Abstract
4-Phenylethynyl-6-phenyl-1,4-dihydropyridine derivatives are selective antagonists at human A3 adenosine receptors, with Ki values in a radioligand binding assay vs [125I]AB-MECA [N6-(4-amino-3-iodobenzyl)-5′-N-methylcarbamoyl-adenosine] in the submicromolar range. In this study, functionalized congeners of 1,4-dihydropyridines were designed as chemically reactive adenosine A3 antagonists, for the purpose of synthesizing molecular probes for this receptor subtype. Selectivity of the new analogues for cloned human A3 adenosine receptors was determined in radioligand binding in comparison to binding at rat brain A1 and A2A receptors. Benzyl ester groups at the 3- and/or 5-positions and phenyl groups at the 2- and/or 6-positions were introduced as potential sites for chain attachment. Structure–activity analysis at A3 adenosine receptors indicated that 3,5-dibenzyl esters, but not 2,6-diphenyl groups, are tolerated in binding. Ring substitution of the 5-benzyl ester with a 4-fluorosulfonyl group provided enhanced A3 receptor affinity resulting in a Ki value of 2.42 nM; however, a long-chain derivative containing terminal amine functionalization at the 4-position of the 5-benzyl ester showed only moderate affinity. This sulfonyl fluoride derivative appeared to bind irreversibly to the human A3 receptor (1 h incubation at 100 nM resulting in the loss of 56% of the specific radioligand binding sites), while the binding of other potent dihydropyridines and other antagonists was generally reversible. At the 3-position of the dihydropyridine ring, an amine-functionalized chain attached at the 4-position of a benzyl ester provided higher A3 receptor affinity than the corresponding 5-position isomer. This amine congener was also used as an intermediate in the synthesis of a biotin conjugate, which bound to A3 receptors with a Ki value of 0.60 μM.
INTRODUCTION
Medicinal chemists are currently developing agonists and antagonists that interact selectively with adenosine receptors, of which A1, A2A, A2B, and A3 subtypes are known (1). As the only subtype identified through cloning (2, 3), the A3 adenosine receptor has demonstrated unique biological effects, SAR profile, and tissue distribution (4). Intense activation of A3 receptors induces apoptosis (programmed cell death) (4). The A3 receptor has also a cytoprotective action at low agonist concentrations (4). The first cytoprotective effects of an A3 agonist [IB-MECA,1N6-(3-iodobenzyl)-5′-N-methylcarbamoyladenosine] were shown following its chronic administration in gerbils in a model of stroke (5). The agonist was highly cerebroprotective and depressed nitric oxide synthase (5, 6). In cultured chick cardiac myocytes, a brief prior exposure to nanomolar concentrations of the related 2-chloro derivative, Cl-IB-MECA, protected cells from damage induced by subsequent hypoxia (7), thus mimicking a phenomenon termed “preconditioning”. The protective effects of an A3 receptor agonist on cardiac myocytes were also evident when added during the prolonged ischemia (8). A3 receptor agonists at micromolar concentrations were found to induce apoptosis a variety of cell types, including tumor cell lines (9) and rat cardiac myocytes (10). Thus, the varied effects of A3 receptor agonists appear to be dual and opposite, i.e., either cytoprotective or cytotoxic, depending on the level of receptor activation and the system studied.
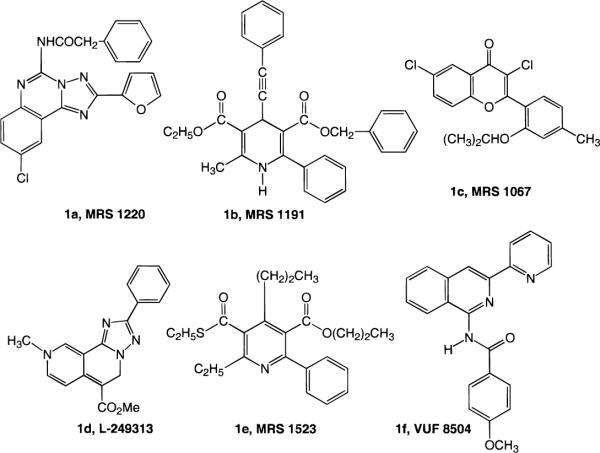
A3 adenosine receptor antagonists, although only recently introduced (11–16), were previously hypothesized to act as potential antiasthmatic (17), antiinflammatory (17), or cerebroprotective agents (18). The most promising leads for A3 receptor antagonists have appeared recently among chemically diverse nonxanthine heterocycles (Figure 1). For example, the 1,4-dihydropyridines, that are potent blockers of L-type calcium channels (19, 20) and used widely in treating coronary heart disease, were found to bind with intermediate affinity to human A3 receptors and consequently were structurally optimized for subtype selectivity (12). In the present and previous studies, we have used the 1,4-dihydropyridine (DHP) nucleus as a template for probing structure–activity relationships (SAR) at adenosine receptors. By careful structural modification, it has been possible to select for affinity at adenosine receptors and deselect for affinity at L-type Ca2+-channels.
Figure 1.
Structures of key A3 adenosine receptor selective antagonists reported previously.
The most potent antagonist of the human A3 receptor yet reported is MRS 1220 (Figure 1, 1a) (16), a derivative of the nonselective triazoloquinazoline antagonist CGS 15943. Within the series of DHP derivatives, affinity and selectivity for the human A3 receptor were further enhanced in the trisubstituted analogue MRS 1191, 3-ethyl 5-benzyl 2-methyl-6-phenyl-4-phenylethynyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (Table 1, 1b) (12). Other A3 selective antagonists that have been recently reported include a flavonoid derivative (MRS 1067, 1c) (11), a triazolonaphthyridine (L-249313, 1d) (13), a pyridine (MRS 1523, 1e) (14), and a pyridyl isoquinoline (VUF 8504, 1f) (15).
Table 1.
Affinities of 1,4-Dihydropyridine Derivatives in Radioligand Binding Assays at A1, A2A, and A3 Receptorsa–c
|
Ki (μM) or % inhibition |
|||||||
|---|---|---|---|---|---|---|---|
| compd | R3 | R4 | R5 | rA1a | rA2Ab | hA3c | rA1/hA3 |
| 1b e | OCH2CH3 | Ph-C≡C- | OCH2Ph | 40.1 ± 7.5 | d (10−4) | 0.0314 ± 0.0028 | 1300 |
| 2 e | OCH2CH3 | Ph-CH=CH- (trans) | OCH2CH3 | 5.93 ± 0.27 | 4.77 ± 0.29 | 0.108 ± 0.012 | 55 |
| 3 e | OCH2CH3 | Ph-C≡C- | OCH2CH3 | 11.0 ± 0.1 | 26 ± 12% (10−4) | 0.0766 ± 0.0151 | 140 |
| 4 e | OCH2Ph | Ph-C≡C- | OCH2CH3 | 24 ± 4% (10−4) | d (10−4) | 0.169 ± 0.026 | >600 |
| 5 | OCH2Ph | Ph-C≡C- | OCH2Ph | d (10−4) | d (10−4) | 0.195 ± 0.038 | >500 |
| 6, 2-Ph | OCH2CH3 | Ph-C≡C- | OCH2Ph | 36 ± 11% (10−4) | d (10−4) | 20% (10−5) | |
| 7, 2-Ph | OCH2Ph | Ph-C≡C- | OCH2Ph | 19 ± 8% (10−4) | d (10−4) | d (10−5) | |
| 8a | O(CH2)2OCH3 | Ph-C≡C- | OCH2Ph | 48.7 ± 7.3 | 14 ± 7% (10−4) | 0.001 91 ± 0.000 31 | 25 000 |
| 8b | O(CH2)2OCH3 | Ph-C≡C- | OCH2Ph-4–NO2 | 23.6 ± 9.1 | d (10−4) | 0.003 08 ± 0.001 22 | 7700 |
| 9 | O(CH2)2OH | Ph-C≡C- | OCH2Ph | 10.6 ± 1.9 | d (10−4) | 0.002 07 ± 0.0012 | 5100 |
| 10 | O(CH2)2SH | Ph-C≡C- | OCH2Ph | d (10−4) | d (10−4) | 0.197 ± 0.076 | >500 |
| 11 | S(CH2)2SH | Ph-C≡C- | OCH2Ph | 38 ± 11% (10−4) | d (10−4) | 0.178 ± 0.018 | >500 |
| 12 | O(CH2)2S(CH2)2Si(CH3)3 | Ph-C≡C- | OCH2Ph | 13 ± 7% (10−4) | 30% (10−4) | 1.98 ± 0.64 | >50 |
| 13 | OCH2(4-SO2F)Ph | Ph-C≡C- | OCH2CH3 | d (10−4) | 18 | 0.341 ± 0.091 | >300 |
| 14 | OCH2(4-SO2NH2)Ph | Ph-C≡C- | OCH2CH3 | 16.9 ± 5.0 | 13.5 ± 1.3 | 0.187 ± 0.033 | 90 |
| 15 | OCH2(4-CO2CH2CCl3)Ph | Ph-C≡C- | OCH2CH3 | 19% (10−4) | 5.99 ± 1.67 | 8.93 ± 5.63 | >1 |
| 16 | OCH2Ph-4-CO–NH(CH2)2NH2 | Ph-C≡C- | OCH2CH3 | 6.65 ± 1.35 | 7.85 | 0.128 ± 0.018 | 52 |
| 17 | OCH2(4-CO2H)Ph | Ph-C≡C- | OCH2CH3 | 16.0 ± 2.1 | <1 (shallow curves) | 0.799 ± 0.297 | 20 |
| 18 | OCH2Ph-4-CO-NH(CH2)2NH-biotin | Ph-C≡C- | OCH2CH3 | 38 ± 2% (10−4) | 16 ± 8% (10−4) | 0.599 ± 0.151 | >100 |
| 19 f | OCH2CH3 | Ph-C≡C- | OCH2(4-SO2F)Ph | 41 ± 3% (10−4) | 20% (10−4) | 0.002 42 ± 0.000 32 | >30 000 |
| 20 | OCH2CH3 | Ph-C≡C- | OCH2(4-SO2NH2)Ph | 14.4 ± 2.4 | 22.0 ± 6.1 | 0.292 ± 0.030 | 49 |
| 21 | OCH2CH3 | Ph-C≡C- | OCH2 (4-CO2CH2CCl3)Ph | 33 ± 6% (10−4) | 11% (10−4) | 5.00 | >20 |
| 22 g | OCH2CH3 | Ph-C≡C- | OCH2Ph-4-CO-NH(CH2)2NH2 | 3.0 | 39 ± 4% (10−4) | 0.697 ± 0.259 | 4.3 |
| 23 | OCH2CH3 | Ph-C≡C- | OCH2 (4-CO2H)Ph | 19.0 ± 5.3 | 8.22 ± 3.76 | d (10−6) | |
Displacement of specific [3H]R–PIA binding in rat brain membranes, expressed as Ki ± SEM (n = 2–5), or as a percentage of specific binding displaced at the indicated concentration (M).
Displacement of specific [3H]CGS 21 680 binding in rat striatal membranes, expressed as Ki ± SEM (n = 2–6), or as a percentage of specific binding displaced at the indicated concentration (M).
Displacement of specific [125I]AB-MECA binding at human A3 receptors expressed in HEK cells, in membranes, expressed as Ki ± SEM (n = 2–4).
Displacement of ≤10% of specific binding at the indicated concentration (M).
Values taken from Jiang et al. (12) and references therein.
Bound to human A3 receptors irreversibly (see Figure 2), Ki value is apparent.
Same as compound 25 in Jiang et al. (12) Ki values have been redetermined.
In the present study, the SAR of 1,4-dihydropyridines as antagonist ligands for adenosine receptors has been expanded using a “functionalized congener approach” (21). By this approach, a chain substituent is attached to the pharmacophore at a strategic position, such that conjugation to other large molecules is made possible through an easily derivatized functional group, without losing the ability to bind to the receptor. This approach leads to increased flexibility of substitution and enhancement of potency/selectivity via distal interactions at the receptor. This approach has been developed for other selective adenosine receptor agonists and antagonists, such as the potent xanthine amine congener, XAC (8-[4-[[[[(2-aminoethyl)-amino]carbonyl]methyl]oxy]phenyl]-1,3-dipropylxanthine) (34), and ligands for other receptors (35), leading to irreversibly binding affinity probes, fluorescent probes, etc.
A typical means of derivatization in the design of functionalized congeners is to locate a position on the pharmacophore that may readily accommodate substitution with a phenyl group (34), without losing affinity for the receptor. In the present study, we have systematically explored the DHP template for additional sites of incorporation of phenyl substituents and various functional groups followed by chain extension. Fluorosulfonyl groups (29) were included in the analogues as an electrophilic group for potential covalent reaction with the receptor, which was indicated in binding experiments with one derivative. Finally, the incorporation of easily derivatizable amino groups at strategic positions was accomplished.
MATERIALS AND METHODS
Synthesis
Materials and Instrumentation
Phenylpropargyl aldehyde (25), benzyl acetoacetate (26a), ethyl benzoyl acetate (26b), 2-methoxyethyl acetoacetate (26i), 2,2,6-trimethyl-4H-1,3-dioxin-4-one (27), ethylene glycol (28d), 2-mercaptoethanol (28e), ethylene dithiol (28f), 2-trimethylsilylethanol (28h), p-nitrobenzyl alcohol (29), p-toluenesulfonyl fluoride (30a), p-toluenesulfonamide (30b), NBS, 2-(trimethylsilyl)ethanethiol, α-bromo-p-toluic acid, chloromethyl methyl ether, and 2-bromoethanol were purchased from Aldrich (St. Louis, MO). 2-Trimethylsilylethylthioethanol (28g) was prepared by reaction of 2-(trimethylsilyl)ethanethiol (1.1 mmol), 2-bromoethanol (1.0 mmol), and potassium hydroxide (1.5 mmol) in CH3CN at room temperature for 4 h (yield, 78%). Compounds 1b–4 were prepared as described Jiang et al. (12). Dihydropyridines 21 [3-ethyl 5-[4-(2,2,2-trichloroethoxycarbonyl])benzyl] 2-methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate], 22 [3-ethyl 5-[4-(2-aminoethyl)aminocarbonyl-benzyl]-2-methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarbox-ylate], 1-(ethoxymethyl)-3-(ethoxycarbonyl)-2-methyl-4-(phenylethynyl)-6-phenyl-1,4-dihydropyridine-5-carboxylic acid (32b), compound 30c, and bromide 31c were prepared according to the method of Jiang et al. (12). Compounds 24a, 24b, and benzyl benzoyl acetate (26c) were prepared in our previous paper (14). All other materials were obtained from commercial sources.
Proton nuclear magnetic resonance spectroscopy was performed on a Varian GEMINI-300 spectrometer and all spectra were taken in CDCl3. Chemical ionization (CI) mass spectrometry was performed with a Finnigan 4600 mass spectrometer and electron-impact (EI) mass spectrometry with a VG7070F mass spectrometer at 6 kV. Elemental analysis was performed either by Atlantic Microlab Inc. (Norcross, GA) or by Galbraith Laboratories, Inc. (Knoxville, TN).
General Procedure for Preparation of Substituted 1,4-Dihydropyridine (5–8a, 9–12, and 33, Scheme 1)
Scheme 1. Synthesis of 6-Phenyl-4-phenylethynyl-1,4-dihydropyridine Derivatives Using the Hantzsch Reactiona.
aSilyl-, hydroxyl-, and thiol-containing ester groups are included. The 2-position contains either a methyl or a phenyl group.
Equimolar amounts (0.5–1.0 mmol) of the appropriate β-enaminoester (24), aldehyde (25), and β-ketoester (26) were dissolved in 2–5 mL of absolute ethanol. The mixture was sealed in a Pyrex tube and heated, with stirring, at 80 °C for 18–24 h. After cooled to room temperature, the solvent was evaporated and the residue was purified by preparative TLC [silica 60; 1000 or 2000 μm; Analtech, Newark, DE; petroleum ether-ethyl acetate (1:1–4:1)].
3,5-Dibenzyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (5)
1H NMR (CDCl3): 2.34 (s, 3 H, 2-CH3), 5.01 (AB, 2 H, J = 12.6 Hz, 3-OCH2Ph), 5.24 (s, 1 H, 4-H), 5.27 (AB, 2 H, J = 13.8 Hz, 5- OCH2Ph), 6.05 (s, br, 1 H, NH), 7.04–7.11 (m, 2 H, aromatic H's), 7.15–7.30 (m, 9 H, aromatic H's), 7.33–7.40 (m, 7 H, aromatic H's), 7.44–7.47 (m, 2 H, aromatic H's). MS (EI): m/z 539 (M+), 448 (M+ – CH2−Ph), 404 (M+ – CO2CH2Ph), 91 (+CH2Ph, base).
3-Ethyl 5-Benzyl 4-phenylethynyl-2,6-diphenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (6)
1H NMR (CDCl3): 1.10 (t, 3 H, J = 7.8 Hz, 3-OCH2CH3), 4.16 (q, 2 H, J = 7.8 Hz, 3- OCH2CH3), 5.11 (s, 1 H, 4-H), 5.14 (AB, 2 H, J = 12.9 Hz, 5-OCH2Ph), 6.86–8.10 (m, 21 H, NH and aromatic H's). MS (EI): m/z 539 (M+), 448 (M+ – CH2−Ph), 404 (M+ – CO2CH2Ph), 91 (+CH2Ph, base).
3,5-Dibenzyl 4-Phenylethynyl-2,6-diphenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (7)
1H NMR (CDCl3): 5.07 (AB, 4 H, J = 12.6 Hz, 3- and 5-OCH2Ph), 5.35 (s, 1 H, 4-H), 6.15 (s, br, 1 H, NH), 7.07–7.45 (m, 25 H, aromatic H's). MS (EI): m/z 601 (M+), 510 (M+ – CH2−Ph), 466 (M+ – CO2CH2Ph), 91 (+CH2Ph, base). MS (CI–NH3): m/z 619 (M+ + NH4), 602 (M+ + 1). HRMS calcd: 601.2253. Found: 601.2255.
3-(2-Methoxyethyl) 5-Benzyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (8a)
1H NMR (CDCl3): 2.35 (s, 3 H, 2-CH3), 3.37 (s, 3 H, 3-OCH2CH2OCH3), 3.67 (s, 2 H, J = 5.7 Hz, 3-OCH2CH2−OCH3), 4.34 (m, 2 H, 3-OCH2CH2OCH3), 5.01 (AB, 2 H, J = 13.0 Hz, 5-OCH2Ph), 5.18 (s, 1 H, 4-H), 6.06 (s, br, 1 H, NH), 7.05–7.09 (m, 2 H, aromatic H's), 7.17–7.22 (m, 3 H, aromatic H's), 7.24–7.26 (m, 3 H, aromatic H's), 7.31–7.39 (m, 7 H, aromatic H's). MS (EI): m/z 507 (M+), 448 (M+ – CH3OCH2CH2), 416 (M+ – CH2Ph), 372 (M+ – CO2CH2Ph), 268 (M+ + 1-CO2CH2Ph-CO2CH2CH2OCH3, base). MS (CI–NH3): m/z 525 (M+ + NH4), 508 (M+ + 1).
3-(2-Methoxyethyl) 5-(4-Nitrobenzyl) 2-Methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (8b)
2-Methoxyethyl acetoacetate (26i, 160 mg, 1 mmol), phenylpropargyl aldehyde (25, 130 mg, 1 mmol), p-nitrobenzyl benzoyl acetate (26j, 299 mg, 1 mmol), and ammonium acetate (154 mg, 2 mmol) were dissolved in 3 mL of absolute ethanol. The mixture was stirred under N2 at 95 °C for 24 h. After cooled to room temperature, the solvent was evaporated and the residue was purified by preparative TLC [silica 60; 1000 μm; Analtech, Newark, DE; petroleum ether-ethyl acetate (1:1)] to give 127 mg (yield = 23%) of the product. 1H NMR (CDCl3): 2.38 (s, 3 H, 2-CH3), 3.40 (s, 3 H, 3-OCH2CH2OCH3), 3.68 (m, 2 H, 3-OCH2CH2OCH3), 4.37 (m, 2 H, 3-OCH2CH2−OCH3), 5.12 (AB, 2 H, J = 13.5 Hz, 5-OCH2Ph), 5.20 (s, 1 H, 4-H), 6.04 (s, br, 1 H, NH), 7.17–7.44 (m, 12 H, aromatic H's), 8.02 (m, 2 H, aromatic H's). MS (CI-NH3): m/z 570 (M+ + NH4), 553 (M+ + 1), 282 (M+ – CH3-CO2CH2C6H4NO2-MeOCH2CH2O, base).
3-(2-Hydroxyethyl) 5-Benzyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (9)
1H NMR (CDCl3): 2.35 (s, 3 H, 2-CH3), 3.84 (m, 2 H, 3-OCH2CH2OH), 4.17 (m, 1 H, 3-OCH2CH2OH), 4.45 (m, 1 H, 3-OCH2CH2OH), 4.99 (AB, 2 H, J = 12.6 Hz, 5-OCH2Ph), 5.17 (s, 1 H, 4-H), 6.20 (s, br, 1 H, NH), 7.02–7.39 (m, 15 H, aromatic H's). MS (EI): m/z 493 (M+), 448 (M+ – HOCH2CH2), 432 (M+ – HOCH2CH2O), 404 (M+ – HOCH2CH2O2C), 402 (M+ – CH2Ph), 358 (M+ – CO2−CH2Ph), 91 (+CH2Ph, base).
3-(2-Mercaptoethyl) 5-Benzyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (10)
1H NMR (CDCl3): 1.77 (t, 1 H, J = 8.7 Hz, SH), 2.36 (s, 3 H, 2-CH3), 2.82 (m, 2 H, 3-OCH2CH2SH), 4.23 (m, 1 H, 3-OCH2CH2SH), 4.42 (m, 1 H, 3-OCH2CH2SH), 5.01 (AB, 2 H, J = 12.6 Hz, 5-OCH2Ph), 5.17 (s, 1 H, 4-H), 6.07 (s, br, 1 H, NH), 7.05–7.37 (m, 15 H, aromatic H's). MS (EI): m/z 509 (M+), 448 (M+ – HSCH2CH2), 419 (MH+–CH2Ph), 404 (M+ – HSCH2CH2O2C), 374 (M+ – CO2CH2−Ph), 91 (+CH2Ph, base).
3-(2-Mercaptoethylsulfanyl) 5-Benzyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (11)
1H NMR (CDCl3): 1.69 (t, 1 H, J = 8.7 Hz, SH), 2.36 (s, 3 H, 2-CH3), 2.75 (dt, 2 H, J = 8.7, 6.9 Hz, 3-SCH2CH2SH), 3.20 (t, 1 H, J = 6.9 Hz, 3-SCH2CH2−SH), 5.04 (AB, 2 H, J = 12.6 Hz, 5-OCH2Ph), 5.32 (s, 1 H, 4-H), 6.14 (s, br, 1 H, NH), 7.05–7.41 (m, 15 H, aromatic H's). MS (EI): m/z 525 (M+), 432 (M+ – HSCH2−CH2S), 404 (M+ – HSCH2CH2SOC), 330 (MH+–CO2CH2−Ph-SCH2CH2SH, base), 91 (+CH2Ph). HRMS calcd for MH+: 526.1511. Found: 526.1466.
3-(2-Trimethylsilylethylthioethyl) 5-Benzyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (12)
1H NMR (CDCl3): 0.00 (m, 9 H, 3-SiCH3), 0.85 (m, 2 H, 3-SCH2CH2SiMe3), 2.34 (s, 3 H, 2-CH3), 2.60 (m, 2 H, 3-SCH2CH2SiMe3), 2.82 (m, 2 H, 3-CO2CH2-CH2SCH2CH2SiMe3), 4.33 (m, 2 H, 3-CO2CH2CH2SCH2-CH2SiMe3), 5.00 (AB, 2 H, J = 12.9 Hz, 5-OCH2Ph), 5.15 (s, 1 H, 4-H), 6.03 (s, br, 1 H, NH), 7.04–7.40 (m, 15 H, aromatic H's). MS (CI–NH3): m/z 609 (M+), 518 (M+ − CH2Ph), 448 (M+ – CH2CH2SCH2CH2SiMe3, base).
3-(2-Trimethylsilylethyl) 5-Ethyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (33)
1H NMR (CDCl3): 0.00 (m, 9 H, 3-SiCH3), 0.99 (t, 3 H, J = 7.8 Hz, 5-OCH2CH3), 1.12 (t, 2 H, J = 8.8 Hz, 3-OCH2CH2SiMe3), 2.57 (s, 3 H, 2-CH3), 4.02 (m, 2 H, 3-CO2CH2CH2SiMe3), 4.33 (t, 2 H, J = 7.8 Hz, 5-OCH2-CH3), 5.13 (s, 1 H, 4-H), 5.87 (s, br, 1 H, NH), 7.25–7.44 (m, 10 H, aromatic H's).
General Procedure for Preparation of 1,4-Dihydropyridine-3,5-dicarboxylate Esters 13–15, 32c–d, and 19–20 (Scheme 3)
Scheme 3. Synthesis of Sulfonyl-, Carboxy-, and Amine-Functionalized Congeners of the A3 Adenosine Receptor Selective Antagonists Based on Dihydropyridinesa.
a Reagents: (a) NBS; (b) CH3OCH2Cl, Et3N; (c) K2CO3, acetone, reflux; (d) 1 N HCl, acetone, 45 °C; (e) H2N(CH2)2NH2, rt., 4 h; (f) N-hydroxysuccinimidyl biotin, DMF, rt.
(Condition a) Compound 32a or 32b (0.04 mmol) was dissolved in dry acetone (2 mL) and anhydrous potassium carbonate (0.1 g), and the appropriate benzyl bromide (31a–d, 5 equiv.) was added, and the mixture was refluxed for 2–6 h. After filtering the mixture, the solvent was evaporated in vacuo. The residue was separated by preparative TLC (4:1 hexanes/EtOAc), and the desired N-protected 3- or 5-benzyl ester DHP was isolated. (Condition b): Deprotection was achieved by dissolving these N-ethoxymethyl-protected DHPs in acetone (1 mL) and adding excess 1 N HCl (200 μL) and heating at 45 °C for 2–3 h. The solvent was evaporated followed by purification with preparative TLC (4:1 hexanes/EtOAc or 10:1 CHCl3/MeOH) to afford the desired 3- or 5-benzyl ester DHP.
3-(4-Fluorosulfonylbenzyl) 5-Ethyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate (13)
1H NMR (CDCl3): δ 0.93 (t, 3H, J = 6.84 Hz, 5-CH2CH3), 2.39 (s, 3H, 2-CH3), 4.12 (q, 2H, J = 6.83 Hz, 5-OCH2), 5.20 & 5.58 (AB, 2H, J = 14.65 Hz, 3-OCH2), 5.21 (s, 1H, H-4), 6.23 (br, 1H, NH), 7.28–7.44 (m, 10H, 4-C6H5 and 6-C6H5), 7.71–7.89 (m, 4H, 3-C6H4).
3-(4-Sulfonylamidobenzyl) 5-Ethyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate (14)
1H NMR (CDCl3): δ 0.93 (t, 3H, J = 6.84 Hz, 5-CH2CH3), 2.38 (s, 3H, 2-CH3), 3.99 (m, 2H, 5-OCH2), 4.77 (br, 2H, NH2), 5.20 & 5.49 (AB, 2H, J = 13.67 Hz, 3-OCH2), 5.19 (s, 1H, H-4), 5.97 (br, 1H, NH), 7.27–7.84 (m, 14H, 3-C6H4, 4-C6H5 and 6-C6H5). HPLC retention time: 7.66 min (> 95% purity) using solvent system 0.1 M TEAA (pH 5.0)/CH3CN, 80:20 to 20:80, in 20 min with flow rate 1 mL/min.
3-[4-(2,2,2-Trichloroethoxycarbonyl])benzyl] 5-ethyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate (15)
1H NMR (CDCl3): δ 0.95 (t, 3H, J = 6.84 Hz, 5-CH2CH3), 2.38 (s, 3H, 2-CH3), 4.00 (m, 2H, 5-OCH2), 4.96 (s, 2H, CH2CCl3), 5.22 and 5.51 (AB, 2H, J = 14.65 Hz, 3-OCH2), 5.21 (s, 1H, H-4), 5.97 (br, 1H, NH), 7.25–8.07 (m, 14H, 3-C6H4, 4-C6H5 and 6-C6H5).
3-[4-(2-Aminoethyl)aminocarbonylbenzyl] 5-Ethyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate (16)
Compound 15 (13.4 mg, 0.02 mmol) was mixed with ethylenediamine (0.175 mL). After stirring at room temperature for 4 h, the ethylenediamine was evaporated and the product purified by preparative TLC (5:1 CHCl3/MeOH) to yield 7.0 mg of a white solid (59%). Compound 16 (Rf = 0.68, EtOAc) was shown to be pure by TLC.
1H NMR (CDCl3): δ 0.95 (t, 3H, J = 6.84 Hz, 5-CH2CH3), 2.37 (s, 3H, 2-CH3), 2.78 (s, 2H, NH2), 2.96 (t, 2H, J = 5.86 Hz, –CH2−), 3.50 (m, 2H, –CH2−), 4.00 (m, 2H, 5-OCH2), 5.21 and 5.45 (AB, 2H, J = 13.68 Hz, 3-OCH2), 5.20 (s, 1H, H-4), 5.92 (br, 1H, NH), 6.75 (br, 1H, –CH2NH-), 7.27–7.73 (m, 14H, 3-C6H4, 4-C6H5 and 6-C6H5).
3-(4-Carboxybenzyl) 5-Ethyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate (17)
Compound 32c [3-(4-methoxyethoxycarbonylbenzyl) 5-ethyl 2-methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate] was prepared according to the general procedure using 30 mg of 32a (0.067 mmol) and 86.8 mg of 31d (0.335 mmol). The crude product was dissolved in 2 mL of acetone. To 1 mL of such acetone solution was added 0.2 mL of 1 N HCl. The mixture was stirred at 45 °C for 2 h. The solution was removed in vacuo and residue was purified by preparative TLC (silica 60; 1000 μM, 10:1 CHCl3/MeOH) to give 13.2 mg of white solid, yield = 38%. Compound 17 (Rf = 0.17, MeOH) was shown to be pure by TLC.
1H NMR (CDCl3): δ 0.89 (t, 3H, J = 6.84 Hz, 5-CH2CH3), 2.38 (s, 3H, 2-CH3), 4.01 (m, 2H, 5-OCH2), 4.61 (s, 1H, OH), 5.21 and 5.49 (AB, 2H, J = 13.67 Hz, 3-OCH2), 5.21 (s, 1H, H-4), 5.96 (br, 1H, NH), 7.35–8.04 (m, 14H, 3-C6H4, 4-C6H5 and 6-C6H5).
3-[4-(2-d-biotinylaminoethyl)aminocarbonylbenzyl] 5-Ethyl 2-Methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate (18)
Compound 16 (6.1 mg, 0.01 mmol) and N-hydroxysuccinimide biotin (4.4 mg, 0.01 mmol) were dissolved in 0.5 mL of anhydrous DMF and stirred at room temperature overnight. The solvent was evaporated and the product was purified by preparative TLC [12:1 CHCl3/MeOH followed by 6:1 CHCl3/MeOH] to yield 5.6 mg of a yellow solid (64%). Compound 18 (Rf = 0.73, MeOH/EtOAc = 1/3) was shown to be pure by TLC.
1H NMR (CDCl3): δ 0.90 (t, 3H, J = 6.84 Hz, 5-CH2CH3), 1.32 (br, 1H, H-4′), 1.60 (t, 1H, J = 6.84 Hz, H-2′), 1.74 (br, 1H, H-3′), 2.17 (m, 4H, 2 X –CH2−), 2.37 (s, 3H, 2-CH3), 2.63 (d, 2H, J = 12.70 Hz, –CH2−5′), 2.82 (m, 2H, –CH2−;), 2.97 (m, 2H, –CH2−), 3.47 (br, 1H, NH), 3.55 (br, 1H, NH), 3.94 (q, 2H, J = 6.84 Hz, 5-OCH2), 4.12 (br, 2H, –CH2−), 4.39 (br, 2H, –CH2−), 5.16 (s, 1H, H-4), 5.21 and 5.40 (AB, 2H, J = 13.67 Hz, 3-OCH2), 5.60 (br, 1H, NH), 6.29 (br, 1H, NH), 6.54 (br, 1H, NH), 7.16–7.77 (m, 14H, 3-C6H4, 4-C6H5 and 6-C6H5).
3-Ethyl 5-(4-Fluorosulfonylbenzyl) 2-Methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate (19)
1H NMR (CDCl3): δ 1.36 (t, 3H, J = 6.83 Hz, 3-CH2CH3), 2.39 (s, 3H, 2-CH3), 4.30 (m, 2H, 3-OCH2), 5.04 and 5.13 (AB, 2H, J = 14.66 Hz, 5-OCH2), 5.19 (s, 1H, 4-H), 6.32 (br, 1H, NH), 7.23–8.00 (m, 14H, 4-C6H5, 5-C6H4, and 6-C6H5).
3-Ethyl 5-(4-Sulfonamidobenzyl) 2-Methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (20)
1H NMR (CDCl3): δ 1.35 (t, 3H, J = 6.84 Hz, 3-CH2CH3), 2.38 (s, 3H, 2-CH3), 4.29 (m, 2H, 3-OCH2), 4.74 (br, 2H, NH2), 5.10 and 5.18 (AB, 2H, J = 13.68 Hz, 5-OCH2), 5.18 (s, 1H, 4-H), 5.93 (br, 1H, NH), 7.16–7.83 (m, 14H, 4-C6H5, 5-C6H4, and 6-C6H5). HPLC retention time: 7.42 min (>95% purity) using solvent system 0.1 M TEAA (pH 5.0)/CH3CN, 80:20 to 20:80, in 20 min with flow rate 1 mL/min.
3-Ethyl 5-(4-Carboxybenzyl) 2-Methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate (23)
Compound 32d [3-ethyl 5-(4-methoxyethoxycarbonylbenzyl) 2-methyl-4-phenylethynyl-6-phenyl-1,4(±)-dihydropyridine-3,5-dicarboxylate] was prepared in quantitative yield according to the general procedure using 35 mg of 32b (0.0786 mmol) and 102 mg of 31d (0.392 mmol). An acetone solution (1 mL) of 25.3 mg (0.041 mmol) of 32d was mixed with 1 N HCl (0.15 mL), and the mixture was stirred at 45 °C for 2 h. The solution was removed in vacuo and residue was purified by preparative TLC (silica 60; 1000 μM, 10:1 CHCl3/MeOH) to give 10.9 mg of light yellow solid, yield ) 51%.
1H NMR (CDCl3): δ 1.34 (t, 3H, J = 6.84 Hz, 3-CH2CH3), 2.37 (s, 3H, 2-CH3), 4.26 (m, 2H, 3-OCH2), 5.00 & 5.15 (AB, 2H, J = 13.68 Hz, 5-OCH2), 5.17 (s, 1H, 4-H), 5.99 (br, 1H, NH), 7.07–7.92 (m, 14H, 4-C6H5, 5-C6H4, and 6-C6H5).
Synthesis of β-Ketoesters 26d–h (Scheme 2)
Scheme 2.
Synthesis of β-Ketoesters
β-Ketoesters 26d–h were prepared by the reaction of 2,2,6-trimethyl-4H-1,3-dioxin-4-one (27) and an alcohol or a thiol (eq 1 in Scheme 2). Equimolar amounts (for example, 3 mmol) of compound 27 and an alcohol or a thiol were heated with a little toluene (1–2 mL) at 80 °C in a sealed tube overnight. After cooled to room temperature, the solvent was removed under reduced pressure and the residue was chromatographed to give desired products in satisfactory yields (57% for 26d, 39% for 26e, 39% for 26f, 95% for 26g, 95% for 26h).
2-Hydroxyethyl Acetoacetate (26d)
1H NMR (CDCl3/ TMS): δ 2.29 (s, 3 H, CH3CO), 2.43 (s, br, 1 H, OH), 3.54 (s, 2 H, CH3COCH2CO), 3.84 (m, 2 H, CO2CH2CH2OH), 4.31 (t, J = 4.8 Hz, 2 H, CO2CH2CH2OH). MS (CI/NH3): 164 (M+ + NH4, base), 147 (M+ + 1), 129 (M+ − OH).
2-Mercaptoethyl Acetoacetate (26e)
1H NMR (CDCl3/ TMS): δ 1.54 (t, J = 8.7 Hz, 1 H, SH), 2.29 (s, 3 H, CH3-CO), 2.78 (dt, J = 8.7, 6.9 Hz, 2 H, CO2CH2CH2SH), 3.50 (s, 2 H, CH3COCH2CO), 4.28 (t, J = 6.9 Hz, 2 H, CO2CH2−CH2SH). MS (CI/NH3): 180 (M+ + NH4, base).
S-(2-Mercaptoethyl) 3-Oxothiobutyrate (26f)
1H NMR (CDCl3/TMS): δ 1.64 (t, J = 8.7 Hz, 1 H, SH), 2.28 (s, 3 H, CH3CO), 2.74 (dt, J = 8.7, 6.9 Hz, 2 H, COSCH2CH2−SH), 3.16 (t, J = 6.9 Hz, 2 H, COSCH2CH2SH), 3.70 (s, 2 H, CH3COCH2CO). MS (CI/NH3): 196 (M+ + NH4, base), 179 (M+ + 1).
(2-Trimethylsilylethylthio)ethyl Acetoacetate (26g)
1H NMR (CDCl3/TMS): δ 0.03 (s, 9 H, SiMe3), 0.86 (m, 2 H, Me3SiCH2CH2S), 2.28 (s, 3 H, CH3CO), 2.60 (m, 2 H,Me3−SiCH2CH2S), 2.78 (t, J = 6.8 Hz, 2 H, SCH2CH2O2C), 3.48 (s, 2 H, CH3COCH2CO), 4.29 (t, J = 6.8 Hz, 2 H, CO2CH2−CH2S). MS (CI/NH3): 280 (M+ + NH4), 161 (M+ − CH2−CH2SiMe3).
2-Trimethylsilylethyl Acetoacetate (26h)
1H NMR (CDCl3/TMS): δ 0.03 (s, 9 H, SiMe3), 1.01 (m, 2 H, Me3−SiCH2CH2), 2.26 (s, 3 H, CH3CO), 3.43 (s, 2 H, OCCH2−CO), 4.22 (m, 2 H, Me3SiCH2CH2O).
Preparation of p-Nitrobenzyl Benzoyl Acetate (26j) (eq 2 in Scheme 2)
Ethyl benzoyl acetate (26b, 1.92 g, 10 mmol) and p-nitrobenzyl alcohol (29, 1.53 g, 10 mmol) in toluene (10 mL) were heated with stirring for 24 h. The solvent was removed, and the residue was chromatographed [silica 60, petroleum ether-ethyl acetate (2:1)] to give 26j (2.08 g, yield ) 82%). 1H NMR (CDCl3/TMS): δ 4.11 (s, 2 H, PhCOCH2CO), 5.30 (s, 2 H, CH2C6H4NO2−p), 7.47–7.65 (m, 5 H, aromatic H's), 7.94 (d, J = 9.0 Hz, 2 H, aromatic H's), 8.20 (d, J = 9.0 Hz, 2 H, aromatic H's). MS (CI/NH3): 317 (M+ + NH4, base), 300 (M+ + 1).
Preparation of Bromides 31a and 31b (Scheme 3) (17)
A solution of p-toluenesulfonyl fluoride (30a, 1.0 equiv) or p-toluenesulfonamide (30b, 1.0 equiv), N-bromosuccinimide (1.0 equiv), and a catalytic amount of benzoyl peroxide dissolved in benzene (for example, 6 mmol of NBS using 10 mL of benzene) was stirred at 60 °C for 1 h. After the mixture cooled, the succinimide was filtered off and the filtrate chromatographed with pre parative TLC to yield the desired products (yield ) 65% for 31a and 62% for 31b).
p-(Bromomethyl)benzenesulfonyl Fluoride (31a)
1H NMR (CDCl3/TMS): δ 4.52 (s, 2 H, CH2), 7.65 (d, J = 7.8 Hz, 2 H, aromatic H's), 8.00 (d, J = 7.8 Hz, 2 H, aromatic H's).
(p-Bromomethyl)benzenesulfonamide (31b)
MS (EI): 249 and 251 (M+).
Preparation of Methoxymethyl 4-(Bromomethyl)-benzoate (31d) (Scheme 3)
α-Bromo-p-toluic acid (3.37 g, 15.7 mmol) was dissolved in DMF (10 mL). Chloromethyl methyl ether (1.26 g, 1.19 mL, 15.7 mmol) was then added, followed by adding triethylamine (1.9 g, 2.62 mL, 18.8 mmol). The reaction was stirred at room temperature overnight. Water (10 mL) was added, and the mixture was extracted with dichloromethane, washed with water, brine, and dried over MgSO4. Column chromatography (hexanes/ethyl acetate = 5/1) gave 0.588 g of light yellowish oil, yield = 15%. 1H NMR (CDCl3/TMS): δ 3.55 (s, 3 H, OCH3), 4.62 (s, 2 H, BrCH2), 5.50 (s, 2 H, OCH2O), 7.48 (d, J = 7.8 Hz, 2 H, aromatic H's), 8.08 (d, J = 7.8 Hz, 2 H, aromatic H's). MS (EI): 259 (M+).
Preparation of 1-Ethoxymethyl-2-methyl-5-ethoxycarbonyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3-carboxylic acid (32a) (Scheme 4)
Scheme 4.

Synthesis of a 3-Carboxylic Acid Dihydropyridine Intermediate for Alkylation, as Shown in Scheme 3
Sodium hydride (60% in mineral oil, 107 mg, 4.46 mmol) was added to compound 33 in solution of DMF (10 mL). The mixture was stirred for 30 min, chloromethyl ethyl ether (422 mg, 0.414 mL, 4.46 mmol) was added slowly to the solution under N2 at room temperature and stirred for 2h. The reaction was quenched by adding cold water (10 mL), extracted with ethyl acetate (10 mL × 2), the organic layer was washed with water (10 mL × 2), brine (10 mL × 2), dried with sodium sulfate. The solvent was evaporated and residue was purified with preparative TLC (silica gel 60, hexanes/EtOAc: 4/1) to give N-protected product 34 497 mg, yield = 49%. 1H NMR (CDCl3): 0.00 (m, 9 H, 3-SiCH3), 0.88 (t, 3 H, J = 6.8 Hz, 5-CH2CH3), 0.95 (t, 2 H, J = 6.8 Hz, 1-OCH2CH3), 1.12 (t, 2 H, J = 8.8 Hz, 3-OCH2CH2SiMe3), 2.62 (s, 3 H, 2-CH3), 3.15 (m, 1 H, 1-OCH2CH3), 3.69 (m, 1 H, 1-OCH2CH3), 3.92 (q, 2 H, J = 6.8 Hz, 5-OCH2CH3), 4.33 (t, 2 H, J = 6.8 Hz, 3-CO2CH2CH2SiMe3), 4.47 and 4.88 (AB, 2 H, J = 10.7 Hz, N-CH2O), 5.09 (s, 1 H, 4-H), 7.24–7.42 (m, 10 H, aromatic H's). MS (CI–NH3): m/z 563 (M+ + NH4), 546 (M+ + 1).
TBAF (hydrate, 2.49 mmol, 1 M solution in THF) was added to a solution of 34 (340 mg, 0.623 mmol) in DMF (2 mL). The mixture was stirred under N2 at room temperature for 2 h, diluted with ethyl acetate (20 mL), washed with 1 N HCl (5 mL), H2O (20 mL × 2) and brine (20 mL × 2), and dried with magnesium sulfate. The solvent was evaporated, and residue was separated with preparative TLC to give 271 mg of compound 32a as a yellow solid, yield = 98%. 1H NMR (CDCl3): δ 0.95 (m, 6 H, OCH2CH3x2), 2.67 (s, 3H, 2-CH3), 3.16 and 3.69 (m, 2 H, N–CH2OCH2CH3), 3.94 (q, 2 H, J = 6.8 Hz, 5-OCH2), 4.50 and 4.90 (AB, 2 H, N-CH2O), 5.13 (s, 1 H, 4-H), 7.18 (br, 1 H, COOH), 7.24–7.43 (m, 10 H, 2 × C6H5). MS (EI): m/z 445 (M+).
Pharmacology. Radioligand Binding Studies
Binding of [3H]R-N6-phenylisopropyladenosine ([3H]R-PIA) to A1 receptors from rat cerebral cortex membranes and of [3H]-2-[4-[(2-carboxyethyl)phenyl]ethylamino]-5′-N-ethylcarbamoyladenosine ([3H]CGS 21680) to A2A receptors from rat striatal membranes was performed as described previously (22, 23). Adenosine deaminase (3 units/mL) was present during the preparation of the brain membranes, in a preincubation of 30 min at 30 °C and during the incubation with the radioligands.
Binding of [125I]N6-(4-amino-3-iodobenzyl)-5′-N-methylcarbamoyladenosine ([125I]AB-MECA) to membranes prepared from HEK-293 cells stably expressing the human A3 receptor (2), clone HS-21a (Receptor Biology, Inc., Beltsville, MD) or to membranes prepared from CHO cells stably expressing the rat A3 receptor was performed as described (11, 24). The assay medium consisted of a buffer containing 10 mM Mg2+, 50 mM Tris, and 1 mM EDTA, at pH 8.0. The glass incubation tubes contained 100 μL of the membrane suspension (0.3 mg of protein/mL, stored at −80 °C in the same buffer), 50 μL of [125I]AB-MECA (final concentration 0.3 nM), and 50 μL of a solution of the proposed antagonist. Nonspecific binding was determined in the presence of 100 μM N6-phenylisopropyladenosine (R-PIA).
All nonradioactive compounds were initially dissolved in DMSO and diluted with buffer to the final concentration, where the amount of DMSO never exceeded 2%.
Incubations were terminated by rapid filtration over Whatman GF/B filters, using a Brandell cell harvester (Brandell, Gaithersburg, MD). The tubes were rinsed three times with 3 mL of buffer each.
At least five different concentrations of competitor, spanning 3 orders of magnitude adjusted appropriately for the IC50 of each compound, were used. IC50 values, calculated with the nonlinear regression method implemented in the Prism program (Graph-PAD, San Diego, CA), were converted to apparent Ki values using the Cheng–Prusoff equation (25) and Kd values of 1.0 and 14 nM for [3H]R-PIA and [3H]CGS 21680, respectively, and 0.59 nM for binding of [125I]AB-MECA at human A3 receptors, respectively.
For studies of irreversible binding, membranes were incubated with the indicated (Figure 2) concentration of ligand for 1 h at RT. Membranes were then washed 10 times by sequential resuspension and centrifugation with buffer (50 mM Tris, 10 mM Mg2+, and 1 mM EDTA) at pH 7.4 containing 0.02% CHAPS. Adenosine deaminase (3 units/mL) was present during all steps. The final pellet was suspended in a fixed volume of the above buffer solution for the A3 receptor binding assay as described.
Figure 2.

Irreversible inhibition of radioligand ([125I]ABMECA) binding to human A3 adenosine receptors in HEK-293 cell membranes, following a 1 h incubation with the sulfonyl fluoride derivative, 19 (n) 2−6).
RESULTS
Synthesis
The structures of the 1,4-dihydropyridines and related derivatives (1b–23) tested for affinity in radioligand binding assays at adenosine receptors are shown in Table 1. As in the previous studies (12, 14), the basic synthesis of the DHP nucleus consisted of the Hantzsch condensation (Scheme 1, reviewed in ref 19). This method involves a three component reaction of a 3-amino-2-propenoate ester, such as benzyl 3-amino-3-phenyl-2-propenoate (Scheme 1), 24a, an aldehyde, such as phenylpropargyl aldehyde, 25, and a β-ketoester, such as benzyl acetoacetate, 26a, that were dissolved in ethanol and refluxed. β-Ketoesters containing free hydroxyl and thio groups, 26d–f, for use in the unprotected form in the condensation reaction, were synthesized as shown (Scheme 2). A silyl-protected thiol, 26g, was also included.
The synthesis of DHP derivatives containing benzyl esters at the 3- and 5-positions, that were substituted with various acyl and sulfonyl groups, some of which were chemically reactive, was best approached through the alkylation of a free carboxylic acid using the corresponding benzyl bromide (Scheme 3). The benzyl bromides, 31a–c, were synthesized by bromination of the corresponding toluene derivatives, 30a–c, using N-bromosuccinimide. Use of compounds 30c and 31c in this context was described previously (12). The appropriate 3- or 5-carboxy derivative of the 6-phenyl-4-phenylethynyl-DHP was prepared by the methodology described (for the 5-carboxy derivative) in Jiang et al. (12). This approach incorporates a protecting group, a (2-trimethylsilylethyl)ester, at the 5-ester (12) or 3-ester (Scheme 4) position, that allows for facility of substitution of this ester and protection of the 1-position with the ethoxymethyl group (12). The 5-(2-trimethylsilylethyl)ester group was introduced at the stage of the Hantzsch condensation and subsequently removed using TBAF to provide 32b. The resulting free carboxylic acid was then esterified in high yield by means of alkylation using the appropriate benzyl bromide, 31, with potassium carbonate in acetone. The 1-ethoxymethyl group was finally removed upon heating at 45 °C for 1 h with 1 N HCl in acetone, to provide 5-position substituted benzyl esters, 19–21, or 3-position esters, 13–15.
Several additional modifications of functionality at the p-position of these benzyl esters were carried out. The 2,2,2-trichloroethyl derivatives (12), 15 and 21 (Scheme 3 and Table 1), were aminolyzed selectively using neat ethylenediamine at room temperature, to provide the amine functionalized congeners, 16 and 22, respectively. As demonstrated by Jiang et al. (12), the ester groups at the 3- and 5-positions of the DHP were not susceptible to reaction with ethylenediamine under these conditions. Also, the methoxymethyl groups of 33 and 34 were removed using 1 N HCl to provide the corresponding carboxylic acid derivatives, 17 and 23 (Table 1). One of the primary amine congeners, 16, was also acylated using a biotin-active ester to provide the biotin conjugate, 18.
Pharmacology
Compounds 2 and 3 are 3,5-diethyl ester derivatives of 6-phenyl-1,4-dihydropyridines previously shown to act as selective A3 receptor antagonists. Furthermore, the incorporation of a single benzyl ester at the 3- or 5-position was shown to enhance selectivity for the A3 subtype. Several related benzyl esters, 4 and 5, were included. As previously reported, the 3-benzyl ester, 4, is >600-fold selective, although not as potent as the 3,5-diethyl ester, 3, while the 5-benzyl ester, 1b, is twice as potent at A3 receptors as 3. Combining both benzyl esters, in 5, did not alter the receptor affinity of the 3-benzyl ester, 4. However, at the 2- and 6-positions, double substitution with phenyl groups, as in compounds 6 and 7, prevented binding to adenosine receptors. Transformation of the 3-ethyl ester of 1b into a β-methoxyethyl ester, 8a, enhanced affinity at A3 receptors by ~15-fold. Incorporation of a p-nitro group in the 5-benzyl ester substituent, 8b, which was shown previously to enhance A3 receptor affinity by roughly an order of magnitude (12), had no further enhancing effect on the affinity of the β-methoxyethyl ester. Incorporation of a β-hydroxyl group in the 3-ethyl ester substituent, 9, had an affinity-enhancing effect at A3 adenosine receptors. Curiously, a thiol group at the same position was much less favorable for affinity at A3 receptors, both for the 3-ester analogue, 10, and the corresponding 3-thioester, 11. A β-trimethylsilylethyl derivative, 12, only weakly bound to adenosine A3 receptors.
The structure activity relationships of p-substituted 3-and 5-ester positions were probed, as potential sites for attachment of functionalized chains. Ring substitution of the 5-benzyl ester with a 4-fluorosulfonyl group, 19, provided enhanced selectivity of >30000-fold for the A3 receptor, while similar substitution of the corresponding 3-benzyl ester, 13, was not affinity enhancing. The corresponding sulfonamides, 14 and 20, displayed only moderate potency and selectivity, with only minor affinity differences between 3- and 5-isomers. The corresponding 3- and 5- carboxylates, 17 and 23, were prepared, and only 17 bound to A3 receptors with moderate potency. A DHP containing a long amine-functionalized chain attached at the 3-position, 16, displayed moderate affinity, while the corresponding 5-isomer, 22, was 5-fold less potent at A3 receptors and nonselective.
Since an amine-functionalized chain attached at the 4-position of a benzyl ester at the 3-position of the DHP ring provided higher affinity A3 receptors, 16 was used as an intermediate in the synthesis of a biotin conjugate, 18. The biotin derivative showed >100-fold selectivity for human A3 receptors.
Unlike related derivatives not containing a reactive, electrophilic group, the sulfonyl fluoride derivative, 19, appeared to irreversibly bind to the A3 receptor in a concentration-dependent manner (Figure 2). A 1 h preincubation with this derivative resulted in the loss of binding in a subsequent experiment following exhaustive washing of the membranes. At 100 nM 19, approximately 56% of human A3 receptor radioligand binding sites in transfected HEK-293 cell membranes were irreversibly inhibited. As control experiments, similar preincubation with other A3 receptor antagonists was carried out. Preincubation with 8-p-sulfophenyltheophylline (10 μM), CGS 15943 (1 μM) (16), and MRS 1067 (1 μM) (12) failed to affect the level of subsequent A3 receptor radioligand binding. Preincubation with compound 1b, or its highly potent 4-nitrobenzyl derivative (12), at 100 nM did not significantly affect the level of subsequent A3 receptor radioligand binding (loss of <10% of total binding). Compound 13, another fluorosulfonyl derivative which bound considerably more weakly to human A3 receptors, resulted in only 11 and 62% irreversible inhibition of radioligand binding at 1 and 5 μM, respectively.
DISCUSSION
Previously, we have applied a “functionalized congener approach” (21) to the design of ligands for G protein-coupled receptors, such as adenosine receptors (12), muscarinic receptors (35), and nucleotide receptors (36). By this approach, an easily derivatized functional group is incorporated at the end of a strategically designed and attached chain-substituent Several of the derivatives in Table 1, such as the amines, 16 and 22, and the carboxylic acids, 17 and 23, represent easily reacted functionalized congeners of dihydropyridines, derivatives of which would retain moderate affinity and/or selectivity as A3 receptor antagonists.
The indication that the 5-ester position is the most flexible for substitution in relation to A3 receptor affinity has suggested the design of the amine-functionalized congener, 22. As in our previous studies of purines derivatized with long chains for the purpose of conjugating with other molecules while retaining the biological potency, this derivative may prove to be a key intermediate in the design of much higher molecular weight derivatives bearing “carrier” moieties, reporter groups, prosthetic groups, etc. The presence of the amino group in 22 also increased water solubility; unfortunately, it was not potent in binding at human A3 receptors.
Irreversible inhibitors of binding of A1 and A2A subtypes of adenosine receptors have been reported (26–29). Furthermore, an irreversibly binding A3 receptor agonist, MRS 1163, derived from IB-MECA was characterized (30). Among the pharmacological applications of such affinity labels are establishing the levels of spare receptors in native tissues, such as the heart (31–33). The irreversibly binding A3 receptor antagonists presented in this study may prove to be useful tools in the characterization of this receptor subtype. Since the DHP antagonists (12) are selective for the human A3 receptor vs other species, the design of irreversible ligands for A3 receptors in rat (14) and other species remains to be accomplished.
Supplementary Material
Table 2.
Characterization of Dihydropyridine Derivatives
| no | formula | MS | analysis | yield (%) |
|---|---|---|---|---|
| 5 | C36H29NO4 | 539 (EI) | HRMS | 11 |
| 6 | C36H29NO4 | 539 (EI) | HRMS | 40 |
| 7 | C41H31NO4·0.5H2O | 601 (EI) | C, H, N | 50 |
| 8a | C32H29NO5 | 507 (EI) | HRMS | 15 |
| 8b | C32H28N2O7 | 570 (CI, M+ + NH4) | C, H, N | 23 |
| 9 | C31H27NO5 | 493 (EI) | C, H, N | 72 |
| 10 | C31H27NO4S | 509 (EI) | C, H, N | 22 |
| 11 | C31H27NO3S2·1.0H2O | 525 (EI) | C, H, N | 16 |
| 12 | C36H39NO4SSi | 609 (CI) | HRMS | 28 |
| 13 | C31H26NFO6S·0.71EtOAc | 559 (EI) | C, H, N | 78 |
| 14 | C31H28N2O6S | 556 (EI) | HRMS(FAB) | 49 |
| 15 | C34H28NCl3O6 | 652 (CI) | C, H, N | 89 |
| 16 | C34H33N3O5 | 564 (FAB) | HRMS(FAB) | 59 |
| 17 | C32H27NO6 | 521 (EI) | HRMS(FAB) | 38 |
| 18 | C44H47N5O7S | 790 (CI) | HRMS(FAB) | 64 |
| 19 | C31H26NFO6S | 559 (EI) | C, H, N | 75 |
| 20 | C31H28N2O6S | 556 (EI) | HRMS(FAB) | 56 |
| 23 | C32H27NO6·0.92H2O | 521 (EI) | C, H, N | 51 |
ACKNOWLEDGMENT
We thank the Cystic Fibrosis Foundation (Silver Spring, MD) for financial support and Dr. Mark Olah and Dr. Gary L. Stiles of Duke University Medical Center for helpful discussions.
Footnotes
Supporting Information Available: Elemental analysis or HRMS data. This material is available free of charge via the Internet at http://pubs.acs.org.
Abbreviations: [125I]AB-MECA, [125I]N6-(4-amino-3-iodobenzyl)-5′-N-methylcarbamoyladenosine; CGS 21680, 2-[4-[(2-carboxyethyl)phenyl]ethyl-amino]-5′-N-ethylcarbamoyl-adenosine, CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]–1-propanesulfonate; CHO cells, Chinese hamster ovary cells; DHP, 1,4-dihydropyridine; DMAP, N,N-dimethylaminopyridine; DMSO, dimethyl sulfoxide; DPPA, diphenylphosphoryl azide; EDAC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; HEK cells, human embryonic kidney cells; IB-MECA, N6-(3-iodobenzyl)-5′-N-methylcarbamoyladenosine; Ki, equilibrium inhibition constant; MRS 1097, 3,5-diethyl 2-methyl-6-phenyl-4-[2-phenyl-(E)-vinyl]-1,4-(±)-dihydropyridine-3,5-dicarboxylate; MRS 1163, N6-(3-isothiocyanatobenzyl)-5′-N-methylcarbamoyladenosine; MRS 1191, 3-ethyl 5-benzyl 2-methyl-6-phenyl-4-phenylethynyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate; NBS, N-bromosuccinimide; R-PIA, R-N6-phenylisopropyladenosine; SAR, structure–activity relationship; TBAF, tetrabutylammonium fluoride; TMS, tetramethylsilane; Tris, tris(hydroxymethyl)aminomethane.
LITERATURE CITED
- (1).Jacobson KA, Suzuki F. Recent developments in selective agonists and antagonists acting at purine and pyrimidine receptors. Drug Dev. Res. 1997;39:289–300. doi: 10.1002/(sici)1098-2299(199611/12)39:3/4<289::aid-ddr8>3.0.co;2-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Zhou QY, Li CY, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor–The A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Jacobson KA. A3 adenosine receptors: Novel ligands and paradoxical effects. Trends Pharmacol. Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).von Lubitz DKJE, Lin RCS, Popik P, Carter MF, Jacobson KA. Adenosine A3 receptor stimulation and cerebral ischemia. Eur. J. Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).von Lubitz DKJE, Lin R-C, Boyd M, Bischofberger N, Jacobson KA. Chronic administration of adenosine A3 receptor agonist and cerebral ischemia: neuronal and glial effects. Eur. J. Pharmacol. 1999;367:157–163. doi: 10.1016/s0014-2999(98)00977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Strickler J, Jacobson KA, Liang BT. Direct preconditioning of cultured chick ventricular myocytes: novel functions of cardiac adenosine A2A and A3 receptors. J. Clin. Invest. 1996;98:1773–1779. doi: 10.1172/JCI118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Stambaugh K, Jacobson KA, Jiang J.-l., Liang BT. A novel cardioprotective function of adenosine A1 and A3 receptors during prolonged stimulated ischemia. Am. J. Physiol. 1997;273:H501–H505. doi: 10.1152/ajpheart.1997.273.1.H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Kohno Y, Sei Y, Koshiba M, Kim HO, Jacobson KA. Induction of apoptosis in HL-60 human promyelocytic leukemia cells by selective adenosine A3 receptor agonists. Biochem. Biophys. Res. Commun. 1996;219:904–910. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Shneyvays V, Nawrath H, Jacobson KA, Shainberg A. Induction of apoptosis in cardiac myocytes by an A3 adenosine receptors agonist. Exp. Cell Res. 1998;243:383–397. doi: 10.1006/excr.1998.4134. [DOI] [PubMed] [Google Scholar]
- (11).Karton Y, Jiang JL, Ji XD, Melman N, Olah ME, Stiles GL, Jacobson KA. Synthesis and biological-activities of flavonoid derivatives as as adenosine receptor antagonists. J. Med. Chem. 1996;39:2293–2301. doi: 10.1021/jm950923i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Jiang J.-l., van Rhee AM, Chang L, Patchornik A, Evans P, Melman N, Jacobson KA. Structure activity relationships of 4-phenylethynyl-6-phenyl-1,4-dihydropyridines as highly selective A3 adenosine receptor antagonists. J. Med. Chem. 1997;40:2596–2608. doi: 10.1021/jm970091j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Jacobson MA, Chakravarty PK, Johnson RG, Norton R. Novel selective nonxanthine selective A3 adenosine receptor antagonists. Drug Dev. Res. 1996;37:131. [Google Scholar]
- (14).Li A-H, Moro S, Melman N, Ji X.-d., Jacobson KA. Structure activity relationships and molecular modeling of 3,5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J. Med. Chem. 1998;41:3186–3201. doi: 10.1021/jm980093j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Van Muijlwijk-Koezen JE, Timmerman H, Link R, Van der Goot H, IJzerman AP. A novel class of adenosine A3 receptor ligands. II. Structure affinity profile of a series of isoquinoline and quinazolinecompounds. J. Med. Chem. 1998;41:3994–4000. doi: 10.1021/jm980037i. [DOI] [PubMed] [Google Scholar]
- (16).Kim Y-C, Ji X.d., Jacobson KA. Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. J. Med. Chem. 1996;39:4142–4148. doi: 10.1021/jm960482i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Beaven MA, Ramkumar V, Ali H. Adenosine-A3 receptors in mast-cells. Trends Pharmacol. Sci. 1994;15:13–14. doi: 10.1016/0165-6147(94)90124-4. [DOI] [PubMed] [Google Scholar]
- (18).von Lubitz DKJE, Lin RCS, Sei Y, Boyd M, Abbracchio M, Bischofberger N, Jacobson KA. A3 adenosine receptors and ischemic brain injury: a hope or a disaster? Drug Dev. Res. 1996;37:140. [Google Scholar]
- (19).Stout DM, Myers AI. Recent advances in the chemistry of dihydropyridines. Chem. Rev. 1982;82:223–243. [Google Scholar]
- (20).Goldmann S, Stoltefuss J. 1,4-Dihydropyridines: Effect of chirality and conformation on the calcium antagonist and calcium agonist activities. Angew. Chem., Int. Ed. Engl. 1991:1559–1578. [Google Scholar]
- (21).Jacobson KA. Molecular probes for adenosine receptors. In: Jacobson KA, Daly JW, Manganiello V, editors. Purines in Cellular Signaling: Targets for New Drugs. Springer; New York: 1990. pp. 54–64. [Google Scholar]
- (22).Schwabe U, Trost T. Characterization of adenosine receptors in rat brain by (−) [3H]N6-phenylisopropyladenosine. Naunyn-Schmiedeberg's Arch. Pharmacol. 1980;313:179–187. doi: 10.1007/BF00505731. [DOI] [PubMed] [Google Scholar]
- (23).Jarvis MF, Schutz R, Hutchison AJ, Do E, Sills MA, Williams M. [3H]CGS 21680, an A2 selective adenosine receptor agonist directly labels A2 receptors in rat brain tissue. J. Pharmacol. Exp.Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- (24).Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL. [125I]AB-MECA, a high affinity radioligand for the rat A3 adenosine receptor. Mol. Pharmacol. 1994;45:978–982. [PMC free article] [PubMed] [Google Scholar]
- (25).Cheng YC, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (IC50) of an enzyme reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- (26).Jacobson KA, Fischer B, Ji X-D. “Cleavable trifunctional” approach to receptor affinity labeling: regeneration of binding to A1-adenosine receptors. Bioconjugate Chem. 1995;6:255–263. doi: 10.1021/bc00033a004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Niiya K, Jacobson KA, Silvia SK, Olsson RA. Covalent binding of a selective agonist irreversibly activates guinea pig coronary artery A2 adenosine receptors. Naunyn-Schmeideberg's Arch. Pharamacol. 1993;347:521–526. doi: 10.1007/BF00166745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ji X-D, Gallo-Rodriguez C, Jacobson KA. 8-(3-Isothiocyanatostyryl)caffeine is a selective irreversible inhibitor of striatal A2-adenosine receptors. Drug Dev. Res. 1993;29:292–298. doi: 10.1002/ddr.430290407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Morey TE, Belardinelli L, Dennis DM. Validation of Furchgott's method to determine agonist-dependent A1-adenosine receptor reserve in guinea-pig atrium. Br. J. Pharmacol. 1998;7:1425–1433. doi: 10.1038/sj.bjp.0701747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ji X-D, Gallo-Rodriguez C, Jacobson KA. A selective affinity label for A3 adenosine receptors. Biochem. Biophys. Res. Commun. 1994;203:570–576. doi: 10.1006/bbrc.1994.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Dennis D, Jacobson K, Belardinelli L. Evidence of spare A1-adenosine in guinea pig atrioventricular node. Am. J. Physiol. 1992;262:H661–H671. doi: 10.1152/ajpheart.1992.262.3.H661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zhang J, Belardinelli L, Jacobson KA, Otero DH, Baker SP. Persistent activation of A1 adenosine receptors by an acylating adenosine derivative and its receptor reserve in DDT1 MF-2 cells and heart. Mol. Pharmacol. 1997;52:491–498. doi: 10.1124/mol.52.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Shryock JC, Snowdy S, Baraldi PG, Cacciari B, Spalluto G, Monopoli A, Ongini E, Baker SP, Belardinelli L. A2A-adenosine receptor reserve for coronary vasodilation. Circulation. 1998;7:711–718. doi: 10.1161/01.cir.98.7.711. [DOI] [PubMed] [Google Scholar]
- (34).Jacobson KA, Kirk KL, Padgett WL, Daly JW. Functionalized congeners of 1,3-dialkylxanthines: preparation of analogues with high affinity for adenosine receptors. J. Med. Chem. 1985;28:1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Karton Y, Baumgold J, Handen JS, Jacobson KA. Molecular probes for muscarinic receptors: Derivatives of the M1-antagonist telenzepine. Bioconjugate Chem. 1992;3:234–240. doi: 10.1021/bc00015a006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Fischer B, Boyer JL, Hoyle CHV, Ziganshin AU, Brizzolara AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA. Identification of potent, selective P2Y-purinoceptor agonists: Structure activity relationships for 2-thioether derivatives of adenosine-5′-triphosphate. J. Med. Chem. 1993;36:3937–3946. doi: 10.1021/jm00076a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.