Abstract
Lysophosphatidic acid (LPA) has been implicated as causative in phenotypic modulation (PM) of cultured vascular smooth muscle cells (VSMC) in their transition to the dedifferentiated phenotype. We evaluated the contribution of the three major LPA receptors, LPA1 and LPA2 GPCR and PPARγ, on PM of VSMC. Expression of differentiated VSMC-specific marker genes, including smooth muscle α-actin, smooth muscle myosin heavy chain, calponin, SM-22α, and h-caldesmon, was measured by quantitative real-time PCR in VSMC cultures and aortic rings kept in serum-free chemically defined medium or serum- or LPA-containing medium using wild-type C57BL/6, LPA1, LPA2, and LPA1&2 receptor knockout mice. Within hours after cells were deprived of physiological cues, the expression of VSMC marker genes, regardless of genotype, rapidly decreased. This early PM was neither prevented by IGF-I, inhibitors of p38, ERK1/2, or PPARγ nor significantly accelerated by LPA or serum. To elucidate the mechanism of PM in vivo, carotid artery ligation with/without replacement of blood with Krebs solution was used to evaluate contributions of blood flow and pressure. Early PM in the common carotid was induced by depressurization regardless of the presence/absence of blood, but eliminating blood flow while maintaining blood pressure or after sham surgery elicited no early PM. The present results indicate that LPA, serum, dissociation of VSMC, IGF-I, p38, ERK1/2, LPA1, and LPA2 are not causative factors of early PM of VSMC. Tensile stress generated by blood pressure may be the fundamental signal maintaining the fully differentiated phenotype of VSMC.
Keywords: LPA, Dedifferentiation, Vascular smooth muscle cell, Aortic ring, Blood pressure, Common carotid artery
1. Introduction
Distinct from skeletal and cardiac muscle cells, smooth muscle cells (SMC) have a unique property of plasticity referred to as phenotypic modulation (PM). PM, although bidirectional, in the present study refers to the process of transition from differentiated phenotype to dedifferentiated phenotype [1]. The differentiated SMC show a high level of expression of a unique repertoire of marker genes that includes smooth muscle α-actin (SMA) [2], smooth muscle myosin heavy chain (SMMHC) [3], calponin (CN) [4], SM-22α (SM22) [4,5], and h-caldesmon (CALD1) [6]. They also exhibit an extremely low rate of proliferation and synthetic activity. In sharp contrast, the dedifferentiated SMC show low expression of these marker genes and also exhibit a high rate of proliferation and synthetic activity. In the process of vascular tissue repair, PM of vascular SMC (VSMC) provides cells with the ability to rapidly fill or replace damage to the vessel. This plasticity of VSMC is physiologically advantageous. On the other hand, in the process of atherosclerosis, PM of VSMC provides these cells with the ability to migrate from the tunica media to the intima and to proliferate there. In this latter case, the plasticity is disadvantageous and pathological because it leads to neointima formation, an early step in atherosclerotic disease [7–11].
Because PM is a critical process in atherogenesis and vascular injury repair, numerous studies have attempted to elucidate its causes and mechanism in vivo and in vitro. Mechanical factors, soluble biochemical factors, and extracellular matrix components have been proved to induce PM. Studies with cultured cells [12–17] and with intact cultured vessels [18–21] have shown that mechanical stimulation is able to maintain VSMC in the differentiated phenotype, typified by a high level of SMC-specific marker genes or a low proliferation rate. Soluble biochemical factors, including platelet-derived growth factor (PDGF) [22–25], transforming growth factor (TGF)-β [26,27], and retinoic acid [28–31] have been shown to affect PM. Extracellular matrix molecules, such as heparin, fibrillar collagen type I, collagen type IV, and laminin have also been shown to have significant effects on PM [32–39].
Hayashi and colleagues [40–42] developed a chemically defined culture system for aortic VSMC containing insulin-like growth factor I (IGF-I) and laminin substratum that they proposed maintains the differentiated phenotype of VSMC as judged by semiquantitative reverse transcriptase (RT)-PCR of marker genes. These authors proposed that changes in the balance between the phosphatidyl inositol 3 kinase (PI3K)/Akt pathway and the extracellular signal-regulated mitogen-activated kinase 1/2 (ERK1/2 MAPK) pathway determine the phenotype of VSMC in vitro and in vivo. These investigators also proposed that unsaturated fatty acyl species of lysophosphatidic acid (LPA) are the single ingredient of serum that elicits PM of dissociated VSMC in vitro [42] and causes neointima formation in vivo through activation of ERK and p38 MAPK pathways [42,43]. These interesting findings raise two questions: First, does the loss of mechanical forces during the dissociation of VSMC have anything to do with the PM in their model? The nature of blood pressure and flow creates stimuli in the form of stretch and shear stress that act on the constituents of the blood vessel wall. While endothelial cells are primarily subjected to the shear stress resulting from blood flow, VSMC are primarily subjected to the stretch resulting from blood pressure. When VSMC are dissociated, they become deprived of the mechanical stimuli, which have been proved by numerous studies [12–21] to play a role in PM. Second, if inhibition of ERK and the p38 MAPK pathways is a prerequisite for maintaining the differentiated phenotype [40–43], how can one explain the findings that stretch not only increased ERK and p38 MAPK activity [44–46], but also at the same time was sufficient for maintaining the differentiated phenotype [16,17]?
Furthermore, most of these in vitro studies used 1-day-old or older primary cultures or early-passage VSMC as the benchmark of the differentiated phenotype [40–42,47,48]. This selection is based on a fundamental assumption that primary or early-passage VSMC have maintained the fully differentiated phenotype; and this assumption ignores the possibility that primary or early-passage VSMC, which have been dissociated from the animal for at least several hours, would not maintain the in vivo differentiated phenotype. This begs the question, once VSMC are removed from their in vivo physiological cues, of how early the VSMC begin to undergo PM. At the present time, how early PM begins remains unclear.
To answer these questions and reconcile the paradoxical findings with those reports demonstrating that activation of the ERK and p38 MAPK pathways induces PM [40–43] and that stretch increases ERK and p38 MAPK activity while maintaining the differentiated phenotype [16,17], we hypothesized that there may exist an early PM of dissociated VSMC that occurs within hours after the removal of the physiological cues acting on the vessel wall, which may have escaped detection. In addition, we posited that early PM has a different molecular mechanism from that of late PM, in which activation of ERK and p38 MAPK pathways is important. Here we introduce the concept of an early phase of PM that covers the interval from the point of vessel isolation to one day after VSMC culture. With the availability of LPA1 and LPA2 knockout (KO) mice as well as LPA1&2 double KO (DKO) mice [64,65], we set out to examine the role of LPA GPCR from these mice along with stimulation of the PI3K/Akt pathway by IGF-I and MAPK inhibitors PD98059 and SB203580 in the PM of VSMC.
Here, we examined cultured VSMC [42] and thoracic aortic rings of mice cultured in serum-free medium for 1, 3, 6, 12, 24, 48, and 72 h. The expression of VSMC-specific marker genes was determined by quantitative RT-PCR (QPCR) and Western blot analysis. We found that early PM which manifested in significant downregulation of the expression of these VSMC-specific marker genes as early as 3 h was followed by substantial decrease in protein levels by 12 h. Finally, we determined whether loss of mechanical force is the cause of early PM. Our results suggest that VSMC undergo an early PM within hours after deprivation of their physiological cues regardless of the expression of LPA1 and LPA2 or their exposure to the conditions and culture ingredients examined in the present study. In vivo experiments suggest that this early PM is caused by the loss of signals generated by blood pressure, which is absent in cells or aortic rings cultured in vitro.
2. Materials and methods
2.1. Materials
Dulbecco's modified Eagle's medium (DMEM)-Ham's F12 50:50 mixture and modified Eagle's medium were purchased from Mediatech (Herndon, VA). Fetal bovine serum (FBS) and antibiotics were obtained from GIBCO-BRL (Grand Island, NY). Collagenase type I and elastase were from Worthington Biochemical Corporation (Lakewood, NJ). Laminin and IGF-I were from BD Bioscience (Franklin Lakes, NJ). Trypsin inhibitor cocktail and fatty acid-free bovine albumin, GW9662 were purchased from Sigma (St. Louis, MO). Antibodies to SM22 and SMA were obtained from Abcam (Cambridge, MA) and Sigma, respectively. The SuperSignal West Pico Chemiluminescent Substrate system was purchased from Pierce (Rockford, IL). The Cell Death Detection kit was purchased from Roche (Nutley, NJ). The Thermo-Script RT-PCR System for first-strand cDNA synthesis, TRIzol, PCR nucleotide mix, and random primers were purchased from Invitrogen (Carlsbad, CA). The RT2 Real-Time SYBR Green/ROX was from Super-Array (Frederick, MD). MAPK inhibitors PD98059 and SB203580 were bought from CalBiochem (San Diego, CA). LPA (18:1) was from Avanti (Alabaster, AL). All other chemicals were of the highest purity commercially available.
2.2. Animals
Animal experiments were approved by the University of Tennessee Health Science Center (UTHSC) Institutional Animal Care and Use Committee. Wild-type (WT) male C57BL/6 mice (4–6 months of age) and SD male rats (250–300 g) were purchased from Charles River LAB (Indianapolis, IN). Breeders for LPA1 and LPA2 KO mice were generously provided by Dr. Jerold Chun (Scripps Inst.; San Diego, CA), and LPA1&2 DKO mice were bred in the UTHSC animal facility. KO and DKO animals age-matched to the WT mice were used in the studies described.
2.3. Aortic VSMC cultures
The procedure originally described by Hayashi et al. was followed [42]. Dissociated VSMC were washed twice with MEM supplemented with 200 µM calcium chloride and 0.1% bovine serum albumin and resuspended in base medium (DMEM-Ham's F12 50:50 mixture supplemented with 500 U/ml penicillin, and 500 µg/ml streptomycin) plus 2 ng/ml IGF-1 and plated on 20 µg/ml laminin at a cell density of 0.5×106/ml.
2.4. Aortic ring cultures
The thoracic aorta was isolated as described previously [42]. The attached loose fat and connective tissue were gently peeled away and cut from the aorta under a dissecting microscope. The vessel was cut into three equal segments. The proximal aortic ring from an aorta was mixed with the middle and the distal ring isolated from two other mice; thus, each sample was derived from three animals representing the entire length of the thoracic aorta. The rings were cultured in either the base medium, base medium plus 2 ng/ml IGF-1, base medium plus PD98059 (30 µM) and SB203580 (20 µM), base medium plus 3 µM LPA (18:1), base medium supplemented with 10% FBS or base medium plus 1 µM GW9662. The rings were then incubated at 37 °C in a humidified atmosphere of 95% air–5% CO2 for various periods (1–72 h) or immediately processed for RNA extraction (0 h).
2.5. Elimination of blood flow, pressure, and blood in the rat common carotid artery in vivo
The non-injury rat model originally developed by Yoshida et al. [43] was modified. Briefly, the right common carotid artery (CCA), the proximal end of the internal carotid artery (ICA), and the external carotid artery (ECA) of anesthetized rats were surgically exposed. Three treatments were performed on the CCA. To eliminate the blood flow, pressure, and blood in the rat CCA, the proximal end of the CCA and ICA were occluded using vessel clips (Biomedical Research Instruments, Inc.; Silver Spring, MD). The ECA was ligated distally and cut open to release pressure in the CCA without mechanical injury to it. A cannula was retrogradely inserted into the ECA and the clip occluding the CCA was temporarily released during simultaneous injection of 500 µl Krebs solution (120 mM NaCl, 5 mM KCl, 0.62 mM MgSO4, 1.8 mM CaCl2, 10 mM HEPES, 6 mM glucose; pH 7.4) to flush out residual blood. The CCA was again clipped and depressurized by withdrawing the cannula, followed by ligation at both ends. To eliminate the pressure and blood flow in the rat CCA, the ECA was cut open to release pressure, but without cannulating and flushing with Krebs solution. To eliminate flow only, the ECA was not cut open, and the distal end of the CCA was ligated first followed by the proximal end. The wounds were closed by continuous suture, and the rats were allowed to recover on a heating pad for 12 h. At the end of the recovery period, the CCA was dissected out, cleaned of adventitia, rinsed with saline, and subjected to RNA extraction followed by QPCR.
2.6. TUNEL assay
Aortic rings were fixed in 10% formalin for 48 h, dehydrated, embedded in paraffin, and subsequently processed for TUNEL assay. Staining of apoptotic cells was performed with the Cell Death Detection kit according to the manufacturer's instructions. The ratio of apoptotic cells was evaluated by counting the TUNEL-positive cells/total cells. The relative incidence of TUNEL-positive apoptotic cells for each group was normalized to 0-h group.
2.7. Real-time quantitative polymerase chain reaction analysis
Aortic rings were homogenized in TRIzol, and total RNA was prepared according to manufacturer's instructions. Three micrograms of total RNA were used for the subsequent synthesis of cDNA using the ThermoScript RT-PCR system for first-strand synthesis as recommended by the manufacturer. Quantitative PCR reactions were performed using 0.5 µl of the cDNA mix with 300 nmol of each primer in a final volume of 25 µl of 2×RT2 Real-Time SYBR Green/ROX master mix. The following primer pairs were used: SMA, AGAGCAAGAGAGGGATCCTGA (forward) and GTCGTCCCAGTTGGTGATGAT (reverse); SMMHC, GCAATGCGAAAACCGTCAA (forward) and GATGCGAATGAACTTGCCAAA (reverse); SM22, (forward) TGAAGAAAGCCCAGGAGCAT and TGCTTCCCCTCCTGCAGTT (reverse); CN, ACCAAGCGGCAGATCTTTGA (forward) and CATCTGCAAGCTGACGTTGA (reverse); CALD1, GGAGGCTGATCGAAAAGCTA (forward) and AGCTTCTGCCCTTCTCCTTT (reverse); GAPDH, CTGCACCACCAACTGCTTAG (forward) and GGGCCATCCACAGTCTTCT (reverse); HPRT1, AAGCTTGCTGGTGAAAAGGA (forward) and TTTCAAATCCAACAAAGTCTGG (reverse); RPL13a, ACAAGAAAAAGCGGATGGTG (forward) and GCTGTCACTGCCTGGTACTTC (reverse); LPA1 receptor, GTCTTCTGGGCCATTTTCAA (forward) and (TCATAGTCCTCTGGCGAACA (reverse); LPA2 receptor, GGGCCAGTGCTACTACAACG (forward) and ACCAGCAGATTGGTCAGCA (reverse); and PPARγ GGGCGATCTTGACAGGAA (forward) and CACCTCTTTGCTCTGCTCCT (reverse). The primer sets were designed by using Primer Express software from ABI (Foster City, CA) and Primer 3 software from Whitehead Institute (Cambridge, MA). Amplification was performed for 40 cycles at 94 °C/15 s and 60 °C/60 s. Quantitative values were obtained from the threshold cycle value (Ct), which is the point where a significant increase of fluorescence is first detected.
For the in vitro culture experiments, the transcript number of GAPDH was quantified as an internal RNA control, and each sample was normalized on the basis of its GAPDH gene content. The relative gene expression level of each gene was then normalized to the 0-h group (calibrator). Final results, expressed as N-fold difference in gene expression relative to GAPDH of the 0-h group, termed N, were calculated as: N=2(Ctgroup−Ctcalibrator) (http://dorakmt.tripod.com/genetics/realtime.html), where Ct values of the group and calibrator were determined by subtracting the average Ct value of a target gene from the corresponding Ct value of the GAPDH gene.
For the in vivo surgical treatment experiments, the average transcript numbers [66] of GAPDH, HPRT1, and RPL13a were quantified as an internal RNA control to counteract the relatively larger variability inherited by in vivo experiments. To take advantage of the paired experimental design, the ratio of each SMC-specific marker gene expression level (treatment side/sham side) was calculated for each rat. The mean ratio was then calculated for each treatment group. We named this mean ratio the “phenotype index”. If the treatment had no effect on PM, the phenotype index should be close to 1. If the treatment caused PM, the phenotype index should be significantly less than 1.
2.8. Western blot
Aortic rings were washed twice with ice-cold PBS and homogenized in a reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer on ice. Tissue lysates were centrifuged at 10,000 ×g for 10 min, separated using 10% SDS-PAGE, and transferred to a polyvinylidene difluoride membrane. Immunocomplexes were visualized and quantified using the SuperSignal West Pico chemiluminescent substrate system. Anti-GAPDH immunoreactivity was used as the loading control.
2.9. Statistical analysis
Data are presented as mean±SEM. All experiments were done on with three to ten animals (n=3–10). Western blots are representative of three experiments, and each experiment represents tissue collected from three mice. Student's t-test (for samples with unequal variances) or one-way ANOVA (for samples with equal variances) with appropriate post hoc testing was used to determine the significance of the differences between means. P<0.05 was regarded as statistically significant.
3. Results
3.1. Early PM in cultured dissociated aortic VSMC
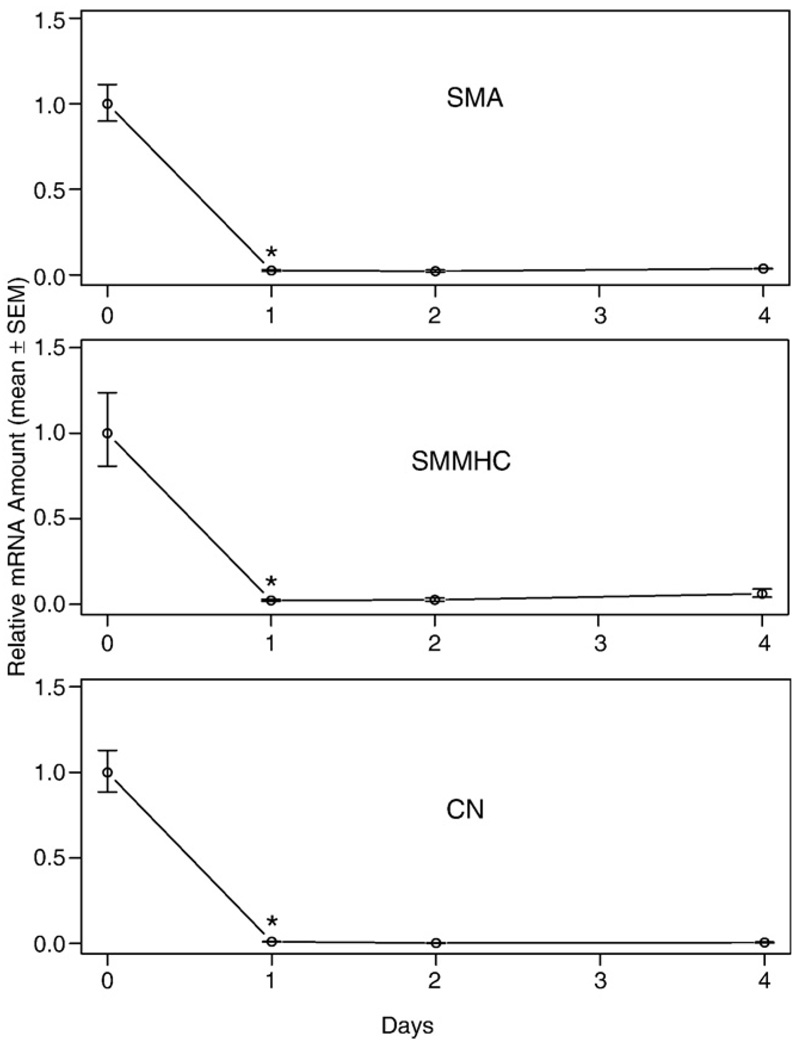
IGF-I and laminin has been shown to prevent the PM of dissociated VSMC [41,42,67]. Following these procedure to the best of our ability, our results showed a clear decrease in the VSMC marker gene expression after 1 day of culture (Fig. 1). Varying IGF-I and laminin concentration, including micromolar concentrations of LPA or 10% serum in the medium, did not alter this trend in our hands (data not shown). This apparent contradiction between our data and those previously reported can potentially be explained by the difference in the reference point chosen. We used freshly isolated cells within 2 h of dissociation from mice as the reference point. In contrast, other authors used 1-day cultured cells as the reference point [41,42,67]. By choosing this later time as the reference point, they may have missed the early PM that took place during the first 24 h. Because it takes 2–3 h to obtain dissociated VSMC, to further elucidate the early phase of PM and to eliminate effects due to disruption of physiological cell-to-cell contacts present in the vessel wall, we chose to culture aortic rings, which could be isolated within 30 min.
Fig. 1.
Vascular smooth muscle cell (VSMC)-specific marker gene mRNA expression in cultured VSMC. VSMC isolated from the thoracic aorta were incubated on plates covered with 20 µg/ml laminin in DMEM-Ham's F12 50:50 mixture medium supplemented with 2 ng/ml IGF for various periods. The 0-day sample represents freshly isolated VSMC dissociated from the mouse body using a procedure lasting approximately 2 h. Values are means ± SEM for 3 replicates. *Denotes significant difference relative to preceding time point (P<0.05).
3.2. Apoptosis in cultured aortic rings
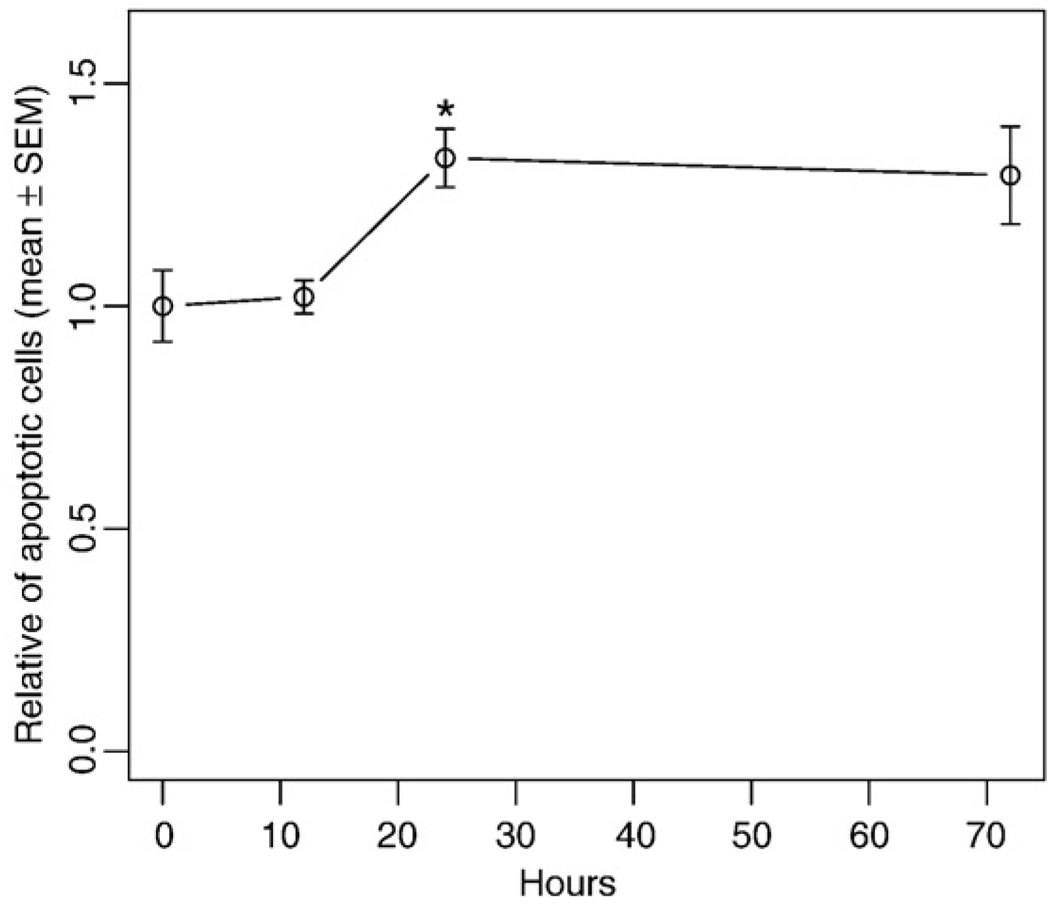
It is well known that once cells are deprived of their physiological cues, they will undergo apoptosis. To determine the extent of apoptosis in the cultured aortic rings, we assessed apoptosis during the culture using TUNEL staining. The data showed that even though there was a slight increase in apoptosis after 24 h culture, this increase was not statistically significant up to 12 h (Fig. 2).
Fig. 2.
Ratio of apoptotic cells in cultured aortic rings. The thoracic aorta was surgically isolated, cultured in serum-free medium for 0–72 h, and fixed. Apoptotic cells were visualized using TUNEL staining. The percentage of apoptotic cells was evaluated by counting the stained cells/total cells. The number of apoptotic cells in each group was normalized to the 0-h group. Values are means±SEM for 4 random fields. *Denotes significant difference from the preceding time point (P<0.05).
3.3. Early PM in cultured aortic rings
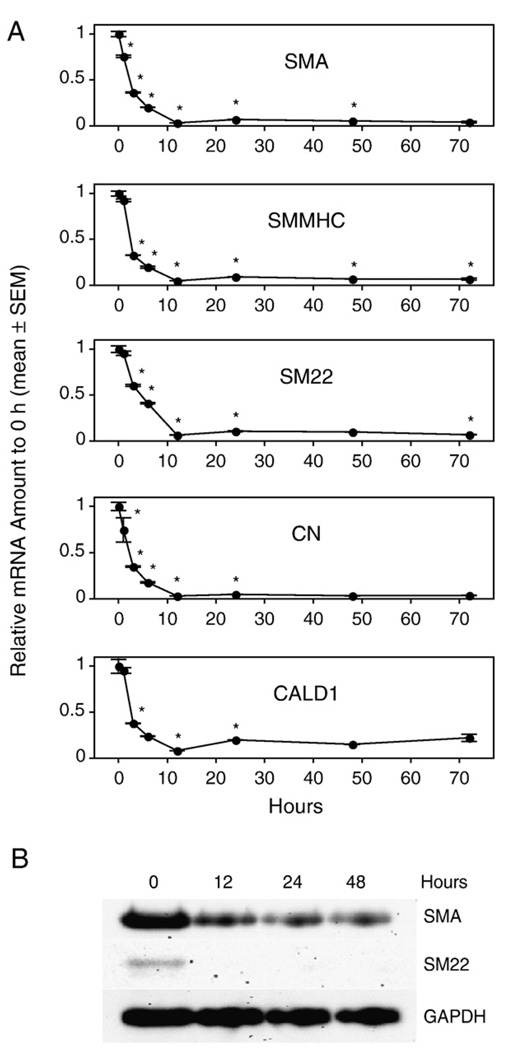
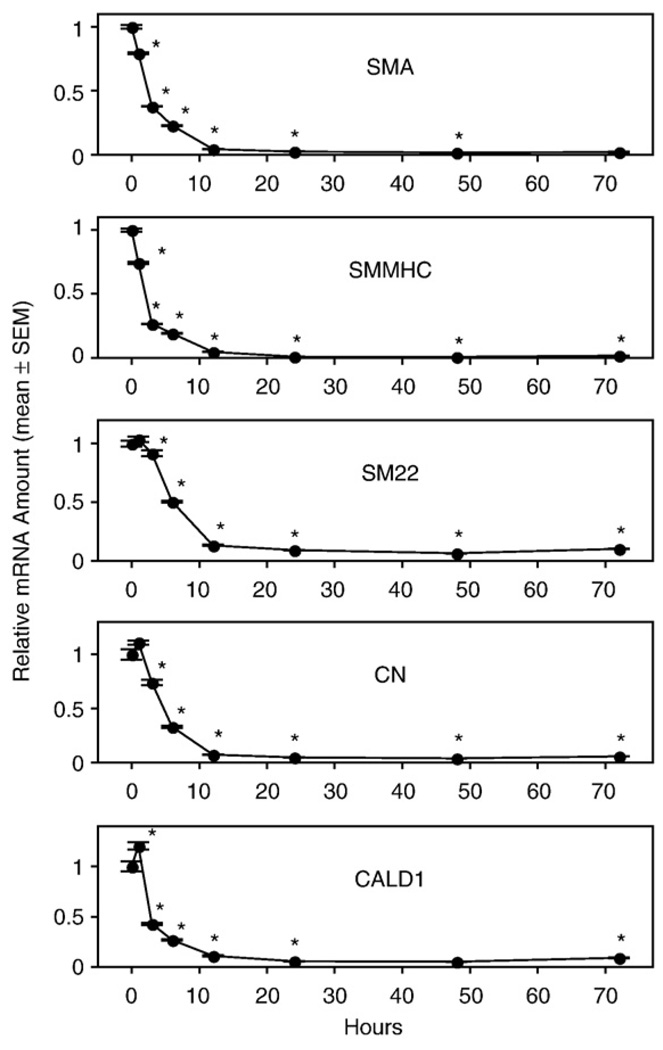
To determine the early time course of PM of VSMC, we cultured the aortic rings from WT mice for 0 h to 72 h and quantified SMC-specific marker gene expression. We found that every marker gene we chose to monitor showed a statistically significant decrease within 3 h of culture. The decrease reached nadir after 12 h of culture and then leveled off (Fig. 3A). The expression level after 12 h of culture was less than 25% of the expression level in vivo. To monitor whether there was an accompanying change at the protein level, we selected the 12 h, 24 h, and 48 h cultured aortic rings to assess protein expression for SMA and SM22. We found that SMA and SM22 proteins had already decreased significantly after 12 h of culture (Fig. 3B). These results indicate that PM of VSMC that begins at the mRNA level within 3 h after dissection is followed by a decrease at the protein level within 12 h after dissociation.
Fig. 3.
Vascular smooth muscle cell (VSMC)-specific marker gene mRNA (A) and protein (B) expression in cultured aortic rings of wild-type (WT) mice. Rings were cultured in serum-free base medium for 0–72 h prior to RNA or protein extraction. Quantitative RT-PCR was used to assess the amount of mRNA, and Western blot was used to quantify the expression of marker proteins. Values are means ± SEM for 3 replicates. *Denotes significant difference from the preceding time point (P<0.05).
3.4. Effects of IGF-I and MAPK inhibitors on early PM
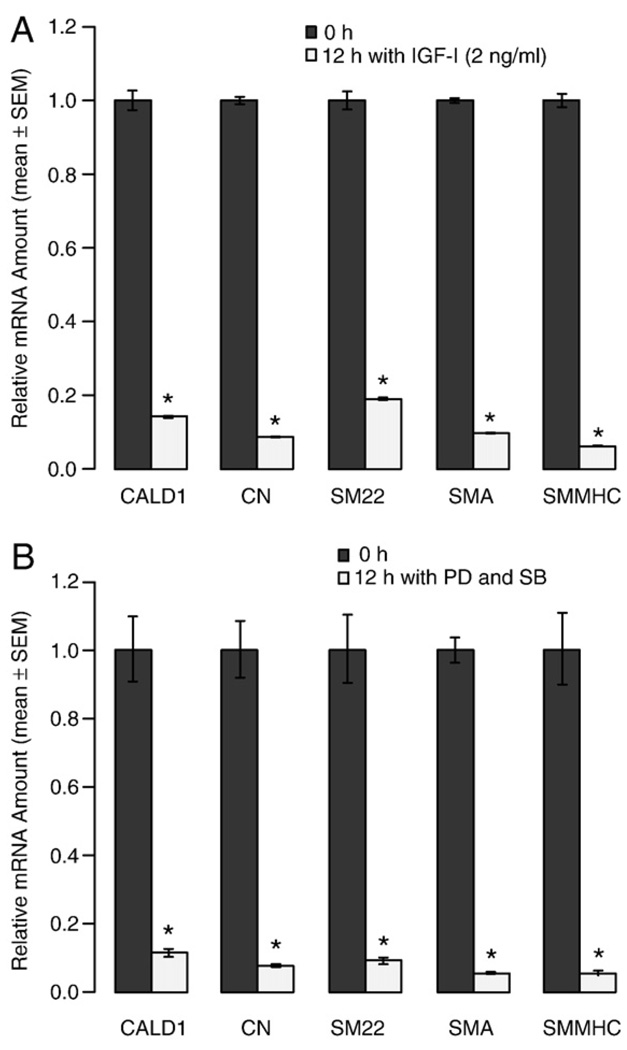
IGF-I plus laminin or the combined application of the MAPK inhibitors PD98059 plus SB203580 has been shown to prevent modulation of VSMC in vitro [40–42]. To determine whether these mechanisms play a role in early PM, we cultured the aortic rings in the presence of IGF-I or PD98059 plus SB203580 for 12 h. Under these conditions, we found that all the SMC-specific marker genes we monitored decreased to less than 20% of that level in the 0-h group (Fig. 4). These results showed that neither IGF-I nor PD98059 plus SB203580 could block the early PM of VSMC in the aortic ring cultures. This implicates that early PM is not likely to be affected by the balance between the PI3K/Akt and the ERK1/2 and p38 MAPK pathways.
Fig. 4.
The effect of IGF-I (A) and mitogen-activated protein kinase (MAPK) inhibitors (B) on early phenotypic modulation (PM) of vascular smooth muscle cells (VSMC). The segments of the thoracic aorta from wild-type (WT) mice were cultured in serum-free base medium supplemented with 2 ng/ml IGF-I and PD98059 (PD. 30 µM) plus SB203580 (SB, 20 µM) for times ranging from 0 to 12 h prior to RNA extraction. Quantitative RT-PCR was used to assess mRNA levels of marker genes. Values are means ± SEM for 3 replicates. *Denotes significant differences relative to the base medium (P<0.05).
3.5. Effect of LPA and serum on early PM
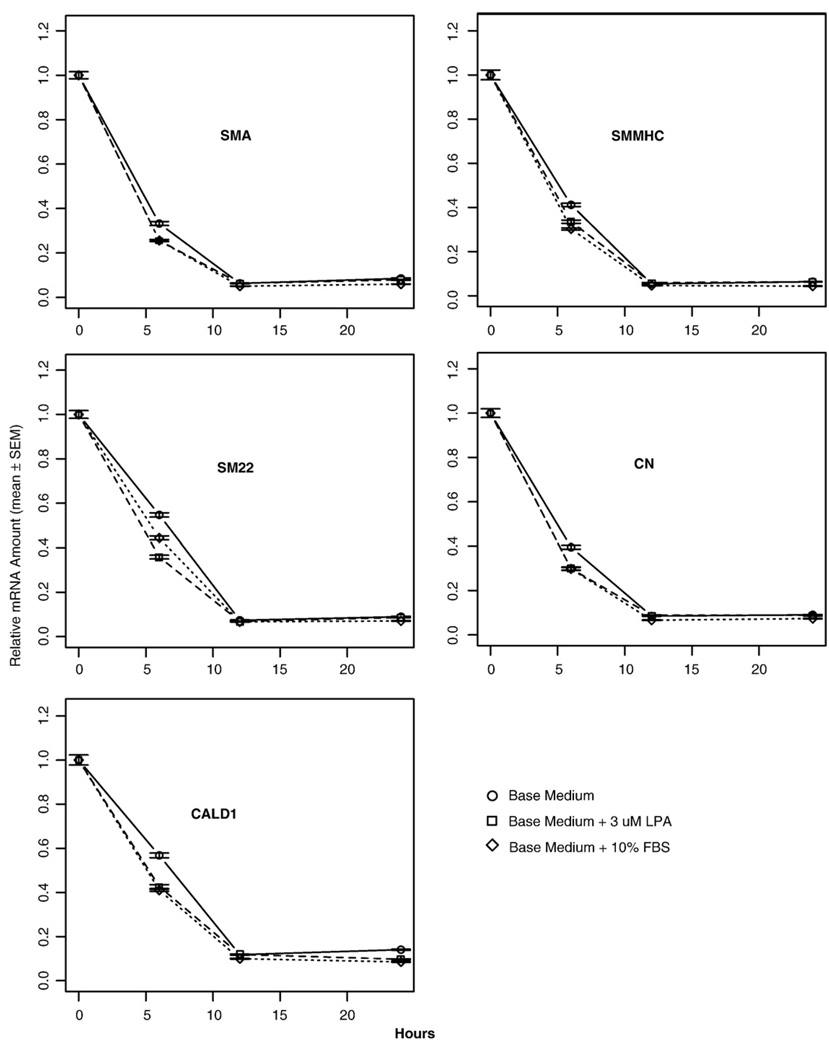
LPA is a mitogen to subcultured VSMC [50]. It has also been reported that unsaturated species of LPA present in serum promote PM in cultured VSMC [42]. To clarify whether unsaturated LPA 18:1 also promotes early PM, we cultured the aortic rings in base medium, base medium plus 3 µM LPA (18:1), and base medium plus 10% FBS. In all cases, we observed a rapid decrease in marker gene expression (Fig. 5). However, after 6 h of culture, both LPA and serum marginally accelerated the downregulation of VSMC-specific marker gene expression (Fig. 5). Nonetheless, after 12 h of culture, there was no statistically significant effect of LPA or serum on the expression of VSMC marker genes. These results indicate that the early PM at the end of the initial 12 h period is not significantly affected by LPA or serum.
Fig. 5.
The effects of lysophosphatidic acid (LPA) and serum on the early phenotypic modulation (PM) of vascular smooth muscle cells (VSMC). Rings from thoracic aortas of wild-type (WT) mice were cultured in serum-free base medium, base medium plus 3 µM LPA (18:1), or base medium plus 10% fetal bovine serum (FBS) for 0–24 h prior to RNA extraction. Quantitative RT-PCR was used to assess mRNA levels of the VSMC-specific marker genes. Values are means ± SEM for 3 replicates.
3.6. LPA1, LPA2 and PPARγ receptors do not mediate early PM
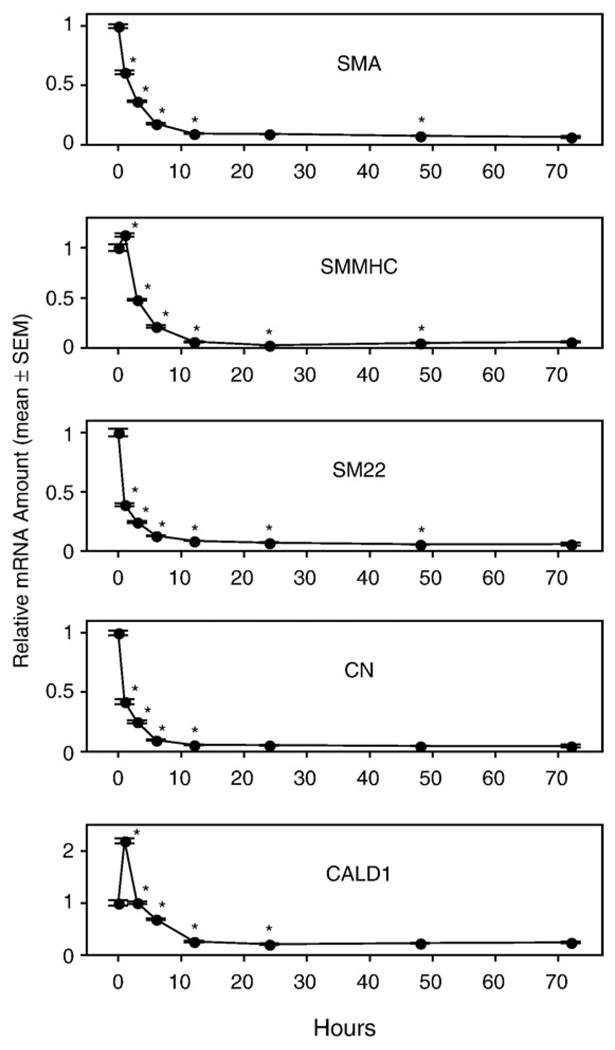
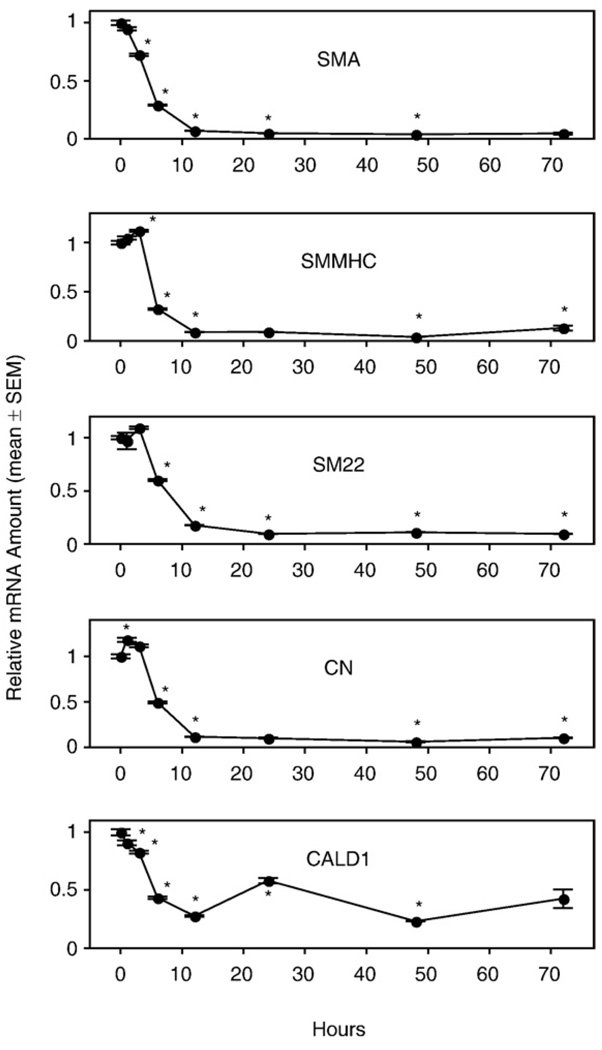
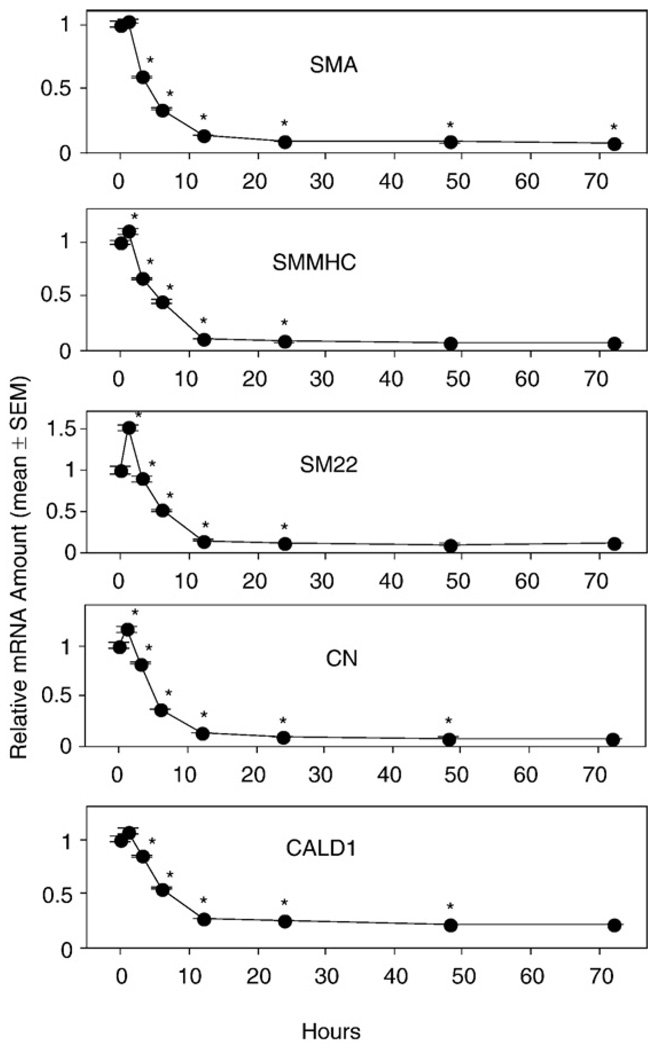
To obtain direct evidence for the involvement of LPA GPCR in the PM of VSMC, we performed the same experiments as described above for aortas from WT mice but using aortic rings isolated from KO mice. In LPA1, LPA2, and DKO mouse lines, the mRNA of all SMA-specific marker genes decreased significantly after 6 h of culture compared to the 0-h expression level. Regardless whether GAPDH, β-actin, or 18S-RNA was used as a reference quantitatively similar trends were observed for each VSMC marker gene (data not shown). The decrease reached nadir after 12 h of culture and then leveled off (Figs. 6–8), exactly as seen in the aortic rings of WT mice (Fig. 3). We noticed some variability of the initial mRNA levels during the first 3 h of culture between different mouse lines. For example, there was an increased expression of the CALD1 gene in the LPA1 KO mouse line (Fig. 6). This variability could be explained by individual variation between the mice, or it could be due to a difference due to knocking down the LPA1 GPCR. We prefer the former explanation because we found that there was noticeable variability of the expression pattern of SMC-specific marker genes for the first three time points (0 h, 1 h, and 3 h) between independent experiments using the same mouse line. However, the significant decrease of all the marker genes after 6 h of culture was consistently detected in every experiment for every mouse line tested.
Fig. 6.
Vascular smooth muscle cell (VSMC)-specific marker gene mRNA expression in cultured aortic rings of LPA1 knockout mice. Rings from thoracic aortas of wild-type (WT) mice were cultured in serum-free base medium for 0–72 h prior to RNA extraction. Quantitative RT-PCR was used to assess mRNA levels of the VSMC-specific marker genes. Values are means ± SEM for 3 replicates. *Denotes significant differences relative to the 0-h group (P<0.05).
Fig. 8.
Vascular smooth muscle cell (VSMC)-specific marker gene mRNA expression in cultured aortic rings of LPA1&2 double knockout mice. Rings from thoracic aortas were cultured in serum-free base medium for 0–72 h prior to RNA extraction. Quantitative RT-PCR was used to assess mRNA level. Values are means ± SEM for 3 replicates. *Denotes significant differences relative to the 0-h group (P<0.05).
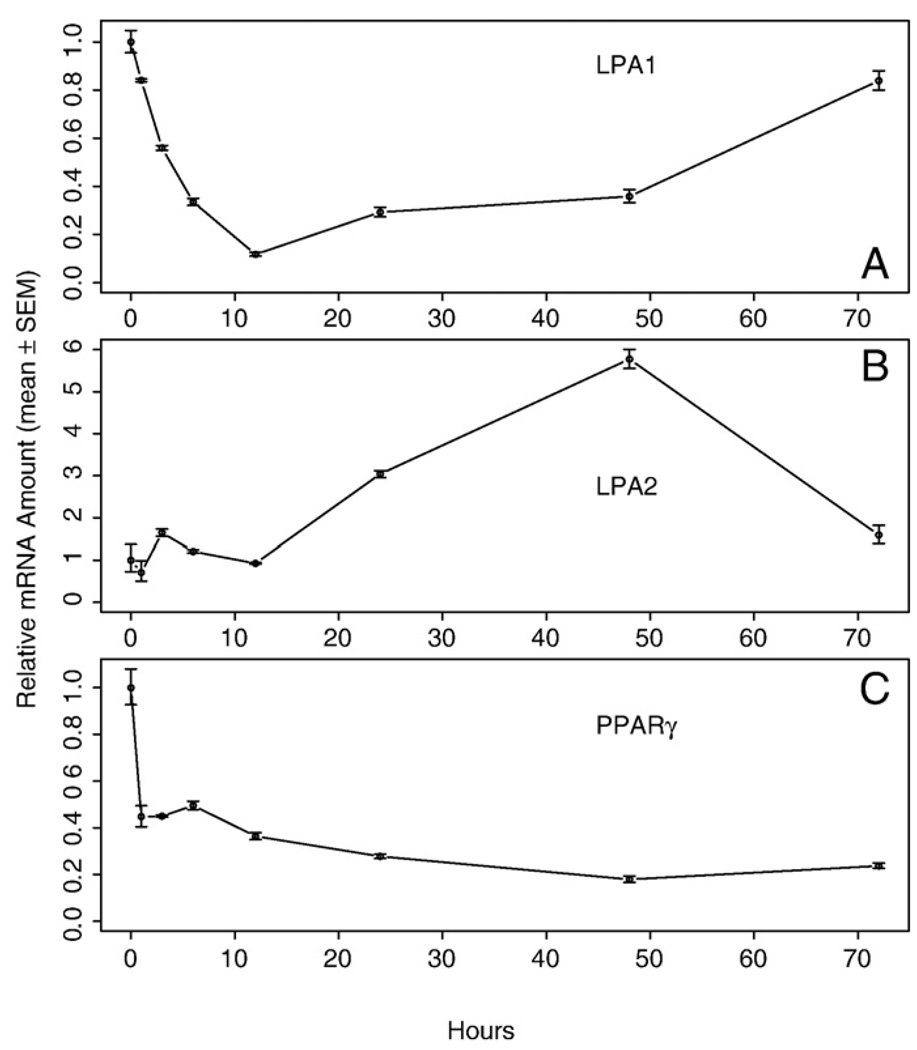
We also examined the LPA1, LPA2, and PPARγ receptor mRNA abundance in aortic rings isolated from WT mice maintained in serum-free medium for up to 72 h (Fig. 9). LPA1 and PPARγ showed a similar decrease over the first 12 h of incubation as seen for the VSMC marker genes, whereas LPA2 showed no significant changes. At the end of the 72-h incubation period, both LPA1 and LPA2 mRNA levels returned to a level similar to that at the beginning of the experiment. In summary, the present results indicate that early PM is not affected by the presence or absence of LPA1, LPA2, or both receptors in the vessel wall.
Fig. 9.
Expression of lysophosphatidic acid (LPA) G protein-coupled receptors (GPCR) and peroxisome proliferator-activated receptor gamma (PPARγ) in aortic rings isolated from wild-type (WT) mice and cultured in serum-free DMEM-Ham's F12 50:50 medium. Quantitative RT-PCR was used to assess mRNA levels, and values represent the mean ± SEM for 3 animals.
Our earlier results with cultured VSMC suggested a role for the nuclear hormone receptor PPARγ in the late stage PM [55]. To test the contribution of PPARγ to the early PM we treated aortic rings with the specific irreversible antagonist 1 µM GW9662 (Fig. 10). GW9662 treatment showed no significant attenuation in the decrease of marker gene expression discounting the role of PPARγ in this stage of PM. These observations mirrored our findings obtained using aortic rings obtained from PPARγ conditional knockout mice (data not shown).
Fig. 10.
Effect of PPARγ antagonist GW9662 (1 µM) on VSMC marker gene expression in cultured aortic rings. Quantitative RT-PCR was used to assess mRNA levels, and values represent the mean ± SEM for 3 animals.
3.7. Loss of blood pressure mediates early PM
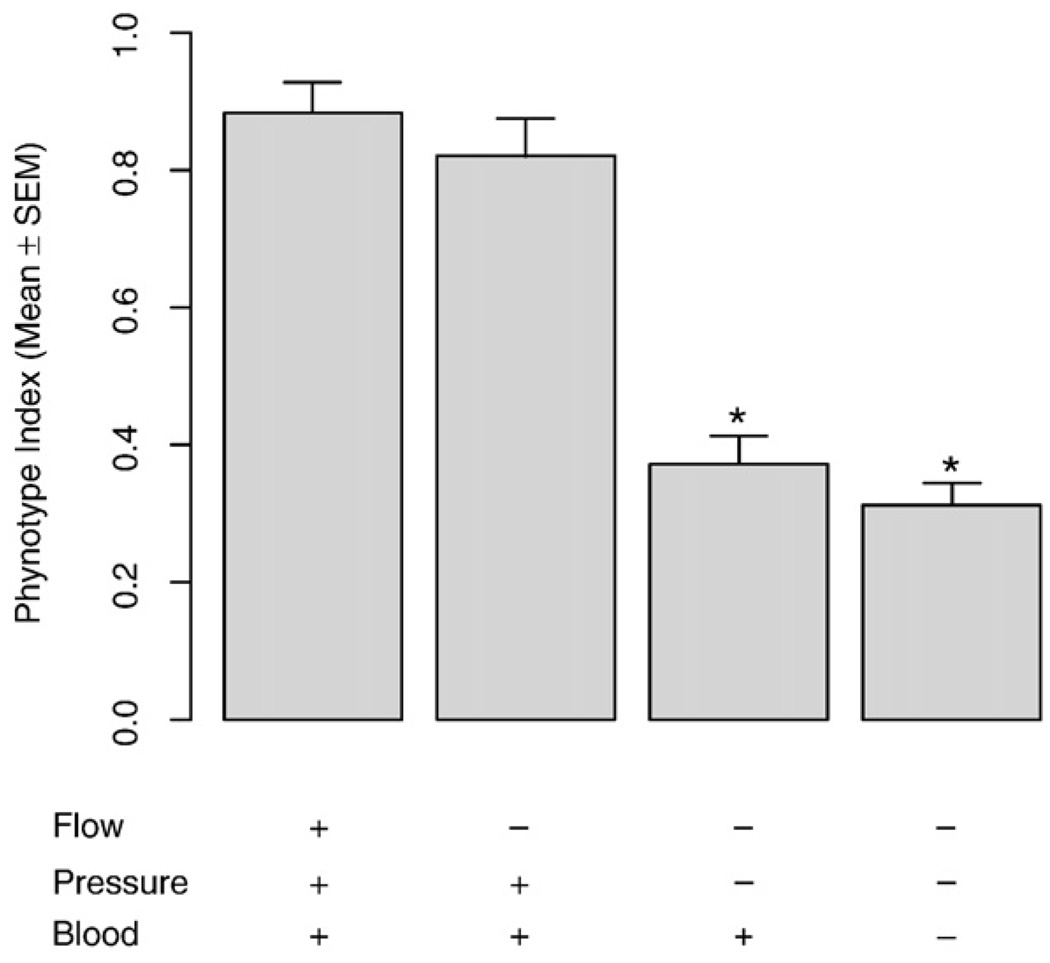
The results up to this point implied that early PM was not mediated by dissociation, LPA or other serum factors. Because the VSMC continuously encounter mechanical stimuli that have important effects on their phenotype, we modified the non-injury rat model developed by Yoshida [43] to permit testing the roles of flow, pressure, and blood in early PM in vivo. As expected, in the presence of all three factors in the control group, early PM did not occur (Fig. 11). Eliminating flow alone did not induce early PM after 12 h of treatment. Eliminating both flow and pressure for 12 h induced early PM, and the additional elimination of blood did not significantly affect the already induced early PM. These results imply that loss of flow and exposure to blood does not elicit early PM in cultured VSMC or aortic rings. The loss of stretch induced by static blood pressure alone may be the causal factor of early PM.
Fig. 11.
The phenotype index of vascular smooth muscle cells (VSMC) in four surgical treatment groups in vivo. Of the paired common carotid arteries in each rat, one side is treated to eliminate either flow, pressure, or blood or a combination of these. The other side is subjected to sham surgical manipulation. After 12 h, rats were sacrificed and common carotid arteries were used for Quantitative RT-PCR to quantify the expression level of VSMC-specific marker genes. The ratio of each VSMC-specific marker gene expression level (treatment side/sham side) was calculated for each rat. The average ratio was then calculated for each treatment group and termed the “phenotype index”. The values represent the mean±SEM for 4–10 rats. *Denotes significant differences relative to the control group with the presence flow, pressure, and blood.
4. Discussion
LPA has not only been implicated in PM but also in normal vascular development [49], SMC and VSMC mitogenesis [50,51], and contractility [52,53]. Alkyl-LPA has been identified as a biologically active lipid in mildly oxidized low-density lipoprotein, human atherosclerotic lesions, and the supernatant of activated platelets [54]. Unsaturated acyl and alkyl species of LPA have been reported to elicit neointima formation in a non-injury model of the rat carotid artery [43,55]. Only unsaturated acyl species of LPA cause in vitro PM of cultured VSMC from the rat aorta [42]. Extracellularly applied LPA is known to elicit its biological responses through a family of seven GPCRs [56–61]. The LPA receptor subtype that the rat aorta expresses is the LPA1 receptor subtype, whereas in C57BL/6 mice, LPA1 and LPA2 receptors were detected [55]. The peroxisome proliferator-activated receptor gamma (PPARγ) has been demonstrated to be an intracellular receptor for LPA and it shows a structure–activity relationship that matches that of neointima induction in vivo [55,62,63]. Inhibition of PPARγ prevents neointima formation elicited by unsaturated and alkyl species of LPA [55].
PM is defined as the process of transformation of VSMC from the differentiated phenotype to a dedifferentiated phenotype [1]. LPA has been implicated in the PM of VSMC as the single ingredient of serum that elicits PM [42]. In vivo, unsaturated and alkyl ether species of LPA topically applied to the carotid artery briefly (1 h) cause a profound remodeling and neointima formation [43,55].PM of VSMC is considered to be an essential event in the pathogenesis of atherosclerotic disease [7–11]. To gain a better understanding of the role of LPA in the pathophysiology of the vascular wall, in the present study we have evaluated the role of LPA GPCR and PPARγ in the transcriptional regulation of VSMC marker genes that characterize the differentiated phenotype.
First, by culturing the dissociated mouse VSMC, we found that there is an early PM within 24 h of isolation in DMEM-F12 medium supplemented with 2 ng/ml IGF-I on 20 µg/ml laminin substratum. These conditions were established by Hayashi and colleagues [40–42,67], aimed at sustaining the differentiated phenotype of rat aortic VSMC. However, these conditions and variations in the IGF-I and laminin concentrations (data not shown) in our hands failed to maintain the high level of marker gene expression characteristic of the differentiated VSMC.
These negative findings prompted us to change from dissociated VSMC cultures to organotypic cultures of aortic rings, hypothesizing that elimination of the potentially harsh conditions lasting for up to 2 h during cell dissociation would prevent the rapid loss of the marker gene expression characteristic to the differentiated phenotype. By using cultured aortic rings, the processing time was markedly reduced to 15–30 min. Hence, it is reasonable to assume that our starting materials, designated as “0 h” cultured aortic rings, possess a phenotype closest to that of VSMC in vivo. We also excluded apoptosis as a confounding factor in the cultured aortic rings within 12 h (Fig. 2). We chose DMEM-Ham's F12 50:50 mixture serum-free medium to eliminate any chemical stimulus that could positively or negatively affect PM. We also tested a variety of supplements ranging from IGF-I, through LPA to inhibitors of MAPK. Using 0 h cultured aortic rings as the benchmark of the differentiated phenotype, we were able to reveal that early PM occurs within hours (Fig. 3). QPCR demonstrated a rapid significant decrease in marker gene expression as early as 3 h after isolation. This decrease leveled off after 12 h and remained steady for up to 72 h, the longest time point tested in this study. Our observation concerning the abundance of marker gene mRNAs was confirmed by Western blotting at 12 h, which corroborates the rapid decline in the function of these markers. We therefore designated these changes in marker gene expression as the early phase of PM. Early PM has been implicated in earlier studies in which the authors noticed a small number of phenotypically modulated cells with fewer myofilaments in the 1-day culture of free-floating explants [68,69]. To the best of our knowledge, this finding has not been pursued further. Subsequent studies on the PM of VSMC did not reveal this early PM because they used at least 1-day-old cultured VSMC as the benchmark of the differentiated phenotype [40–42,47,48] or they monitored the gene expression level change using wide time intervals (several days after dissociation from the animal body). If early PM modulation occurs within 1 day, as shown by our results (Figs. 1 and 3), it is easy to understand why most of the studies have failed to recognize this phenomenon.
Our studies using dissociated VSMC and isolated aortic ring cultures revealed that the PM manifested regardless of the presence of LPA. The total amount of LPA in plasma is in the low nanomolar range [70–74], and the portion derived from isolated platelets remains only a fraction of that generated ex vivo during blood clotting [70].In the course of blood clotting, large amounts of LPA are generated, reaching concentrations as high as 5–10 µM [70,71,75,76], and the molecular species are dominated by the 18:2 and 20:4 unsaturated acyl species (20% and 34% of total LPA, respectively) [70]. LPA 20:4 is a more potent activator of platelets than other saturated or mono-unsaturated acyl species [77–79]. Among the acyl species, 20:4 and 18:2 acyl-LPA are the most potent activators of PPARγ and elicit neointima formation [55]). In contrast, the saturated acyl species are inactive in eliciting vascular wall remodeling [43,55]. Using atherosclerotic tissue specimens from patients who underwent operations for high-grade carotid stenosis, we analyzed the LPA content and composition of the lipid-rich core that is characterized histologically by extracellular lipid deposits and beds of foam cells [80]. This region had the highest LPA content [77,81]. The unsaturated but not the saturated LPA species have been implicated in eliciting the PM of VSMC [42].
Because it is now well established that extracellular LPA evokes its biological responses through a subfamily of GPCR, it was important to know which GPCR mediates the early PM in VSMC. Using LPA1 KO, LPA2 KO, and LPA1&2 DKO mouse lines, we demonstrated early PM of VSMC in all of these mouse lines (Figs. 6–8). These results imply that early PM is not mediated through LPA1 or LPA2 receptors, which are the two most abundantly expressed subtypes in the mouse aorta. Interestingly, we also found that the level of the intracellular LPA receptors PPARγ also decreased during PM (Fig. 9) and that the PPARγ antagonist GW9662 failed to prevent early PM (Fig. 10).
In addition, we found that neither IGF-I nor PD98059 plus SB203580 could block the early PM in cultured aortic rings (Fig. 4). It has been shown that IGF-I or PD98059 plus SB203580 could block the late PM of primary cultured VSMC derived from rat aortas and concluded that changes in the balance between the PI3K/Akt pathway and the ERK and p38 MAPK pathways would determine phenotypes of VSMC [40–42]. Although there are several differences between our study and those previous reports, which include the difference in species, the lack of dissociation in the organotypic aortic ring cultures, and the time scale, the major difference is that we studied the early phase of the PM, whereas the other reports and our earlier report [55] examined the late phase. At present, definitions of the “differentiated” and “dedifferentiated” phenotypes are highly arbitrary. However, it is now clearly established that VSMC exhibit a broad phenotypic spectrum with differentiated and dedifferentiated phenotypes at the two ends of this spectrum. While PM in one direction represents the transit from differentiated phenotype to a dedifferentiated phenotype, this transition seemingly involves an early phase and a late phase. Taking our present study together with previous reports on this topic, we suggest that the changes in the balance between the PI3K/Akt pathway and the ERK and p38 MAPK pathways may be involved in the late stage of PM, but not in early PM.
Furthermore, although LPA and serum have been found to significantly accelerate late PM of VSMC [42,82], LPA only marginally affected the rate of early PM after 6 h but had no further effect at 12 h (Fig. 5). It is important to note that under every culture condition that we have tested, including serum-free, as well as serum-, LPA-, IGF-I-, MAPK and PPARγ inhibitor-supplemented cultures, the cultured aortic rings consistently showed early PM. These findings imply that the early phase of PM has a different molecular mechanism than the late phase does and that other physiological cues may play a fundamental role in maintaining the differentiated phenotype in vivo.
In addition to biochemical factors, VSMC in vivo are exposed to mechanical factors that are represented by tensile stress due to blood pressure and shear stress due to flow. To independently test the effects of blood, pressure, and flow on early PM, we modified the non-injury rat model developed by Yoshida et al. [43]. By eliminating these physiological cues in vivo, we were able to show that blood pressure alone maintained the differentiated phenotype and that loss of the blood pressure might be the cause of early PM in cultured vascular cells or organs (Fig. 11). After distinguishing early PM from late PM and demonstrating that early PM is not dependent on the activation of the ERK and p38 MAPK pathways, we were able to reconcile the paradoxical experimental findings that activation of ERK and p38 MAPK pathways induces PM and that stretch increases the ERK and p38 MAPK activity while maintaining the differentiated phenotype. Once the cells or arteries are taken out of the animal and cultured in vitro, the VSMC undergo within hours an early PM mediated by loss of mechanical stretch that does not involve activation of the MAPK pathways, and a late PM (within days) mediated by biochemical factors in serum, including LPA, through activation of MAPK pathways [42,43].
In summary, the important findings in this study are that (1) PM of cultured VSMC can be divided into an early stage (within hours) and a late stage (within days); (2) early PM is not mediated by LPA or other blood factors; rather (3) it is mediated by signals due to the loss of mechanical forces to which VSMC are subjected in vivo; and (4) early PM is not mediated by the activation of the ERK and p38 MAPK pathways.
Fig. 7.
Vascular smooth muscle cell (VSMC)-specific marker gene mRNA expression in cultured aortic rings of LPA2 knockout mice. The thoracic aortas of LPA2 knockout mice were cultured in serum-free base medium for 0–72 h prior to RNA extraction. Quantitative RT-PCR was used to assess mRNA levels of the VSMC-specific marker genes. Values are means ± SEM for 3 replicates. *Denotes significant difference from the preceding time point (P<0.05).
Acknowledgements
We thank Ms. Jian Yang for her expert assistance with the histology, Ms. Lillian Zalduondo and Ms. Melinda McCarty for animal care, and Drs. Ken-ichiro Hayashi and Kenji Sobue (Osaka University) for helpful discussions concerning our data. This study was supported by NIH grant HL79004 (GT), a Thomas Gerwin Graduate Student Fellowship (SE), and American Heart Association grant 0530106N (CZ).
References
- 1.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol. Rev. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Gabbiani G, Schmid E, Winter S, Chaponnier C, de Ckhastonay C, Vandekerckhove J, Weber K, Franke WW. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc. Natl. Acad. Sci. U. S. A. 1981;78:298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagai R, Kuro-o M, Babij P, Periasamy M. Identification of two types of smooth muscle myosin heavy chain isoforms by cDNA cloning and immunoblot analysis. J. Biol. Chem. 1989;264:9734–9737. [PubMed] [Google Scholar]
- 4.Gimona M, Sparrow MP, Strasser P, Herzog M, Small JV. Calponin and SM 22 isoforms in avian and mammalian smooth muscle. Absence of phosphorylation in vivo. Eur. J. Biochem. 1992;205:1067–1075. doi: 10.1111/j.1432-1033.1992.tb16875.x. [DOI] [PubMed] [Google Scholar]
- 5.Solway J, Seltzer J, Samaha FF, Kim S, Alger LE, Niu Q, Morrisey EE, Ip HS, Parmacek MS. Structure and expression of a smooth muscle cell-specific gene. SM22 alpha, J. Biol. Chem. 1995;270:13460–13469. doi: 10.1074/jbc.270.22.13460. [DOI] [PubMed] [Google Scholar]
- 6.Frid MG, Shekhonin BV, Koteliansky VE, Glukhova MA. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev. Biol. 1992;153:185–193. doi: 10.1016/0012-1606(92)90104-o. [DOI] [PubMed] [Google Scholar]
- 7.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 8.Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int. Rev. Cytol. 1996;169:183–265. doi: 10.1016/s0074-7696(08)61987-7. [DOI] [PubMed] [Google Scholar]
- 9.Shanahan CM, Weissberg PL. Smooth muscle cell heterogeneity: patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 1998;18:333–338. doi: 10.1161/01.atv.18.3.333. [DOI] [PubMed] [Google Scholar]
- 10.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 11.Stegemann JP, Hong H, Nerem RM. Mechanical,biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J. Appl. Physiol. 2005;98:2321–2327. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 12.Kanda K, Matsuda T. Mechanical stress-induced orientation and ultrastructural change of smooth muscle cells cultured in three-dimensional collagen lattices. Cell Transplant. 1994;3:481–492. doi: 10.1177/096368979400300605. [DOI] [PubMed] [Google Scholar]
- 13.Birukov KG, Shirinsky VP, Stepanova OV, Tkachuk VA, Hahn AW, Resink TJ, Smirnov VN. Stretch affects phenotype and proliferation of vascular smooth muscle cells. Mol. Cell Biochem. 1995;144:131–139. doi: 10.1007/BF00944392. [DOI] [PubMed] [Google Scholar]
- 14.Reusch P, Wagdy H, Reusch R, Wilson E, Ives HE. Mechanical strain increases smooth muscle and decreases nonmuscle myosin expression in rat vascular smooth muscle cells. Circ. Res. 1996;79:1046–1053. doi: 10.1161/01.res.79.5.1046. [DOI] [PubMed] [Google Scholar]
- 15.Hipper A, Isenberg G. Cyclic mechanical strain decreases the DNA synthesis of vascular smooth muscle cells. Pflugers. Arch. 2000;440:19–27. doi: 10.1007/s004240000246. [DOI] [PubMed] [Google Scholar]
- 16.Tock J, Van Putten V, Stenmark KR, Nemenoff RA. Induction of SM-alpha-actin expression by mechanical strain in adult vascular smooth muscle cells is mediated through activation of JNK and p38 MAP kinase. Biochem. Biophys. Res. Commun. 2003;301:1116–1121. doi: 10.1016/s0006-291x(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 17.Qu MJ, Liu B, Wang HQ, Yan ZQ, Shen BR, Jiang ZL. Frequency-dependent phenotype modulation of vascular smooth muscle cells under cyclic mechanical strain. J. Vasc. Res. 2007;44:345–353. doi: 10.1159/000102278. [DOI] [PubMed] [Google Scholar]
- 18.Bardy N, Karillon GJ, Merval R, Samuel JL, Tedgui A. Differential effects of pressure and flow on DNA and protein synthesis and on fibronectin expression by arteries in a novel organ culture system. Circ. Res. 1995;77:684–694. doi: 10.1161/01.res.77.4.684. [DOI] [PubMed] [Google Scholar]
- 19.Birukov KG, Bardy N, Lehoux S, Merval R, Shirinsky VP, Tedgui A. Intraluminal pressure is essential for the maintenance of smooth muscle caldesmon and filamin content in aortic organ culture. Arterioscler. Thromb. Vasc. Biol. 1998;18:922–927. doi: 10.1161/01.atv.18.6.922. [DOI] [PubMed] [Google Scholar]
- 20.Zeidan A, Nordstrom I, Albinsson S, Malmqvist U, Sward K, Hellstrand P. Stretch-induced contractile differentiation of vascular smooth muscle: sensitivity to actin polymerization inhibitors. Am. J. Physiol. Cell Physiol. 2003;284:C1387–1396. doi: 10.1152/ajpcell.00508.2002. [DOI] [PubMed] [Google Scholar]
- 21.Albinsson S, Nordstrom I, Hellstrand P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J. Biol. Chem. 2004;279:34849–34855. doi: 10.1074/jbc.M403370200. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Van Putten V, Zarinetchi F, Nicks ME, Thaler S, Heasley LE, Nemenoff RA. Suppression of smooth-muscle alpha-actin expression by platelet-derived growth factor in vascular smooth-muscle cells involves Ras and cytosolic phospholipase A2. Biochem. J. 1997;327(Pt 3):709–716. doi: 10.1042/bj3270709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deguchi J, Namba T, Hamada H, Nakaoka T, Abe J, Sato O, Miyata T, Makuuchi M, Kurokawa K, Takuwa Y. Targeting endogenous platelet-derived growth factor B-chain by adenovirus-mediated gene transfer potently inhibits in vivo smooth muscle proliferation after arterial injury. Gene. Ther. 1999;6:956–965. doi: 10.1038/sj.gt.3300918. [DOI] [PubMed] [Google Scholar]
- 24.Kingsley K, Huff JL, Rust WL, Carroll K, Martinez AM, Fitchmun M, Plopper GE. ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem. Biophys. Res. Commun. 2002;293:1000–1006. doi: 10.1016/S0006-291X(02)00331-5. [DOI] [PubMed] [Google Scholar]
- 25.Kotani M, Fukuda N, Ando H, Hu WY, Kunimoto S, Saito S, Kanmatsuse K. Chimeric DNA-RNA hammerhead ribozyme targeting PDGF A-chain mRNA specifically inhibits neointima formation in rat carotid artery after balloon injury. Cardiovasc. Res. 2003;57:265–276. doi: 10.1016/s0008-6363(02)00607-7. [DOI] [PubMed] [Google Scholar]
- 26.Hautmann MB, Madsen CS, Owens GK. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J. Biol. Chem. 1997;272:10948–10956. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- 27.Engelse MA, Lardenoye JH, Neele JM, Grimbergen JM, De Vries MR, Lamfers ML, Pannekoek H, Quax PH, De Vries CJ. Adenoviral activin a expression prevents intimal hyperplasia in human and murine blood vessels by maintaining the contractile smooth muscle cell phenotype. Circ. Res. 2002;90:1128–1134. doi: 10.1161/01.res.0000021044.53156.f5. [DOI] [PubMed] [Google Scholar]
- 28.Neuville P, Geinoz A, Benzonana G, Redard M, Gabbiani F, Ropraz P, Gabbiani G. Cellular retinol-binding protein-1 is expressed by distinct subsets of rat arterial smooth muscle cells in vitro and in vivo. Am. J. Pathol. 1997;150:509–521. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Gardner DG. Retinoic acid uses divergent mechanisms to activate or suppress mitogenesis in rat aortic smooth muscle cells. J. Clin. Invest. 1998;102:653–662. doi: 10.1172/JCI3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miano JM, Kelly LA, Artacho CA, Nuckolls TA, Piantedosi R, Blaner WS. All-trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation. 1998;98:1219–1227. doi: 10.1161/01.cir.98.12.1219. [DOI] [PubMed] [Google Scholar]
- 31.Johst U, Betsch A, Wiskirchen J, Schober W, Vonthein R, Rinkert N, Kehlbach R, Claussen CD, Duda SH. All-trans and 9-cis retinoid acids inhibit proliferation, migration, and synthesis of extracellular matrix of human vascular smooth muscle cells by inducing differentiation in vitro. J. Cardiovasc. Pharmacol. 2003;41:526–535. doi: 10.1097/00005344-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Hedin U, Bottger BA, Forsberg E, Johansson S, Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J. Cell Biol. 1988;107:307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thyberg J, Hultgardh-Nilsson A. Fibronectin and the basement membrane components laminin and collagen type IV influence the phenotypic properties of subcultured rat aortic smooth muscle cells differently. Cell Tissue Res. 1994;276:263–271. doi: 10.1007/BF00306112. [DOI] [PubMed] [Google Scholar]
- 34.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 35.Raines EW, Koyama H, Carragher NO. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann. N. Y. Acad. Sci. 2000;902:39–52. doi: 10.1111/j.1749-6632.2000.tb06299.x. [DOI] [PubMed] [Google Scholar]
- 36.Ichii T, Koyama H, Tanaka S, Kim S, Shioi A, Okuno Y, Raines EW, Iwao H, Otani S, Nishizawa Y. Fibrillar collagen specifically regulates human vascular smooth muscle cell genes involved in cellular responses and the pericellular matrix environment. Circ. Res. 2001;88:460–467. doi: 10.1161/01.res.88.5.460. [DOI] [PubMed] [Google Scholar]
- 37.Li S, Lao J, Chen BP, Li YS, Zhao Y, Chu J, Chen KD, Tsou TC, Peck K, Chien S. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. Faseb. J. 2003;17:97–99. doi: 10.1096/fj.02-0256fje. [DOI] [PubMed] [Google Scholar]
- 38.Stegemann JP, Nerem RM. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp. Cell Res. 2003;283:146–155. doi: 10.1016/s0014-4827(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 39.Chai S, Chai Q, Danielsen CC, Hjorth P, Nyengaard JR, Ledet T, Yamaguchi Y, Rasmussen LM, Wogensen L. Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ. Res. 2005;96:583–591. doi: 10.1161/01.RES.0000158963.37132.8b. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi K, Shibata K, Morita T, Iwasaki K, Watanabe M, Sobue K. Insulin receptor substrate-1/SHP-2 interaction, a phenotype-dependent switching machinery of insulin-like growth factor-I signaling in vascular smooth muscle cells. J. Biol. Chem. 2004;279:40807–40818. doi: 10.1074/jbc.M405100200. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi K, Takahashi M, Kimura K, Nishida W, Saga H, Sobue K. Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J. Cell Biol. 1999;145:727–740. doi: 10.1083/jcb.145.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, Aoki J, Arai H, Sobue K. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ. Res. 2001;89:251–258. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, Arai H, Sobue K. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation. 2003;108:1746–1752. doi: 10.1161/01.CIR.0000089374.35455.F3. [DOI] [PubMed] [Google Scholar]
- 44.Loufrani L, Lehoux S, Tedgui A, Levy BI, Henrion D. Stretch induces mitogen-activated protein kinase activation and myogenic tone through 2 distinct pathways. Arterioscler. Thromb. Vasc. Biol. 1999;19:2878–2883. doi: 10.1161/01.atv.19.12.2878. [DOI] [PubMed] [Google Scholar]
- 45.Lehoux S, Esposito B, Merval R, Loufrani L, Tedgui A. Pulsatile stretch-induced extracellular signal-regulated kinase 1/2 activation in organ culture of rabbit aorta involves reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2000;20:2366–2372. doi: 10.1161/01.atv.20.11.2366. [DOI] [PubMed] [Google Scholar]
- 46.Spurrell BE, Murphy TV, Hill MA. Intraluminal pressure stimulates MAPK phosphorylation in arterioles: temporal dissociation from myogenic contractile response. Am. J. Physiol. Heart. Circ. Physiol. 2003;285:H1764–H1773. doi: 10.1152/ajpheart.00468.2003. [DOI] [PubMed] [Google Scholar]
- 47.Han M, Wen JK, Zheng B, Cheng Y, Zhang C. Serum deprivation results in redifferentiation of human umbilical vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2006;291:C50–C58. doi: 10.1152/ajpcell.00524.2005. [DOI] [PubMed] [Google Scholar]
- 48.Poliseno L, Cecchettini A, Mariani L, Evangelista M, Ricci F, Giorgi F, Citti L, Rainaldi G. Resting smooth muscle cells as a model for studying vascular cell activation. Tissue. Cell. 2006;38:111–120. doi: 10.1016/j.tice.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 49.van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokumura A, Iimori M, Nishioka Y, Kitahara M, Sakashita M, Tanaka S. Lysophosphatidic acids induce proliferation of cultured vascular smooth muscle cells from rat aorta. Am. J. Physiol. 1994;267:C204–C210. doi: 10.1152/ajpcell.1994.267.1.C204. [DOI] [PubMed] [Google Scholar]
- 51.Cerutis DR, Nogami M, Anderson JL, Churchill JD, Romberger DJ, Rennard SI, Toews ML. Lysophosphatidic acid and EGF stimulate mitogenesis in human airway smooth muscle cells. Am. J. Physiol. 1997;273:L10–L15. doi: 10.1152/ajplung.1997.273.1.L10. [DOI] [PubMed] [Google Scholar]
- 52.Tokumura A, Yotsumoto T, Masuda Y, Tanaka S. Vasopressor effect of lysophosphatidic acid on spontaneously hypertensive rats and Wistar Kyoto rats. Res. Commun. Mol. Pathol. Pharmacol. 1995;90:96–102. [PubMed] [Google Scholar]
- 53.Toews ML, Ustinova EE, Schultz HD. Lysophosphatidic acid enhances contractility of isolated airway smooth muscle. J. Appl. Physiol. 1997;83:1216–1222. doi: 10.1152/jappl.1997.83.4.1216. [DOI] [PubMed] [Google Scholar]
- 54.Siess W, Tigyi G. Thrombogenic and atherogenic activities of lysophosphatidic acid. J. Cell. Biochem. 2004;92:1086–1094. doi: 10.1002/jcb.20108. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J. Exp. Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2002;54:259–265. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 57.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J. Pharmacol. Exp. Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 58.Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J. Biol. Chem. 2007;282:4310–4317. doi: 10.1074/jbc.M610826200. [DOI] [PubMed] [Google Scholar]
- 59.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 60.Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat. Genet. 2008;40:329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 61.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2007;363:861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 62.McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. U. S. A. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukahara T, Tsukahara R, Yasuda S, Makarova N, Valentine WJ, Allison P, Yuan H, Baker DL, Li Z, Bittman R, Parrill A, Tigyi G. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2006;281:3398–3407. doi: 10.1074/jbc.M510843200. [DOI] [PubMed] [Google Scholar]
- 64.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol. Cell. Biol. 2002;22:6921–6929. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome. Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayashi K, Saga H, Chimori Y, Kimura K, Yamanaka Y, Sobue K. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. J. Biol. Chem. 1998;273:28860–28867. doi: 10.1074/jbc.273.44.28860. [DOI] [PubMed] [Google Scholar]
- 68.Jarmolych J, Daoud AS, Landau J, Fritz KE, McElvene E. Aortic media explants. Cell proliferation and production of mucopolysaccharides, collagen, and elastic tissue. Exp. Mol. Pathol. 1968;9:171–188. doi: 10.1016/0014-4800(68)90033-6. [DOI] [PubMed] [Google Scholar]
- 69.Rossi GL, Alroy J, Rothenmund S. Morphological studies of cultured swine aorta media explants. Virchows. Arch. B. Cell Pathol. 1973;12:133–144. doi: 10.1007/BF02893993. [DOI] [PubMed] [Google Scholar]
- 70.Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J. Biol. Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 71.Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem. 2002;277:48737–48744. doi: 10.1074/jbc.M206812200. [DOI] [PubMed] [Google Scholar]
- 72.Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography–mass spectrometry. Anal. Biochem. 2001;292:287–295. doi: 10.1006/abio.2001.5063. [DOI] [PubMed] [Google Scholar]
- 73.Baker DL, Morrison P, Miller B, Riely CA, Tolley B, Westermann AM, Bonfrer JM, Bais E, Moolenaar WH, Tigyi G. Plasma lysophosphatidic acid concentration and ovarian cancer. Jama. 2002;287:3081–3082. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]
- 74.Saulnier-Blache JS, Girard A, Simon MF, Lafontan M, Valet P. A simple and highly sensitive radioenzymatic assay for lysophosphatidic acid quantification. J. Lipid. Res. 2000;41:1947–1951. [PMC free article] [PubMed] [Google Scholar]
- 75.Gerrard JM, Robinson P. Identification of the molecular species of lysophosphatidic acid in platelets stimulated by thrombin. Biochem. Biphys. Acta. 1989;1001:282–285. doi: 10.1016/0005-2760(89)90112-4. [DOI] [PubMed] [Google Scholar]
- 76.Yatomi Y, Ohmori T, Rile G, Kazama F, Okamoto H, Sano T, Satoh K, Kume S, Tigyi G, Igarashi Y, Ozaki Y. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–3438. [PubMed] [Google Scholar]
- 77.Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, Siess W. Subtype-selective antagonists of lysophosphatidic acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741–747. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- 78.Simon MF, Chap H, Douste-Blazy L. Human platelet aggregation induced by 1-alkyl-lysophosphatidic acid and its analogs: a new group of phospholipid mediators? Biochem. Biophys. Res. Commun. 1982;108:1743–1750. doi: 10.1016/s0006-291x(82)80113-7. [DOI] [PubMed] [Google Scholar]
- 79.Tokumura A, Sinomiya J, Kishimoto S, Tanaka T, Kogure K, Sugiura T, Satouchi K, Waku K, Fukuzawa K. Human platelets respond differentially to lysophosphatidic acids having a highly unsaturated fatty acyl group and alkyl ether-linked lysophosphatidic acids. Biochem. J. 2002;365:617–628. doi: 10.1042/BJ20020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brandl R, Richter T, Haug K, Wilhelm MG, Maurer PC, Nathrath W. Topographic analysis of proliferative activity in carotid endarterectomy specimens by immunocytochemical detection of the cell cycle-related antigen Ki-67. Circulation. 1997;96:3360–3368. doi: 10.1161/01.cir.96.10.3360. [DOI] [PubMed] [Google Scholar]
- 81.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chamley-Campbell JH, Campbell GR, Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J. Cell Biol. 1981;89:379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]