 between the designated groups and was #P< .01 compared with irradiation alone and between the mean crypt survival in wild-type and LPA1 and LPA2 KO mice.
Figure 9.
Representative H&E-stained gut cross sections from (A) nonirradiated control, (B) irradiated vehicle-treated, (C) LPA-treated (2 mg/kg), and (D) OTP-treated wild-type (2 mg/kg) mice, (E and G) vehicle-treated LPA1 and LPA2 KO mice, and OTP-treated (2 mg/kg) (F) LPA1 KO and (H) LPA2 KO mice taken 4 days after irradiation. Calibration bar = ~500 µm.
To further validate LPA2 as the target of the antiapoptotic action of LPA and OTP, we also examined crypt survival in LPA2 KO mice treated the same way as their wild-type counterparts described previously. In contrast to irradiated wild-type mice, LPA1 and particularly LPA2 KO mice showed lower crypt survival. However, in LPA1 KO mice, LPA and OTP both elicited significantly increased crypt survival, which was not significantly different from that seen in wild-type mice treated in the same manner. LPA2 KO mice revealed significantly higher radiation sensitivity. Whereas in wild-type mice exposed to a 15-Gy dose the mean crypt survival per circumference was 10, in LPA2 KO mice it was as little as 1 crypt (Figure 8B). Neither LPA nor OTP applied at 2 mg/kg, the highest dose tested, showed any effect in enhancing intestinal crypt survival in the LPA2 KO mice. This observation extends the increased rate of apoptosis, and the lack of treatment-induced reduction in apoptotic cells observed in the LPA2 KO mice, lending strong support to the hypothesis that LPA2 is the molecular target of the radioprotective effect of LPA and OTP.
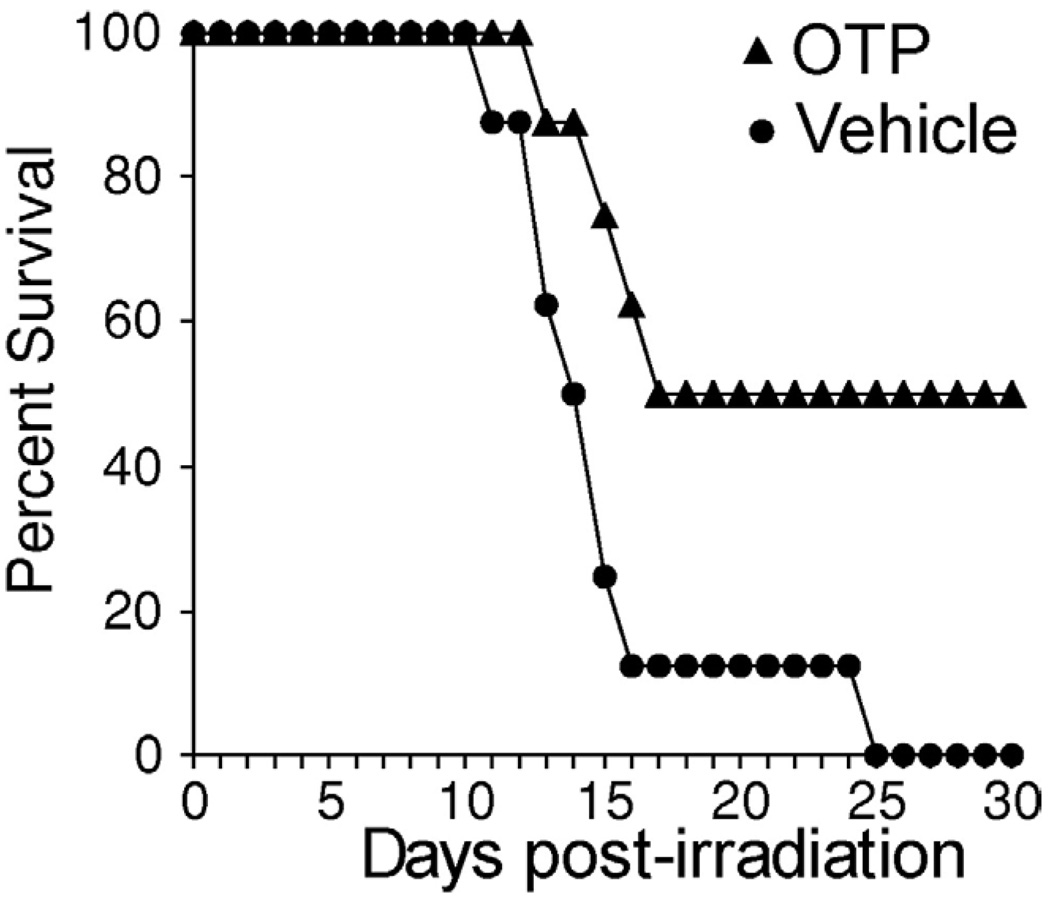
OTP Reduces Radiation-Induced Death
Although orally applied OTP did not get absorbed into the systemic circulation in an effective concentration but reduced radiation-induced cell death in the small intestine, we reasoned that if administered systemically, it might exert a protective effect on survival. To test this hypothesis, we turned to an intraperitoneal route of administration and monitored 30-day survival as the primary end point following a 9-Gy dose of gamma irradiation, which in our system approximately equals an LD100/30 (Figure 10). In the group of mice that received a 0.5-mg/kg dose 30 minutes before irradiation, we observed a 50% survival to the end of the experiment, whereas all animals in the vehicle-treated group died. These results expand the potential usefulness of OTP from the topical protection of the small intestine following an oral route of administration to a radiomitigating agent effectively reducing death caused by the hematopoietic syndrome.
Figure 10.
OTP administered intraperitoneally 30 minutes before irradiation with a 9-Gy (LD100/30) dose increased 30-day survival over vehicle-treated controls.
Discussion
The objective of the present study was to evaluate the effect of OTP, a rationally designed, metabolically stabilized LPA mimic, on radiation-induced apoptosis in vitro and in vivo. Our data indicate that OTP is a highly effective antiapoptotic agent that engages prosurvival pathways similar to those elicited by LPA through the LPA2 receptor subtype. OTP shares a pharmacologic profile similar to that of LPA in that it activated the 3 EDG family LPA receptors expressed heterologously in RH7777 cells. The rank order of the median effective concentration values of OTP was LPA2 (90 nmol/L) < LPA3 < LPA1 based on Ca2+ transients elicited in this heterologous expression system. These values render OTP a considerably weaker ligand of the 3 LPA receptors compared with LPA 18:1. OTP, when compared with LPA in 3 different apoptosis models, which included radiation- and camptothecin-elicited DNA damage–induced and TNF-α/CHX–elicited extrinsic mechanisms, always surpassed the protective effect of LPA. In agreement with the in vitro cellular models of apoptosis, OTP was more effective in reducing the number of apoptotic bodies, caspase-3–positive cells, and caspase-3 activity in C57BL/6 mice exposed to an LD100/15 dose of gamma irradiation.
What may account for the higher efficacy of OTP relative to LPA despite its weaker pharmacologic potency? Although we cannot pinpoint a single reason for the increased efficacy of OTP, there are major differences in its metabolic resistance and bioavailability compared with LPA. First, we found that OTP was not cleaved by pancreatic lipase, the major lipase in the intestine. Second, it is not degraded by LPP1, which is the other major mechanism for the inactivation of LPA.51 Third, due to the lack of a glycerol backbone, unlike LPA, OTP cannot be acylated by lysophosphatidate transacetylases. Fourth, likely due to its polar character, OTP does not transverse the cell membrane readily, and we could not detect radioactively labeled OTP in the blood of experimental animals following application via oral gavage. These differences in its metabolism and bioavailability relative to LPA might represent some of the causes for its higher efficacy in the apoptosis assays. The observation that OTP elicited ERK1/2 and AKT phosphorylation with longer duration in the mouse intestinal tissue certainly supports this hypothesis. At the same time, the lack of absorption of [3H]-OTP into the systemic circulation within a 3-hour period, which appears to be the limit for the duration of its protective time window (data not shown), indicates that it exerts its effect topically from within the intestinal lumen without appearing in a pharmacologically and biologically effective concentration in the bloodstream. Radioactively labeled LPA has been shown to be taken up rapidly (within minutes) into cells where it is rapidly dephosphorylated and reacylated52; nevertheless, ~15% remains intact in the cytoplasm up to 30 minutes after extracellular application.53 These differences in bioavailability could, at least in part, explain the differences in the in vivo efficacy of OTP compared with LPA.
Another important difference was that the LPA2 receptor subtype is sufficient and necessary for the antiapoptotic effect of OTP. In the receptor reconstitution experiments performed in RH7777 cells stably transfected with the individual receptor subtypes, although OTP activated Ca2+ transients through each receptor subtype, only cells expressing LPA2 were protected against apoptosis. In agreement with the role of LPA2 in this model system, experiments conducted in LPA2 KO mice confirmed that this receptor was absolutely necessary for the attenuation of apoptosis and increased crypt survival elicited by OTP. A third line of evidence, namely the increased radiation sensitivity of LPA2 KO gut tissue compared with wild-type animals, suggests that this receptor subtype is uniquely involved in mediating prosurvival signals. Only if we assign the prosurvival function to LPA2 can it explain the lack of protective effect in response to LPA administration observed in the LPA2 KO mice, which continue to express LPA1 and LPA3.20 Reverse-transcription polymerase chain reaction analysis has identified only the LPA1 and LPA2 receptor subtypes in the mouse intestine, as well as in IEC-6 cells used in the present study.8,9 The partial sensitivity of the OTP-elicited anti-apoptotic response to PTX is consistent with the Gq coupling of LPA2.10 However, LPA also showed antiapoptotic protection in RH7777 cells expressing either LPA1 or LPA3 in the TNF-α/CHX-induced apoptosis model. The RH7777 cells showed extreme resistance to radiation (Deng and Tigyi, unpublished data, September 2004) that precluded studying the radioprotective effect exerted by these receptors in this model. We do not understand the reasons for this difference and can only speculate that either the higher than physiologic level of receptor over-expression or the differences in the protective signaling mechanism between the TNF-α/CHX and the DNA damage–induced mechanisms could be part of the reason. We also found that caspase-8 activity was attenuated by OTP, indicating that LPA receptor signaling affects the upstream part of the extrinsic apoptotic pathway. Whether this inhibition of caspase-8 activation is unique to LPA2 or also elicited by other receptor subtypes should be evaluated in future experiments. While this unanswered question does not detract from the importance of LPA2 in the radioprotective mechanisms activated by LPA or OTP and the natural radiation sensitivity of the gut tissue, further experiments will have to be designed to resolve this apparent contradiction. LPA2 is distinct from the other 2 subtypes in that its C-terminus has been shown to interact with PDZ and LIM domain-containing proteins.8,54–57 LPA2 can signal through specific protein-protein interactions in a non–G protein coupled manner and access triple LIM domain-containing proteins, including TRIP6, zyxin, LPP, and Siva-154,56,58 (and Lin, personal communication, September 2006). G protein– linked activation of c-src by LPA2 phosphorylates TRIP6, which in turn augments LPA-induced ERK activation,54 and could lead to increased BAD phosphorylation and inhibition of procaspase-9.16 The potential interaction of LPA2 with Siva-1 offers an exciting possibility, because DNA damage–induced activation of Siva-1 scavenges the antiapoptotic Bcl-XL.59–61 LPA2 could capture Siva-1 and enable Bcl-XL to attenuate apoptosis triggered by DNA damage. Our findings concerning the increased level of Bcl-XL in IEC-6 cells and OTP-treated gut favor this hypothesis. The PDZ domain-mediated interactions including the PDZ-binding protein NHERF2 provide yet another link with antiapoptotic signaling, because stable knockdown of NHERF2 in CaCo-2 cells has been found to attenuate LPA2-induced ERK1/2, AKT, and PLCβ activation.9,55 These potential signaling interactions between LPA2 and LIM or PDZ domain proteins await further investigation.
Orally administered OTP may offer other uses that reach beyond the protection of the gastrointestinal system. Orally applied OTP is unlikely to be useful for reducing apoptosis in the hematopoietic system, because it does not get absorbed to a biologically effective concentration in the systemic circulation. However, experiments performed with intraperitoneal application of OTP in irradiated C57BL/6 mice caused a significant reduction in lethality mitigating the lethality of the LD100/30 dose to equal that of an LD50/30 outcome. Thus, the applicability of OTP for systemic treatment of radiation illness should be the focus of the next experiments.
In summary, the present study identifies OTP as an orally active antiapoptotic agent that targets LPA2 and distinct prosurvival signals coupled to this receptor. The simple chemical nature of OTP, combined with its metabolic resistance, limited systemic bioavailability, and apparently low acute oral toxicity (>10 mg/kg), make this compound a reasonable candidate for treating radiation injury of the gut and warrants further investigation for its potential therapeutic uses.
Acknowledgments
Supported by US Public Health Service grant HL61469 (to G.T.) and RxBio Inc (W.D. and V.G.).
G.T., D.D.M., L.R.J., V.G., and W.D. are stockholders in RxBio Inc.
The authors thank Dr Jerold Chun (Scripps Research Institute, La Jolla, CA) for providing the LPA receptor KO mouse breeders for the study. The intellectual property related to OTP has been licensed to RxBio Inc by the University of Tennessee Research Foundation for commercialization.
Abbreviations used in this paper
- BrdU
bromodeoxyuridine
- BSA
bovine serum albumin
- CHX
cycloheximide
- EDG
endothelial differentiation gene
- ERK
extracellular signal–regulated kinase
- KO
knockout
- LPA
lysophosphatidic acid
- LPP
lipid phosphate phosphatase
- MEK
mitogen-activated protein kinase/extracellular signal–regulated kinase kinase
- OTP
octadecenyl thiophosphate
- PI3K
phosphoinositide 3-kinase
- PTX
pertussis toxin
- TLC
thin-layer chromatography
- TNF
tumor necrosis factor.
References
- 1.Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- 2.Tigyi G, Parrill AL. Molecular mechanisms of lysophosphatidic acid action. Prog Lipid Res. 2003;42:498–526. doi: 10.1016/s0163-7827(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 3.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 4.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 6.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 7.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med. 2005;202:975–986. doi: 10.1084/jem.20050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, Shim H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol. 2005;289:C2–C11. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- 10.Ishii I, Contos JJA, Fukushima N, Chun J. Functional comparisons of the lysophosphatidic acid receptors, LPA1/VZG-1/EDG-2, LPA2/EDG-4, and LPA3/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol Pharmacol. 2000;58:895–902. doi: 10.1124/mol.58.5.895. [DOI] [PubMed] [Google Scholar]
- 11.Yart A, Chap H, Raynal P. Phosphoinositide 3-kinases in lysophosphatidic acid signaling: regulation and cross-talk with the Ras/mitogen-activated protein kinase pathway. Biochim Biophys Acta. 2002;1582:107–111. doi: 10.1016/s1388-1981(02)00144-0. [DOI] [PubMed] [Google Scholar]
- 12.Deng W, Balazs L, Wang DA, Van Middlesworth L, Tigyi G, Johnson LR. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology. 2002;123:206–216. doi: 10.1053/gast.2002.34209. [DOI] [PubMed] [Google Scholar]
- 13.Deng W, Wang DA, Gosmanova E, Johnson LR, Tigyi G. LPA protects intestinal epithelial cells from apoptosis by inhibiting the mitochondrial pathway. Am J Physiol Gastrointest Liver Physiol. 2003;284:G821–G829. doi: 10.1152/ajpgi.00406.2002. [DOI] [PubMed] [Google Scholar]
- 14.Ye X, Ishii I, Kingsbury MA, Chun J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim Biophys Acta. 2002;1585:108–113. doi: 10.1016/s1388-1981(02)00330-x. [DOI] [PubMed] [Google Scholar]
- 15.Kang YC, Kim KM, Lee KS, Namkoong S, Lee SJ, Han JA, Jeoung D, Ha KS, Kwon YG, Kim YM. Serum bioactive lysophospholipids prevent TRAIL-induced apoptosis via PI3K/Akt-dependent cFLIP expression and Bad phosphorylation. Cell Death Differ. 2004;11:1287–1298. doi: 10.1038/sj.cdd.4401489. [DOI] [PubMed] [Google Scholar]
- 16.Fang X, Yu S, Eder A, Mao M, Bast RC, Jr, Boyd D, Mills GB. Regulation of BAD phosphorylation at serine 112 by the Rasmitogen-activated protein kinase pathway. Oncogene. 1999;18:6635–6640. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- 17.Chou CH, Wei LH, Kuo ML, Huang YJ, Lai KP, Chen CA, Hsieh CY. Up-regulation of interleukin-6 in human ovarian cancer cell via a Gi/PI3K–Akt/NF-kappaB pathway by lysophosphatidic acid, an ovarian cancer-activating factor. Carcinogenesis. 2005;26:45–52. doi: 10.1093/carcin/bgh301. [DOI] [PubMed] [Google Scholar]
- 18.Fischer DJ, Liliom K, Guo Z, Nusser N, Virag T, Murakami-Murofushi K, Kobayashi S, Erickson JR, Sun G, Miller DD, Tigyi G. Naturally occurring analogs of lysophosphatidic acid elicit different cellular responses through selective activation of multiple receptor subtypes. Mol Pharmacol. 1998;54:979–988. doi: 10.1124/mol.54.6.979. [DOI] [PubMed] [Google Scholar]
- 19.Contos JJA, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/ lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol Cell Biol. 20002;22:6921–6929. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevreau N, Funk-Archuleta M. Effect of enteral formulas on methotrexate toxicity. Nutr Cancer. 1995;23:185–204. doi: 10.1080/01635589509514374. [DOI] [PubMed] [Google Scholar]
- 23.Funk-Archuleta MA, Foehr MW, Tomei LD, Hennebold KL, Bathurst IC. A soy-derived antiapoptotic fraction decreases meth-otrexate toxicity in the gastrointestinal tract of the rat. Nutr Cancer. 1997;29:217–221. doi: 10.1080/01635589709514627. [DOI] [PubMed] [Google Scholar]
- 24.Logvinova AV, Foehr MW, Pemberton PA, Khazalpour KM, Funk-Archuleta MA, Bathurst IC, Tomei LD. Soy-derived antiapoptotic fractions protect gastrointestinal epithelium from damage caused by methotrexate treatment in the rat. Nutr Cancer. 1999;33:33–39. doi: 10.1080/01635589909514745. [DOI] [PubMed] [Google Scholar]
- 25.Tokumura A, Fukuzawa K, Akamatsu Y, Yamada S, Suzuki T, Tsukatani H. Identification of vasopressor phospholipid in crude soybean lecithin. Lipids. 1978;13:468–472. doi: 10.1007/BF02533615. [DOI] [PubMed] [Google Scholar]
- 26.Sturm A, Dignass AU. Modulation of gastrointestinal wound repair and inflammation by phospholipids. Biochim Biophys Acta. 2002;1582:282–288. doi: 10.1016/s1388-1981(02)00182-8. [DOI] [PubMed] [Google Scholar]
- 27.Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 28.Hines OJ, Ryder N, Chu J, McFadden D. Lysophosphatidic acid stimulates intestinal restitution via cytoskeletal activation and remodeling. J Surg Res. 2000;92:23–28. doi: 10.1006/jsre.2000.5941. [DOI] [PubMed] [Google Scholar]
- 29.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard DM, Watson AJ. Apoptosis and gastrointestinal pharmacology. Pharmacol Ther. 1996;72:149–169. doi: 10.1016/s0163-7258(96)00102-7. [DOI] [PubMed] [Google Scholar]
- 31.Deng W, Poppleton H, Yasuda S, Makarova N, Shinozuka Y, Wang DA, Johnson LR, Patel TB, Tigyi G. Optimal lysophosphatidic acid-induced DNA synthesis and cell migration but not survival require intact autophosphorylation sites of the epidermal growth factor receptor. J Biol Chem. 2004;279:47871–47880. doi: 10.1074/jbc.M405443200. [DOI] [PubMed] [Google Scholar]
- 32.Parrill AL, Wang D, Bautista DL, Van Brocklyn JR, Lorincz Z, Fischer DJ, Baker DL, Liliom K, Spiegel S, Tigyi G. Identification of Edg1 receptor residues that recognize sphingosine 1-phosphate. J Biol Chem. 2000;275:39379–39384. doi: 10.1074/jbc.M007680200. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, Lorincz Z, Bautista DL, Liliom K, Tigyi G, Parrill AL. A single amino acid determines ligand specificity of the S1P1 (EDG1) and LPA1 (EDG2) phospholipid growth factor receptors. J Biol Chem. 2001;276:49213–49220. doi: 10.1074/jbc.M107301200. [DOI] [PubMed] [Google Scholar]
- 34.Sardar VM, Bautista DL, Fischer DJ, Yokoyama K, Nusser N, Virag T, Wang DA, Baker DL, Tigyi G, Parrill AL. Molecular basis for lysophosphatidic acid receptor antagonist selectivity. Biochim Biophys Acta. 2002;1582:309–317. doi: 10.1016/s1388-1981(02)00185-3. [DOI] [PubMed] [Google Scholar]
- 35.Parrill AL, Sardar VM, Yuan H. Sphingosine 1-phosphate and lysophosphatidic acid receptors: agonist and antagonist binding and progress toward development of receptor-specific ligands. Semin Cell Dev Biol. 2004;15:467–476. doi: 10.1016/j.semcdb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara Y, Sardar V, Tokumura A, Baker DL, Murakami-Murofushi K, Parrill AL, Tigyi G. Identification of residues responsible for ligand recognition and regioisomeric selectivity of LPA receptors expressed in mammalian cells. J Biol Chem. 2005;280:35038–35050. doi: 10.1074/jbc.M504351200. [DOI] [PubMed] [Google Scholar]
- 37.Inagaki Y, Pham TT, Fujiwara Y, Kohno T, Osborne DA, Igarashi Y, Tigyi G, Parrill AL. Sphingosine 1-phosphate analogue recognition and selectivity at S1P4 within the endothelial differentiation gene family of receptors. Biochem J. 2005;389:187–195. doi: 10.1042/BJ20050046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, Hla T, Parrill AL, Rosen H. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Virag T, Elrod DB, Liliom K, Sardar VM, Parrill AL, Yokoyama K, Durgam G, Deng W, Miller DD, Tigyi G. Fatty alcohol phosphates are subtype-selective agonists and antagonists of LPA receptors. Mol Pharmacol. 2003;63:1032–1042. doi: 10.1124/mol.63.5.1032. [DOI] [PubMed] [Google Scholar]
- 40.Durgam GG, Virag T, Walker MD, Tsukahara R, Yasuda S, Liliom K, van Meeteren LA, Moolenaar WH, Wilke N, Siess W, Tigyi G, Miller DD. Synthesis, structure-activity relationships, and biological evaluation of fatty alcohol phosphates as lysophosphatidic acid receptor ligands, activators of PPARgamma, and inhibitors of autotaxin. J Med Chem. 2005;48:4919–4930. doi: 10.1021/jm049609r. [DOI] [PubMed] [Google Scholar]
- 41.MOE. Montreal: Chemical Computing Group. 2002 [Google Scholar]
- 42.Halgren TA. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94*. J Comp Chem. 1996;17:490–519. [Google Scholar]
- 43.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 44.Ballesteros JA, Weinstein H. In: Methods in neurosciences. Conn PM, Sealfon SC, editors. San Diego: Academic; 1995. pp. 366–428. Chapter 19. [Google Scholar]
- 45.Kates M. Techniques of lipidology. Amsterdam: Elsevier; 1988. [Google Scholar]
- 46.Yue J, Yokoyama K, Balazs L, Baker DL, Smalley D, Pilquil C, Brindley DN, Tigyi G. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell Signal. 2004;16:385–399. doi: 10.1016/j.cellsig.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Fischer DJ, Nusser N, Virag T, Yokoyama K, Wang D, Baker DL, Bautista D, Parrill AL, Tigyi G. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol Pharmacol. 2001;60:776–784. [PubMed] [Google Scholar]
- 48.Balazs L, Okolicany J, Ferrebee M, Tolley B, Tigyi G. Topical application of the phospholipid growth factor lysophosphatidic acid promotes wound healing in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;280:R466–R472. doi: 10.1152/ajpregu.2001.280.2.R466. [DOI] [PubMed] [Google Scholar]
- 49.Steiner T. The hydrogen bond in the solid state. Angew Chem Int Ed. 2002;41:48–76. doi: 10.1002/1521-3773(20020104)41:1<48::aid-anie48>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 50.Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, Yagi M, Sato M, Suzuki R, Murooka H, Sakai T, Nishitoba T, Im DS, Nochi H, Tamoto K, Tomura H, Okajima F. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- 51.Brindley DN, English D, Pilquil C, Buri K, Ling ZC. Lipid phosphate phosphatases regulate signal transduction through glycerolipids and sphingolipids. Biochim Biophys Acta. 2002;1582:33–44. doi: 10.1016/s1388-1981(02)00135-x. [DOI] [PubMed] [Google Scholar]
- 52.van der Bend RL, de Widt J, van Corven EJ, Moolenaar WH, van Blitterswijk WJ. Metabolic conversion of the biologically active phospholipid, lysophosphatidic acid, in fibroblasts. Biochim Biophys Acta. 1992;1125:110–112. doi: 10.1016/0005-2760(92)90163-p. [DOI] [PubMed] [Google Scholar]
- 53.Tokumura A, Tsutsumi T, Tsukatani H. Transbilayer movement and metabolic fate of ether-linked phosphatidic acid (1-O-Octadecyl-2-acetyl-sn-glycerol 3-phosphate) in guinea pig peritoneal polymorphonuclear leukocytes. J Biol Chem. 1992;267:7275–7283. [PubMed] [Google Scholar]
- 54.Lai YJ, Chen CS, Lin WC, Lin FT. c-Src-mediated phosphorylation of TRIP6 regulates its function in lysophosphatidic acid-induced cell migration. Mol Cell Biol. 2005;25:5859–5868. doi: 10.1128/MCB.25.14.5859-5868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh YS, Jo NW, Choi JW, Kim HS, Seo SW, Kang KO, Hwang JI, Heo K, Kim SH, Kim YH, Kim IH, Kim JH, Banno Y, Ryu SH, Suh PG. NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-beta3 activation. Mol Cell Biol. 2004;24:5069–5079. doi: 10.1128/MCB.24.11.5069-5079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Lai YJ, Lin WC, Lin FT. TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor. J Biol Chem. 2004;279:10459–10468. doi: 10.1074/jbc.M311891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada T, Ohoka Y, Kogo M, Inagaki S. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs) J Biol Chem. 2005;280:19358–19363. doi: 10.1074/jbc.M414561200. [DOI] [PubMed] [Google Scholar]
- 58.Takizawa N, Smith TC, Nebl T, Crowley JL, Palmieri SJ, Lifshitz LM, Ehrhardt AG, Hoffman LM, Beckerle MC, Luna EJ. Supervillin modulation of focal adhesions involving TRIP6/ZRP-1. J Cell Biol. 2006;174:447–458. doi: 10.1083/jcb.200512051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu F, Borthakur A, Sun X, Barkinge J, Gudi R, Hawkins S, Prasad KV. The Siva-1 putative amphipathic helical region (SAH) is sufficient to bind to BCL-XL and sensitize cells to UV radiation induced apoptosis. Apoptosis. 2004;9:83–95. doi: 10.1023/B:APPT.0000012125.01799.4c. [DOI] [PubMed] [Google Scholar]
- 60.Gudi R, Barkinge J, Hawkins S, Chu F, Manicassamy S, Sun Z, Duke-Cohan JS, Prasad KV. Siva-1 negatively regulates NF-kappaB activity: effect on T-cell receptor-mediated activation-induced cell death (AICD) Oncogene. 2006;25:3458–3462. doi: 10.1038/sj.onc.1209381. [DOI] [PubMed] [Google Scholar]
- 61.Xue L, Chu F, Cheng Y, Sun X, Borthakur A, Ramarao M, Pandey P, Wu M, Schlossman SF, Prasad KV. Siva-1 binds to and inhibits BCL-X(L)-mediated protection against UV radiation-induced apoptosis. Proc Natl Acad Sci U S A. 2002;99:6925–6930. doi: 10.1073/pnas.102182299. [DOI] [PMC free article] [PubMed] [Google Scholar]