Abstract
Prostate cancer cell migration is an essential event both in the progression of prostate cancer and in the steps leading to metastasis. We report here that lysophosphatidic acid (LPA), a potent bioactive phospholipid, induces prostate cancer PC3 cell migration via the activation of the LPA1 receptor, which is linked to a PTX-sensitive activation mechanism of the mitogen-activated protein kinases (MAPK). Our results demonstrate that parallel activation of ERK1/2 and p38, but not JNK, is responsible for LPA-stimulated PC3 cell migration. Furthermore, using small interfering RNA (siRNA) technology, and overexpressing dominant-negative mutants of p38 MAPK isotypes of α, β, γ and δ, we have identified that the activation of ERK2 (p42) and p38α, but not of ERK1 and the other isoforms of p38 MAPK, is required for LPA-induced migration. Our study provides the first evidence for a functional role of p42 and p38α in LPA-induced mammalian cell migration, and also demonstrates, for the first time, that the receptor LPA1 mediates prostate cancer cell migration. The results of the present study suggest that LPA, the receptor LPA1, ERK2 and p38α are important regulators for prostate cancer cell invasion and thus could play a significant role in the development of metastasis.
Keywords: Lysophosphatidic acid, Receptors, Cell migration, Protein kinases and prostate cancer cells
1. Introduction
Prostate cancer continues to be the most common lethal malignancy diagnosed in American men and the second leading cause of male cancer deaths [1]. Prostate cancer cells initially proliferate locally, but later may metastasize, preferentially to bone, where the metastases may cause clinical problems such as bone pain, restricted mobility, replacement of hematopoietic tissue, and in the vertebral column, compression of the spinal cord [2–5]. Migration of prostate cancer cells is a critical event in prostate cancer progression and especially metastasis. Understanding the intracellular signaling pathways essential for prostate cancer cell migration is important for the development of therapeutic strategies to prevent prostate carcinoma infiltration and possible metastasis.
Lysophosphatidic acid (LPA) is increasingly being recognized as an important multifunctional mediator, not only because LPA affects diverse cellular functions, including cell proliferation, differentiation, adhesion, migration and contraction [6,7], but also because LPA has been detected in many physiological and pathological biological fluids and tissues, including serum [8], follicular fluids [9], ascites from ovarian cancer patients [10] and atherosclerotic lesions [11]. LPA has recently been reported to be produced by, and released from, prostate cancer cells [12], suggesting that at prostate cancer sites, LPA may serve as an autocrine/paracrine mediator to affect the functions of both cancer and neighboring cells. In support of this, LPA induction of prostate cancer cell proliferation has been reported [13–15].
Previously, it has been reported that the expression of LPA receptors LPA1, LPA2 and LPA3 has been detected in various prostate cancer cells [16–18]; however, the role of these LPA receptors in the migration of prostate cancer cells has not yet been defined. Additionally, while LPA receptors are known to couple to multiple G-proteins, the specific G-protein involved in the activation of downstream signaling molecules that lead to LPA-induced migration of prostate cancer cells remains unknown. Although activation of the mitogen activated protein kinase (MAPK) family has been known to be an essential step in LPA signaling, and notably the activation of MAPK has been detected in prostate cancer tissue [19–21], the role of MAPK in prostate cancer cell migration in response to LPA is unaccounted for.
In the present study, we found that LPA induces prostate cancer PC3 cell migration at very low, nanomolar concentrations. We further investigated the molecular mechanisms involved in this LPA-induced prostate cancer cell migration and identified the specific LPA receptor, specific G protein, and specific groups and subunits of MAPK that regulate LPA-induced migration of prostate cancer cells.
2. Materials and methods
2.1. Materials
LPA (1-oleoyl-2-hydroxy-sn-glycero-3-phosphate) was purchased from Avanti Polar Lipids Inc. (Alabaster, AL); transwell chambers (6.5 mm diameter, with 8.0 µm pore size polycarbonate membranes) were from Corning Costar Corp. (Acton, MA); and phosphate buffer, Harris hematoxylin solution, and eosin Y solution were from Sigma-Aldrich (St. Louis, MO). The LPA receptor subtype-specific antagonists compound 12a, compound 13a and compound 19b were synthesized as described previously [22]. Pertussis toxin (PTX), PD98059 (PD), SB203580 (SB), and U0126 (U0) were from Biomol (Plymouth Meeting, PA). Antibodies against human p42/44 MAPK, human p38 MAPK, human p38α MAPK, p38γ MAPK and Germinal center kinase (GCK); SignalSilence p44 MAPK siRNA Kit, SignalSilence Pool p42 MAPK siRNA Kit; and SignalSilence Pool p38 MAPK siRNA Kit were from Cell Signaling Technology, Inc. (Beverly, MA). The antibody against p38β MAPK (E-20) was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), and the antibody against p38δ MAPK was from Upstate Inc. (Lake Placid, NY). Wild type and dominant negative p38α, β, γ, δ plasmids were constructed as described previously [23–26]. The RNeasy kit was from Qiagen (Valencia, CA) and the ThermoScript RT-PCR system was from Invitrogen (Carlsbad, CA). GeneAmp PCR core reagents were from Applied Biosystems (Foster City, CA).
2.2. RT-PCR assay
Expression of mRNA was evaluated by RT-PCR. Total RNA was isolated from PC3 cells using an RNeasy mini-prep kit (Qiagen Inc. Valencia, CA). The first strand of cDNA was reverse-transcribed using the ThermoScipt RT-PCR system (Invitrogen, Carlsbad, CA). The cDNA products were amplified using GeneAmp PCR core reagents (Applied Biosystems, Forster City, CA). The amplification conditions were as follows: 5 min at 95 °C; 35 cycles of 30 s at 95 °C, 30 s at 56 °C, and 1 min at 72 °C; followed by a final extension for 10 min at 72 °C. The primers were used as follows: LPA1, 5′-ATG GCT GCC ATC TCT ACT TCC ATC CC-3′ (forward) and 5′-CTA AAC CAC AGA GTG GTC ATT GCT GTG-3′ (reverse); LPA2, 5′-ATG GTC ATC ATG GGC CAG TGC TAC TAC-3′ (forward) and 5′-TCA GTC CTG TTG GTT GGG TTG AGC C-3′ (reverse); LPA3, 5′-ATG AAT GAG TGT CAC TAT GAC AAG CAC-3′ (forward) and 5′-TTA GGA AGT GCT TTT ATT GCA GAC TGC-3′ (reverse). The PCR products were analyzed by electrophoresis on a 1.2% agarose gel.
2.3. Cell culture, small interfering RNA (siRNA) transfection and plasmid transfection
PC3 prostate cancer cells (American Type Culture Collection, Manassas, VA) were cultured in RPMI 1640 medium with 10% fetal bovine serum. Cells were starved for 24 h before the migration assay. SignalSilence Control siRNA (non-silencing siRNA, 100 nM), p42 siRNA (20 nM), p44 siRNA (100 nM) and p38α siRNA (40 nM) were transfected according to the manufacturer’s instructions (Cell Signaling Technology). The SignalSilence Control siRNA was used as a negative control. Forty-eight hours after transfection, the cells were starved for 24 h followed by treatment either with or without LPA. Plasmid constructs of p38 MAPK isoforms were transfected into PC3 cells by using Lipofectamine 2000 reagent (Invitrogen). Sixteen hours after transfection, the cells were starved for 24 h, followed by treatment either with or without LPA.
2.4. Cell migration assay
Cell migration was performed using transwell migration plates (Corning). PC3 cells were trypsinized and plated onto transwell plates for migration assays. A volume of 200 µl media containing 2×105 cells was added to the upper chamber. Cells were allowed to migrate through filters (8 µm pore size), which had been precoated on both sides with gelatin, in the presence of either medium (600 µl) alone or medium with LPA at designated concentrations in the lower chamber. Cell migration was carried out at 37 °C in 5% CO2 for 3 h. Cells remaining on the upper surface of the filter were carefully removed by mechanical scraping. The upper chambers were rinsed with PBS, and the cells were fixed with methanol and then stained with Harris hematoxylin and eosin Y. The number of cells that had migrated to the lower surface of the filter was counted in 4 random objective fields (200× magnification) using a Nikon Eclipse E600 microscope.
2.5. Western blot assay
PC3 cells were rinsed with cold PBS and then lysed in Western blot lysis buffer (50 mM Tris–HCl, pH 6.8, 8 M urea, 5%-mercaptoethanol, 2% SDS, and protease inhibitors) with sonication for 20 s on ice. Cellular proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and were transferred to a polyvinylidene fluoride membrane (Immobilon-P, Millipore). The membranes were then probed with the specific antibodies, and the specific protein bands were visualized by ECL-Plus (GE Healthcare) as described previously [27].
2.6. Statistics
Results are means±SE. Comparisons between multiple groups were performed using one-way ANOVA with post-hoc t-tests. Single comparisons were made using two-tailed, unpaired Student’s t-tests. A p value of <0.05 was considered to be statistically significant.
3. Results
3.1. LPA induces migration of PC3 cells in a concentration-dependent manner
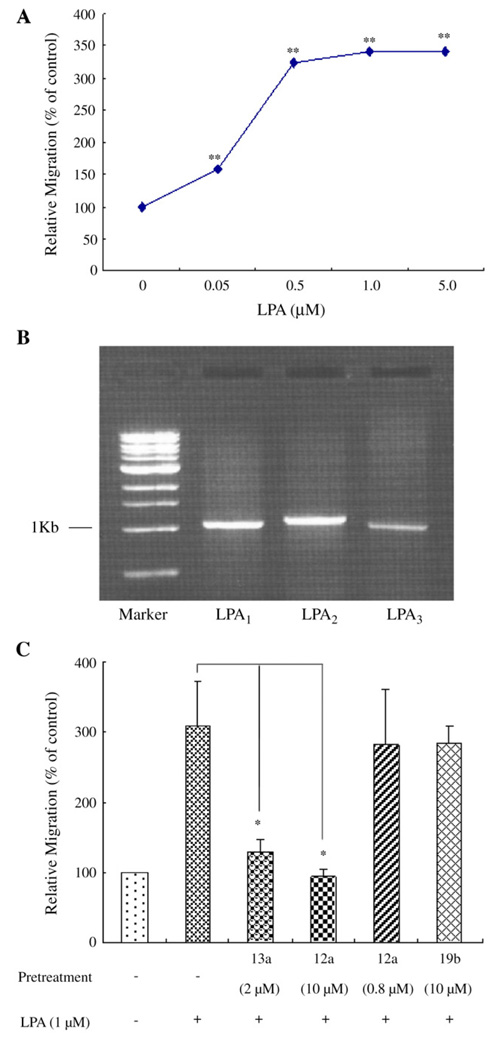
In a transwell chamber assay, we observed that LPA induced PC3 cell migration at a low threshold concentration of 50 nM, and this migration increased in a concentration-dependent manner. The LPA-induced maximal migration (3.2–3.4 fold) was observed in the 0.5 to 5.0 µM concentration range (Fig. 1A). These results are consistent with the hypothesis that LPA-induced PC3 cell migration is receptor dependent.
Fig. 1.
LPA induces migration of prostate cancer PC3 cells, and the migration is mediated by LPA receptor 1 (LPA1), but not by LPA2 or LPA3. (A) The migration of PC3 cells was examined using a transwell chamber. Various doses of LPA were added to the bottom chamber. Human prostate cancer PC3 cells were added to the transwells and allowed to migrate for 3 h. Relative migration rates were mean±SE of three experiments. Data were analyzed using one-way ANOVA with post-hoc t-tests. **p < 0.01 versus control. (B) RT-PCR results show that LPA1, LPA2 and LPA3 are expressed in PC3 cells. (C) LPA1 mediates PC3 cell migration in response to LPA. PC3 cells were pretreated either with or without LPA receptor antagonists for 40 min and then subjected to a migration assay in response to 1 µM LPA. Relative migration rates were mean±SE of three experiments. *p<0.05 versus LPA alone.
3.2. LPA1 mediates LPA-induced PC3 cell migration
Our RT-PCR results show that three EDG-family LPA receptors (LPA1, LPA2 and LPA3) are expressed in PC3 cells (Fig. 1B). These results are in agreement with previous reports [16–18]. To determine which LPA receptor mediates cell migration, we examined the effect of the specific LPA receptor antagonists on LPA-induced PC3 cell migration. The recently developed, short chain stabilized phosphatidate compound 12a (IC50 = 1.58 µM for LPA1; IC50=0.143 µM for LPA3) and compound 13a (IC50=0.328 µM for LPA1; IC50 = 0.184 µM for LPA3) are novel and selective antagonists of LPA1 and LPA3, but have no effect on LPA2 [22]. Therefore compounds 12a and 13a offer pharmacological tools to determine which of these receptors is involved in PC3 cell migration. Pretreatment of PC3 cells with 10 µM of compound 12a completely blocked LPA-stimulated PC3 cell migration; pretreatment of PC3 cells with 2 µM of compound 13a reduced PC3 cell migration by 85±14% (Fig. 1C), suggesting that LPA1 and/or LPA3, but not LPA2 mediate LPA-induced prostate cancer cell migration. To differentiate the functions of LPA1 and LPA3 in LPA-induced PC3 cell migration, we first tested whether a lower concentration of compound 12a could block LPA-induced migration. Since the compound 12a has an IC50 one order of magnitude lower for LPA3 than that for LPA1, the use of compound 12a at the concentration of 0.8 µM, which is exactly 5.6 times the IC50 for LPA3, should efficiently block PC3 cell migration, if LPA3 mediates LPA-induction of PC3 cell migration. The observation that 0.8 µM of compound 12a did not significantly affect LPA-stimulated PC3 cell migration (Fig. 1C) strongly suggests that LPA3 is not involved in LPA-induced migration. To exclude the effect of LPA3 on PC3 cell migration, we pretreated PC3 cells with the recently developed, specific LPA3 antagonist compound 19b (IC50=0.935 µM for LPA3), which has been reported to have no effect on LPA1 and LPA2 [22]. We found that 10 µM of compound 19b had no effect on LPA-induced cell migration (Fig. 1C). This result further diminished the possibility that LPA3 mediates LPA-stimulated PC3 cell migration. Taken together, these pharmacological data, based on the use of a novel specific agonist and selective antagonists, indicate that LPA1, but not LPA2 or LPA3, is responsible for the LPA-induced PC3 cell migration.
3.3. A pertussis toxin-sensitive G protein regulates LPA-stimulated PC3 cell migration
It has been reported that all LPA receptors couple to G proteins. To examine which G protein is responsible for LPA-induced PC3 cell migration, we examined whether pertussis toxin (PTX), a Gi/o protein inhibitor, blocks LPA-induced PC3 cell migration. As shown in Fig. 2, pretreatment of cells overnight with 100 ng/ml PTX did not significantly affect LPA-untreated PC3 cell migration, however, it completely blocked LPA-induced PC3 cell migration, indicating that the Gi/o protein is responsible for LPA-induced PC3 cell migration.
Fig. 2.
Pertussis toxin (PTX)-sensitive Gi protein is involved in LPA-induced PC3 cell migration. PC3 cells were pretreated overnight, either with or without 100 ng/ml PTX, and then a cell migration assay was performed in response to 1 µM LPA. 100 ng/ml PTX completely blocked LPA-induced PC3 cell migration. Results were mean±SE of three experiments. *p < 0.05 versus LPA alone.
3.4. LPA transiently and rapidly induces activation of extracellular signal-regulated kinase (ERK) and p38 MAPK, but not of the stress-activated protein kinase c-Jun/N terminal kinases (JNK) in PC3 cells
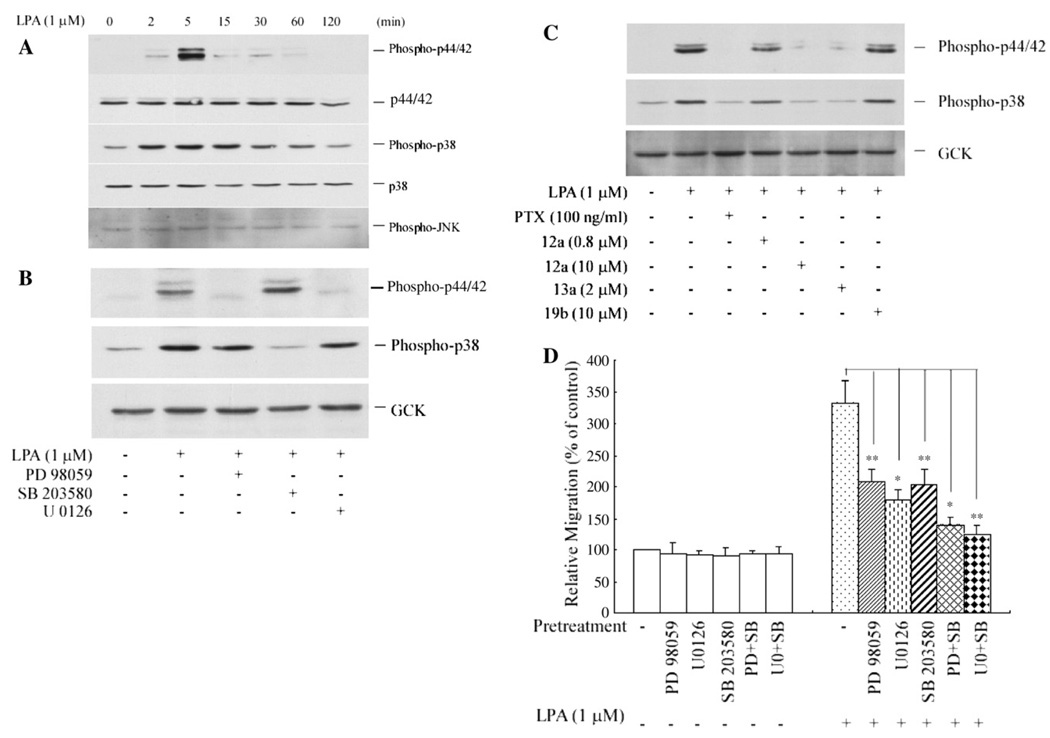
ERK, p38 and JNK are the three major groups of MAPK [28]. LPA induction of ERK activation in PC3 cells has been reported [14,15]; however, whether LPA stimulates activation of other MAPKs in prostate cancer cells has not been documented. As shown in Fig. 3A, we observed that LPA stimulated, marked phosphorylation of p38 MAPK as well as phosphorylation of ERK1/2 (p44/p42). The phosphorylation of these two kinases reached their peaks at 5 min and dropped to the basal level after 2 h of stimulation. In contrast, phosphorylation of JNK was not affected by LPA. These data show for the first time that LPA induces p38 activation in prostate cancer cells and suggest a potential involvement of ERK and p38 in LPA-induced PC3 cell migration.
Fig. 3.
Activation of extracellular signal-regulated kinase (ERK) and p38 mitogen activated protein kinase (MAPK) are required for LPA-induced PC3 cell migration. (A) LPA induces rapid and transient phosphorylation of ERK and p38 MAPK. PC3 cells were stimulated with 1 µM LPA for various time periods. Phosphorylation of ERK, p38 and JNK was detected using phospho-specific antibodies against phospho-p44/42 (ERK1/ERK2), phospho-p38 and phospho-JNK, respectively. (B) LPA induces parallel activation of ERK and p38 in PC3 cells. Cells were pretreated with PD98059 (30 µM), U0126 (10 µM)or SB203580 (10 µM) for 40 min prior to 1 µM of LPA stimulation. Phosphorylation of ERK or p38 was detected after 5 min of LPA treatment. GCK served as a protein loading control. (C) LPA1 and Gi/o proteins mediate phosphorylation of ERK and p38 MAPK. Cells were pretreated with either PTX (100 ng/ml) for 16 h, or one of the antagonists (12a, 13a or 10b, at indicated concentrations) for 40 min prior to LPA stimulation. Phosphorylation of ERK and p38 was measured after 5 min of LPA treatment. GCK served as a protein loading control. (D) Both the ERK kinase inhibitors (PD98059 and U0126) and the p38 MAPK inhibitor (SB203580) inhibited LPA-induced PC3 cell migration. Cells were pretreated with PD98059 (30 µM), U0126 (10 µM), SB203580 (10 µM), PD98059 (30 µM) plus SB203580 (10 µM), or U0126 (10 µM) plus SB203580 (10 µM) for 40 min prior to the transwell migration assay. Results were mean±SE of three experiments. *p<0.05, **p<0.01 versus LPA alone.
3.5. LPA induces parallel activation of ERK and p38 in PC3 cells
Although LPA activates ERK, p38 and JNK MAPK in mammalian cells, the regulatory relationship among these kinases in response to LPA is currently unclear. We examined the regulatory relationship between ERK and p38 in prostate cancer PC3 cells by using the specific inhibitors PD98059 and U0126 for ERK kinase (MEK), and SB203580 for p38 MAPK. As shown in Fig. 3B, 30 µM of PD98059 or 10 µM of U0126 completely blocked ERK phosphorylation, but had no effect on the phosphorylation of p38. These results suggested two possibilities: (1) ERK activation and p38 activation are parallel, or (2) they are in linear positions, in which p38 is upstream from ERK. To further assess the relationship between these two kinases, the cells were pretreated with the p38-specific inhibitor SB203580 (10 µM), after which we observed complete blockage of LPA-induced phosphorylation of p38, but no effect on ERK1/2 phosphorylation (Fig. 3B). These results rule out the possibility that p38 is upstream from ERK. Therefore, activation of the p38 pathway and activation of ERK are independent and run in parallel in LPA-stimulated PC3 cells.
3.6. LPA1 transduces LPA signals to activate ERK and p38, and PTX sensitive Gi/o protein mediates the activation of both ERK and p38 in PC3 cells
The role of LPA receptors in LPA activation of MAPK in prostate cancer cells has not been reported. Utilizing the novel and specific LPA receptor antagonists, 12a, 13a, and 19b [22], we found that LPA1 is required for ERK and p38 activation. Firstly, compound 12a (10 µM) and compound 13a (2 µM), both of which are specific antagonists for LPA1 and LPA3, completely blocked phosphorylation of ERK and p38. This suggests that LPA1 and/or LPA3 but not LPA2 are involved. Secondly, the result that compound 12a, at a concentration of 0.8 µM, which is 5.6 times the IC50 for LPA3 did not significantly affect LPA-induced phosphorylation of ERK and p38 (Fig. 3C), ruled out a role for LPA3. Thirdly, compound 19b, an antagonist specific for LPA3 at 10 µM (10.7 times the IC50 for LPA3), did not affect LPA-induced phosphorylation of either ERK or p38, clearly indicating that LPA3 is not involved. Therefore, LPA1 transduces LPA signals to activate both ERK and p38 MAPK in PC3 cells. To further examine whether the LPA1-transduced signals of phosphorylation of both ERK and p38 in PC3 cells are mediated by PTX sensitive Gi/o-proteins, we pretreated PC3 cells with PTX (100 ng/ml) for 16 h. As shown in Fig. 3C, this treatment completely blocked LPA-induced phosphorylation of ERK and p38, indicating that LPA-induced activation of both ERK and p38 is mediated via Gi/o proteins.
3.7. Activation of both ERK and p38 is required for maximal LPA-induced PC3 cell migration
Reports on the examination of prostate cancer specimens have revealed that increased constitutive activation of MAPK is observed during the progressive development of prostate cancer [20,21], suggesting a role of MAPK in the progression of prostate cancer. A previous study has shown that LPA-induced ERK activation is involved in prostate cancer cell proliferation [15], though it is unknown whether p38 activation is involved. We had hypothesized that LPA-triggered MAPK activation contributes to prostate cancer cell migration. This hypothesis was tested by examining the effect of the specific inhibitors of ERK and p38 on LPA-induced PC3 cell migration. As shown in Fig. 3D, ERK kinase inhibitors PD98059 or U0126 alone, p38 MAPK inhibitor SB203580 alone, or a combination of either PD98059 with SB203580, or U0126 with SB203580, did not significantly affect LPA-untreated PC3 migration; however, 30 µM of PD98059 and 10 µM of U0126 reduced LPA-induced PC3 cell migration by 54±5% and 65± 10%, respectively. 10 µM of SB203580 reduced LPA-induced migration by 57±9%. Furthermore, a combination of both ERK and p38 inhibitors, either PD98059 with SB203580 or U0126 with SB203580, inhibited LPA-induced migration by 83± 6% and 89±7% respectively. These results indicate that the activation of both ERK and p38 is required for maximal migration of PC3 cells induced by LPA.
3.8. p42 (ERK2), but not p44 (ERK1), is required for LPA-induced PC3 cell migration
To further determine which subunit(s) of the ERK family mediates LPA-induced PC3 cell migration, we examined the effect of siRNA depletion of each ERK subunit’s expression on LPA-stimulated cell migration. ERK consists of ERK1 (p44) and ERK2 (p42). Transfection of p44 siRNA reduced p44 protein expression by 79±8% (Fig. 4A and C) and had no significant effect on p42 expression (Fig. 4A and B). Transfection of p42 siRNA reduced p42 protein expression by 76±9% (Fig. 4A and B) and p44 protein expression by 23±6% (Fig. 4A and C). We found that transfection of p42 siRNA blocked LPA-induced PC3 cell migration by 64± 7% (Fig. 4D). In contrast, transfection of p44 siRNA, which markedly reduced p44 protein expression (79±8%), had no detectable effect on cell migration (Fig. 4D). These results indicate that activation of p42, but not of p44 MAPK is required for LPA-induced PC3 cell migration.
Fig. 4.
Transfection of small interfering RNA (siRNA) of p42 ERK, but not transfection of siRNA of p44 ERK, affects LPA-induced PC3 cell migration. (A) Effect of siRNA of p42 and p44 on expression of these subunits. siRNA of p42, p44 and non-silencing siRNA were transfected into PC3 cells according to the manufacturer’s instructions described in Materials and methods. Specific antibodies against p42 and p44 were used to detect the expression of the ERK subunits. GCK protein served as a loading control. (B) Bar graph of effect of p42 siRNA, p44 siRNA and non-silencing siRNA on p42 protein expression. Results were based on the scanned data of Western blot analyses as illustrated in Fig. 4A. Data were mean±SE from four experiments. (C) Bar graph of effect of p42 siRNA, p44 siRNA and non-silencing siRNA on p44 protein expression. Data were mean±SE of four experiments. D. Effect of p42 siRNA, p44 siRNA and non-silencing siRNA on LPA-induced PC3 cell migration. Data were mean±SE of three experiments. *p<0.05 versus LPA alone.
3.9. p38α, but not the other subunits of p38, is required for LPA-induced migration of PC3 cells
The p38-specific inhibitor SB203580 reduced LPA-induced PC3 migration by 57±9% (Fig. 3D), suggesting a role for p38 in PC3 cell migration. p38 MAPK consists of 4 subunits, α, β, γ and δ [29]. To determine which of these p38 subunits is responsible for prostate cancer cell migration, we first examined the effect of the dominant negative construct of each p38 subunit on PC3 cell migration in response to LPA by transfection of a series of dominant negative and wild type constructs of each subunit of p38 MAPK. The functional effects of these dominant negative constructs had been established previously [23–26].
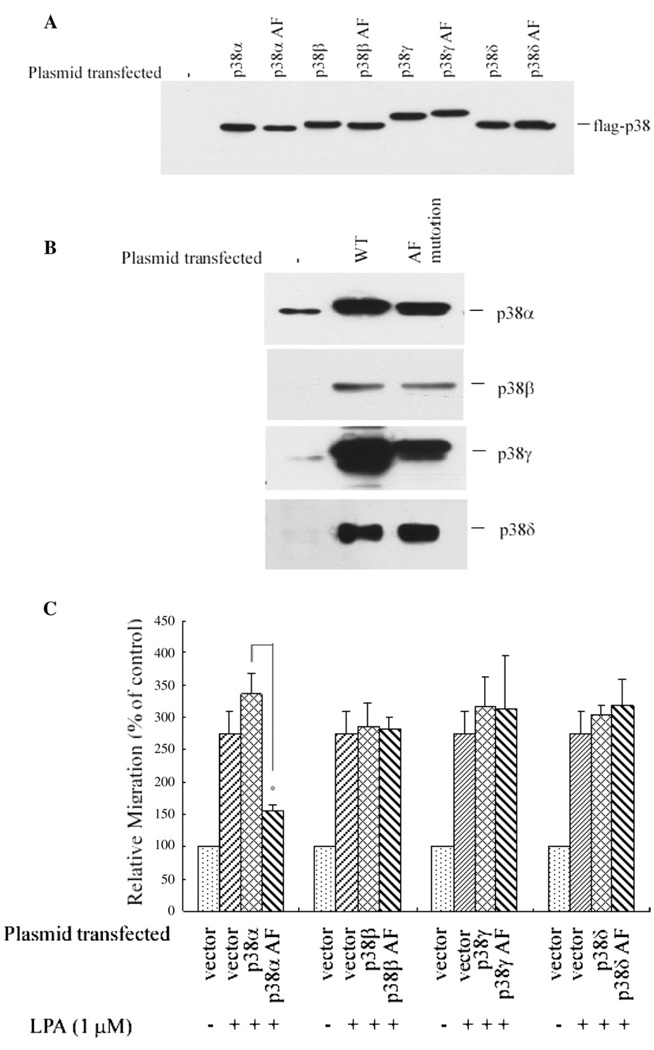
The expression level of these constructs was verified by anti-FLAG epitope-specific antibody (Fig. 5A), since each of the p38 subunits had been tagged with FLAG. The expression of these subunits was further confirmed using the specific antibodies against each subunit (Fig. 5B). Notably, the endogenous p38α and p38γ are the two prominent subunits of p38 MAPK expressed in PC3 cells; the other two subunits, i.e. p38β and p38δ, were undetectable (Fig. 5B), suggesting that very likely the contribution of p38 MAPK to the LPA-induced migration is due to either p38α and/or p38γ MAPK.
Fig. 5.
Overexpression of dominant negative p38α inhibits LPA-induced PC3 cell migration. (A) Overexpression of wild type and dominant negative p38 MAPK subunit constructs in PC3 cells. Dominant negative p38 MAPK used were p38αAF, p38βAF, p38δAF and p38γAF as described previously [23–26]. A specific antibody against FLAG was used, since each subunit of p38 was tagged with FLAG epitope. (B) Overexpression of the wild type and the dominant negative forms of various p38 MAPK subunits was confirmed using specific antibodies against each of the p38 MAPK subunits. (C) Overexpression of the dominant negative p38α inhibits LPA-induced PC3 cell migration. Cells transfected with the vector only and cells transfected with different p38 subunit constructs were used for the migration assay in response to 1 µM of LPA. Data were mean±SE of three experiments. *p<0.05 versus wild type plasmid group.
We found that overexpression of the dominant negative p38α reduced LPA-induced migration by 68±5%, while overexpression of wild type p38α increased cell migration by 37±13% (Fig. 5C). In contrast, overexpression of the dominant negative constructs of the other types of p38 MAPK did not significantly affect PC3 cell migration. These results together indicate that p38α, but not the other subunits of p38 MAPK, such as β, γ or δ, contributes to LPA-induced PC3 cell migration.
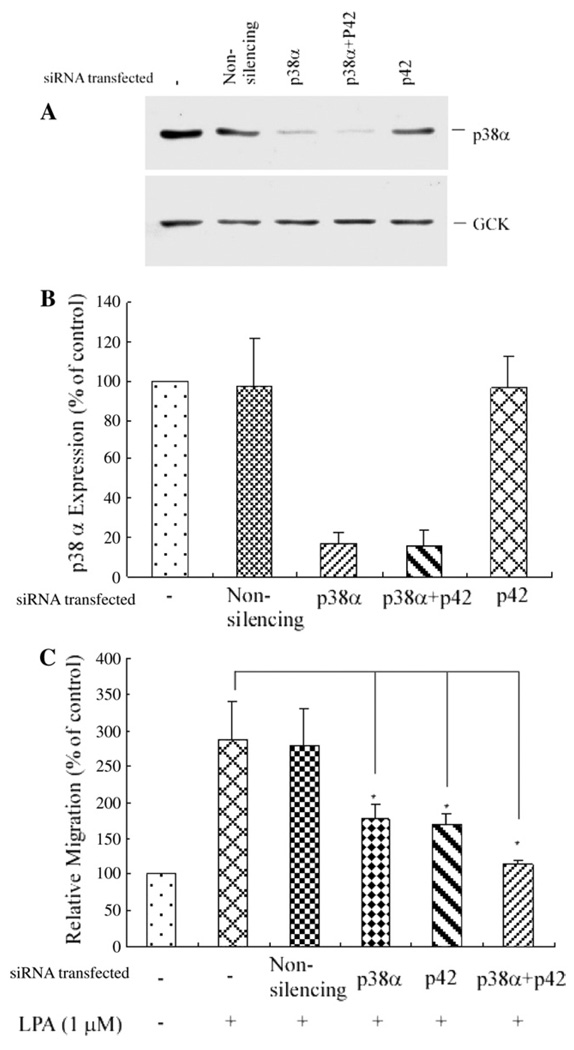
To further confirm the role of p38α, we conducted p38α siRNA knockdown experiments, and observed that transfection of p38α siRNA, which blocked p38α expression by 85±7% (Fig. 6A and B), also reduced LPA-induced cell migration by 59± 8% (Fig. 6C). Furthermore, the combined depletion of the expression of both p42 and p38α, using p42 siRNA and p38α siRNA (Fig. 6A and B), nearly completely inhibited LPA-induced PC3 cell migration (by 93±4%, Fig. 6C). These results indicate that p42 and p38α MAPK, but not p44 or other isotypes of p38 MAPK, mediate LPA-induced PC3 cell migration.
Fig. 6.
Transfection of p38α siRNA blocked endogenous p38α expression and also significantly blocked LPA-induced PC3 cell migration, and transfection of both p38α siRNA and p42 siRNA nearly completely blocked PC3 cell migration. (A) Western blot analysis of the depletion of p38α MAPK expression. Transfection of p38α siRNA alone, or transfection of p38α siRNA and p42 MAPK siRNA depleted the expression of the endogenous p38α compared with either transfection of the non-silencing siRNA (control) or transfection of p42 siRNA alone. GCK was used as a loading control. (B) Bar graph of depletion of endogenous p38α protein expression by transfection of p38α siRNA, and p38α siRNA with p42 siRNA. Data shown are from three experiments. (C) Cell migration assay. Cells were transfected with non-silencing siRNA, p38α siRNA, p42 siRNA, or p38α siRNA plus p42 siRNA. Data were mean±SE of three experiments. *p<0.05 versus LPA alone.
4. Discussion
In the present study, we have identified LPA1 as the primary receptor that couples to the Gi/o proteins to transduce the LPA signal that leads to prostate cancer PC3 cell migration. Our results demonstrate that ERK and p38 MAPK, but not JNK, are the protein kinases necessary for controlling LPA-induced PC3 cell migration. Furthermore, using siRNA and dominant negative mutants, we have identified ERK2 (p42) and p38α as the specific and key intracellular mediators responsible for LPA-induced prostate cancer PC3 cell migration.
Prostate cancer cells produce high levels of LPA and they respond to LPA via an autocrine/paracrine loop [12] suggesting a role of LPA in the development of prostate cancer. Migration of prostate cancer cells is a prerequisite for invasion and metastasis. Prostate cancer cell proliferation, invasion and metastasis are important aspects of prostate cancer progression. The results from the present study of LPA-induced, LPA1 and MAPK dependent prostate cancer cell migration, and from previous reports on LPA induction of prostate cancer cell proliferation [13–15], and on LPA-induced prostate cancer cell in vitro invasion [30], all support a role of LPA in the development of prostate cancer. LPA is also linked to a variety of other types of cancer cell invasion and metastasis (see reviews [7,31]).
The lysophospholipid mediator LPA exerts its function on cells through its specific cell membrane receptors that couple with G-proteins. Of the LPA receptors, LPA1, LPA2 and LPA3 are the best-characterized [32,33] and recent studies have suggested the existence of other LPA receptors, LPA4 (GPR23/P2Y9) [34] and LPA5 [35,36], both of which share no significant identity features with LPA1–3. Notably, expression of LPA1, LPA2 and LPA3 has been detected in prostate cancer cells previously [16–18], though the role of these receptors in prostate cancer cell migration has not been determined. Our study is the first to show that LPA1, but not LPA2 or LPA3, is the functional receptor mediating LPA-induced prostate cancer PC3 cell migration. A recent study has shown that over-expression of LPA1 in androgen-sensitive LNCaP prostate cancer cells, and their subsequent implantation resulted in an increased rate of tumor growth in animals [37]. Our findings that LPA1 is responsible for prostate cancer cell migration in vitro, and the recent report that the overexpression of LPA1 results in increased tumor growth [37] strongly support an important role of LPA1 in the proliferation and migration of prostate cancer cells and thus for the infiltration of the tumor. In addition, our study also provides the first evidence that LPA1 transduces the LPA signal to activate ERK and p38 MAPK in prostate cancer cells.
Our data demonstrated that LPA-induced PC3 cell migration is PTX-sensitive, suggesting that a Gi/o protein, most likely Gi, is required for linking the LPA1 receptor to cell migration. In contrast, LPA-stimulated PC3 cell proliferation has been shown to be mediated by both PTX-insensitive and PTX-sensitive G-proteins [13,14].
ERK activation has been detected in advanced prostate cancer specimens, in contrast to nonneoplastic prostate tissue and normal human prostate tissue [19,20]. These results suggest a role of MAPK in the development of prostate cancer. In support of this, evidence has shown that ERK activation is required for prostate cancer cell mitogenesis induced by growth factors, including the insulin-like growth factor (IGF)-1 and epidermal growth factor (EGF) [38]. It has been shown that LPA-induced proliferation of prostate cancer PC3 cells is also ERK activation-dependent [15]. Although a recent study suggested ERK involvement in prostate cancer cell migration in response to leptin [39,40], the role of p38 in prostate cancer cell migration has not been defined. In the present study of LPA stimulation of prostate cancer cell migration, using pharmacological inhibitors, dominant negative mutants and siRNA depletion, we provide the first evidence of a functional role of p38 in prostate cancer cell migration. The present study also demonstrates for the first time that activation of both ERK and p38 MAPK, but not JNK, is required for LPA-induced prostate cancer cell migration. Notably, a recent report has shown that activation of p38 MAPK was significantly elevated in well-differentiated prostate tumors compared to normal prostates [21]. Therefore, besides ERK, p38 may represent another strategic target for intervention therapy.
Furthermore, we identified p42 and p38α, but not p44 or other subtypes of p38, to be responsible for LPA-induced PC3 cell migration. To our knowledge, this is the first study showing a functional role of p42 and p38α MAPK in LPA-induced mammalian cell migration. In addition, the LPA-triggered parallel activation of ERK and p38 MAPK is different from the ERK-dependent activation of p38, which was observed in ovarian cancer cells, where LPA induces a linear and sequential activation of ERK and p38 [41]. These results indicate that LPA stimulates specific intracellular signaling pathways in specific cell types.
Our finding that LPA induces prostate cancer cell migration and the identification of the specific LPA receptor (LPA1), Gi protein and the specific subunits of MAPK in regulation of prostate cancer cell migration provide new insights into LPA signaling and prostate cancer development. The results of the present study may contribute to the design and use of specific inhibitors to prevent prostate cancer progression.
Acknowledgements
This work was supported by National Institutes of Health grants HL074341 (to M.-Z. Cui) and CA92160 (to G. Tigyi), and a Center of Excellence in Livestock Diseases and Human Health grant (to M.-Z. Cui) from the University of Tennessee College of Veterinary Medicine. The authors wish to thank Dr. Donald McGavin for his critical reading of the manuscript and helpful discussion. The authors also thank Ms. Misty Bailey for her careful reading of the manuscript.
Abbreviations
- LPA
lysophosphatidic acid
- MAPK
mitogen-activated protein kinases
- siRNA
small interfering RNA
- PTX
pertussis toxin
- PD
PD98059
- SB
SB203580
- U0
U0126
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun/N terminal kinases
- IGF
insulin-like growth factor
- EGF
epidermal growth factor
- GCK
Germinal center kinase
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J. Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum. Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, Pienta KJ. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin. Cancer Res. 2000;6:1038–1045. [PubMed] [Google Scholar]
- 4.Tantivejkul K, Kalikin LM, Pienta KJ. Dynamic process of prostate cancer metastasis to bone. J. Cell. Biochem. 2004;91:706–717. doi: 10.1002/jcb.10664. [DOI] [PubMed] [Google Scholar]
- 5.Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J. Clin. Oncol. 2005;23:8232–8241. doi: 10.1200/JCO.2005.03.0841. [DOI] [PubMed] [Google Scholar]
- 6.Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp. Cell Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- 7.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat. Rev., Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 8.Tigyi G, Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J. Biol. Chem. 1992;267:21360–21367. [PubMed] [Google Scholar]
- 9.Tokumura A, Miyake M, Nishioka Y, Yamano S, Aono T, Fukuzawa K. Production of lysophosphatidic acids by lysophospholipase D in human follicular fluids of In vitro fertilization patients. Biol. Reprod. 1999;61:195–199. doi: 10.1095/biolreprod61.1.195. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang XJ, Sharma A, Hurteau J, Casey G, Goodbody A, Mellors A, Holub BJ, Mills GB. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin. Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- 11.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y, Gibbs TC, Mukhin YV, Meier KE. Role for 18:1 lysophosphatidic acid as an autocrine mediator in prostate cancer cells. J. Biol. Chem. 2002;277:32516–32526. doi: 10.1074/jbc.M203864200. [DOI] [PubMed] [Google Scholar]
- 13.Qi C, Park JH, Gibbs TC, Shirley DW, Bradshaw CD, Ella KM, Meier KE. Lysophosphatidic acid stimulates phospholipase D activity and cell proliferation in PC-3 human prostate cancer cells. J. Cell. Physiol. 1998;174:261–272. doi: 10.1002/(SICI)1097-4652(199802)174:2<261::AID-JCP13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Kue PF, Daaka Y. Essential role for G proteins in prostate cancer cell growth and signaling. J. Urol. 2000;164:2162–2167. [PubMed] [Google Scholar]
- 15.Guo C, Luttrell LM, Price DT. Mitogenic signaling in androgen sensitive and insensitive prostate cancer cell lines. J. Urol. 2000;163:1027–1032. [PubMed] [Google Scholar]
- 16.Gibbs TC, Xie Y, Meier KE. Regulation of expression of EDG family receptors in human prostate cancer cell lines. Ann. N.Y. Acad. Sci. 2000;905:290–293. doi: 10.1111/j.1749-6632.2000.tb06563.x. [DOI] [PubMed] [Google Scholar]
- 17.Im DS, Heise CE, Harding MA, George SR, O’Dowd BF, Theodorescu D, Lynch KR. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol. Pharmacol. 2000;57:753–759. [PubMed] [Google Scholar]
- 18.Daaka Y. Mitogenic action of LPA in prostate. Biochim. Biophys. Acta. 2002;1582:265–269. doi: 10.1016/s1388-1981(02)00180-4. [DOI] [PubMed] [Google Scholar]
- 19.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–284. [PubMed] [Google Scholar]
- 20.Price DT, Rocca GD, Guo C, Ballo MS, Schwinn DA, Luttrell LM. Activation of extracellular signal-regulated kinase in human prostate cancer. J. Urol. 1999;162:1537–1542. [PubMed] [Google Scholar]
- 21.Uzgare AR, Kaplan PJ, Greenberg NM. Differential expression and/or activation of P38MAPK, erk1/2, and jnk during the initiation and progression of prostate cancer. Prostate. 2003;55:128–139. doi: 10.1002/pros.10212. [DOI] [PubMed] [Google Scholar]
- 22.Durgam GG, Tsukahara R, Makarova N, Walker MD, Fujiwara Y, Pigg KR, Baker DL, Sardar VM, Parrill AL, Tigyi G, Miller DD. Synthesis and pharmacological evaluation of second-generation phosphatidic acid derivatives as lysophosphatidic acid receptor ligands. Bioorg. Med. Chem. Lett. 2006;16:633–640. doi: 10.1016/j.bmcl.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch RJ, Nemerow GR, Han J. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta) J. Biol. Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, Di Padova F, Ulevitch RJ, Han J. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J. Biol. Chem. 1997;272:30122–30128. doi: 10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38 gamma: a new member of p38 group of MAP kinases. Biochem. Biophys. Res. Commun. 1996;228:334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- 27.Cui MZ, Zhao G, Winokur AL, Laag E, Bydash JR, Penn MS, Chisolm GM, Xu X. Lysophosphatidic acid induction of tissue factor expression in aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:224–230. doi: 10.1161/01.atv.0000054660.61191.7d. [DOI] [PubMed] [Google Scholar]
- 28.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell. Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 30.Hwang YS, Hodge JC, Sivapurapu N, Lindholm PF. Lysophosphatidic acid stimulates PC-3 prostate cancer cell Matrigel invasion through activation of RhoA and NF-kappaB activity. Mol. Carcinog. 2006;45:518–529. doi: 10.1002/mc.20183. [DOI] [PubMed] [Google Scholar]
- 31.Murph M, Tanaka T, Liu S, Mills GB. Of spiders and crabs: the emergence of lysophospholipids and their metabolic pathways as targets for therapy in cancer. Clin. Cancer Res. 2006;12:6598–6602. doi: 10.1158/1078-0432.CCR-06-1721. [DOI] [PubMed] [Google Scholar]
- 32.Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin. Cell Dev. Biol. 2004;15:457–465. doi: 10.1016/j.semcdb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Goetzl EJ, Graeler M, Huang MC, Shankar G. Lysophospholipid growth factors and their G protein-coupled receptors in immunity, coronary artery disease, and cancer. Sci. World J. 2002;2:324–338. doi: 10.1100/tsw.2002.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 35.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 36.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J. Pharmacol. Exp. Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 37.Guo R, Kasbohm EA, Arora P, Sample CJ, Baban B, Sud N, Sivashanmugam P, Moniri NH, Daak Y. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology. 2006;147:4883–4892. doi: 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
- 38.Putz T, Culig Z, Eder IE, Nessler-Menardi C, Bartsch G, Grunicke H, Uberall F, Klocker H. Epidermal growth factor (EGF) receptor blockade inhibits the action of EGF, insulin-like growth factor I, and a protein kinase A activator on the mitogen-activated protein kinase pathway in prostate cancer cell lines. Cancer Res. 1999;59:227–233. [PubMed] [Google Scholar]
- 39.Frankenberry KA, Somasundar P, McFadden DW, Vona-Davis LC. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am. J. Surg. 2004;188:560–565. doi: 10.1016/j.amjsurg.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 40.Wesley UV, McGroarty M, Homoyouni A. Dipeptidyl peptidase inhibits malignant phenotype of prostate cancer cells by blocking basic fibroblast growth factor signaling pathway. Cancer Res. 2005;65:1325–1334. doi: 10.1158/0008-5472.CAN-04-1852. [DOI] [PubMed] [Google Scholar]
- 41.Baudhuin LM, Cristina KL, Lu J, Xu Y. Akt activation induced by lysophosphatidic acid and sphingosine-1-phosphate requires both mitogen-activated protein kinase kinase and p38 mitogen-activated protein kinase and is cell-line specific. Mol. Pharmacol. 2002;62:660–671. doi: 10.1124/mol.62.3.660. [DOI] [PubMed] [Google Scholar]