Abstract
Our objective was to test the hypothesis that aberrantly modified forms of superoxide dismutase (SOD1) influence the disease course for sporadic amyotrophic lateral sclerosis (SALS). We probed for anti-SOD1 antibodies (IgM and IgG) against both the normal and aberrantly oxidized-SOD1 (SODox) antigens in sera from patients with SALS, subjects diagnosed with other neurological disorders and healthy individuals, and correlated the levels of these antibodies to disease duration and/or severity. Anti-SOD1 antibodies were detected in all cohorts; however, a subset of ~5–10% of SALS cases exhibited elevated levels of anti-SOD1 antibodies. Those SALS cases with relatively high levels of IgM antibodies against SODox exhibit a longer survival of 6.4 years, compared to subjects lacking these antibodies. By contrast, SALS subjects expressing higher levels of IgG antibodies reactive for the normal WT-SOD1 antigen exhibit a shorter survival of 4.1 years. Anti-SOD1 antibody levels did not correlate with disease severity in either the Alzheimer’s or Parkinson’s disease cohorts. In conclusion, the association of longer survival with elevated levels of anti-SODox antibodies suggests that these antibodies may be protective. By extension, these data implicate aberrantly modified forms of WT-SOD1 (e.g. oxidized SOD1) in SALS pathogenesis. In contrast, an immune response against the normal WT-SOD1 appears to be disadvantageous in SALS, possibly because the anti-oxidizing activity of normal WT-SOD1 is beneficial to SALS individuals.
Keywords: Sporadic amyotrophic lateral sclerosis, superoxide dismutase, immunotherapy, Parkinson’s disease, Alzheimer’s disease
Introduction
Amyotrophic lateral sclerosis (ALS) is the most common motor neuron disease, marked by progressive degeneration of motor neurons.1 Approximately 20–25% of familial ALS (FALS) cases are due to mutations in SOD1, of which more than 150 have been reported to date (http://alsod.iop.kcl.ac.uk/index.aspx). While 10% of ALS cases are classified as FALS, the vast majority of cases are sporadic in nature. Although FALS and SALS are clinically similar, the causative factors involved with SALS remain largely unknown.
An abundance of evidence supports the view that FALS-linked mutations alter the conformational stability of the SOD1 protein and render it neurotoxic through mechanisms that involve misfolding,2;3 mutant specific protein interactions,4 and/or altered subcellular localization.5 Recent studies suggest that similar cytotoxic mechanisms may be implicated in SALS as a consequence of adverse post-translational modifications of wild-type (WT) SOD1.6 For example, using a monoclonal conformation-specific antibody (C4F6),7 we showed that an oxidized form of WT-SOD1 (SODox)8 is conformationally similar to FALS-linked mutant SOD1 proteins, and that aberrant WT-SOD1 proteins are present in post mortem SALS spinal cord tissues.9 Moreover, SODox and FALS-linked mutant SOD1 proteins exert a similar toxic effect in an in vitro assay for fast-axonal transport.9;10 These observations suggest that a common link between FALS and SALS is the presence of toxic variants of the SOD1 protein, arising respectively from germline mutations and aberrant post-translational processing.
To further address the role of aberrantly modified WT-SOD1 in SALS pathogenesis, we performed an enzyme-linked, immunosorbant assay (ELISA) for the detection of anti-SOD1 antibodies against both normal WT-SOD1 and SODox, and determined whether the levels of these anti-SOD1 antibodies correlate with SALS patient survival. Although SOD1 is normally an intracellular protein, data indicate that mutant SOD1 interacts aberrantly with chromogranin proteins and is thereby secreted into the extracellular milieu where it is potentially accessible to the immune system.3 Since modified forms of WT-SOD1 (e.g. SODox) in SALS mimic mutant SOD1 in FALS, we hypothesized that the modified WT-SOD1 protein is also secreted, and that SALS individuals may generate antibodies against the modified protein. If the putative antibodies reduce the level and toxicity of the offending modified WT-SOD1, then the antibody levels are expected to correlate with phenotypic features of the disease. That these antibodies could be neuroprotective is suggested by immunization and immunotherapy studies in ALS mouse models,7;11 including a recent report demonstrating a neuroprotective effect in G93A-SOD1 transgenic mice that were vaccinated with misfolded WT-apo SOD1.12
Materials and methods
Human subjects
Sera from 298 ALS patients with probable or definite sporadic ALS according to revised El Escorial criteria, were obtained from the Clinical Trials Unit, Massachusetts General Hospital (MGH, Table 1). MGH also provided sera from 61 healthy controls (HC) and 13 disease controls with neurological disorders (OND) other than ALS. Additionally, the sera for 50 individuals with a definite diagnosis of Alzheimer’s disease (AD), 50 patients with Parkinson’s disease (PD) and 50 age-matched HC from the Harvard NeuroDiscovery Center Biomarker Study, Brigham and Women’s Hospital, Cambridge, MA, USA (HBS, Table 5) were obtained. All subjects signed an informed consent. More information is provided in the Supplementary Materials and methods, which are only available in the online version of the journal. Please find this material with the following direct link to the article: http://www.informahealthcare.com/ (DOI: 10.3109/17482968.2011.585163).
Table I.
Demographic characteristics of the study subjects from Massachusetts General Hospital
| SALS | Healthy controls | Other neurological diseases | |
|---|---|---|---|
| Number of subjectsa | 298 | 61 | 13 |
| Ratio white/other (%) | 92/8 | 97/3 | 85/15 |
| Ratio male/female (%) | 38/62b | 62/38 | 54/46 |
| Age of collection (mean years, ± SE) | 56.6 ± 3.5c | 47.3 ± 1.7 | 50.9 ± 4.0 |
Sera samples for all subjects reported in this table were obtained through the Clinical Trials Unit at Massachusetts General Hospital (Methods).
The difference in male/female ratio between the SALS and healthy control cohorts is statistically significant (p=0.0005) as determined by the Chi-square test.
The difference in mean age of serum sample collection (years) between the SALS and healthy control cohorts is statistically significant (p<0.0001) as determined by the Wilcoxon Z-test.
Statistically significant differences between cohorts are highlighted in bold.
Table V.
Mean serum anti-SOD1 antibody concentrations for Alzheimer’s disease, Parkinson’s disease, and healthy control cohorts.
| Cohorta | IgG, SODox | IgG, WT-SOD1 | IgM, SODox | IgM, WT-SOD1 | ||||
|---|---|---|---|---|---|---|---|---|
| μg/ml ± SE | number | μg/ml ± SE | number | μg/ml ± SE | number | μg/ml ± SE | number | |
| Alzheimer’s disease | 0.06 ± 0.03 | 50 | 0.20 ± 0.08 | 50 | 0.05 ± 0.02 | 50 | 0.13 ± 0.04 | 50 |
| Parkinson’s disease | 0.09 ± 0.07b | 50 | 0.16 ± 0.07 | 50 | 0.10 ± 0.04 | 50 | 0.16 ± 0.05 | 50 |
| Healthy controls | 0.04 ± 0.01 | 50 | 0.13 ± 0.03 | 50 | 0.12 ± 0.04 | 50 | 0.20 ± 0.05 | 50 |
Sera samples for all subjects reported in this table were obtained through the Harvard NeuroDiscovery Center (Methods).
This comparison is significant (denoted by bold font) with the Wilcoxon Z-test (p = 0.024), but not after Bonferroni correction, which requires an α=0.05/3=0.0167.
Production of SOD1 antigens
WT-SOD1 was expressed as a glutathione-S-transferase (GST)-fusion protein in Escherichia coli as described.13 GST-SOD1 was purified with glutathione- agarose (Sigma) according to the manufacturer’s instructions. Cleavage of the GST tag was accomplished with the PreScission Protease (GE Healthcare Life Sciences), and glutathione-agarose was subsequently employed to remove the protease and cleaved GST. Q-sepharose fast-flow was used as the final step in the purification of WT-SOD1 as described.14 Recombinant SODox was then prepared from purified WT-SOD1 as described.9
ELISA
Immulux medium binding flat bottom plates (Dynex Technologies, USA) were coated (50 μl/well) with 5 μg/ml WT-SOD1 or SODox in NaCO3 buffer (pH 9.6).15 Coated plates were incubated for 3 h at 37°C, after which they were stored at 4°C overnight, and then washed in 0.01 M phosphate-buffered saline (PBS, pH 7.4) with 0.05% Tween-20 (polysorbant-20, Fisher Scientific) (PBS-T) for 1 h to block the non-specific binding.16 Serial dilution of human serum was prepared in PBS-T and 50 μl of the diluted serum was added to SOD-WT or SODox-coated wells. The remainder of the assay was performed as described.17–20 A detailed description is given in the Supplementary Materials and methods, which are only available in the online version of the journal. Please find this material with the following direct link to the article: http://www.informahealthcare.com/ (DOI: 10.3109/17482968.2011.585163).
Statistical analysis
All statistical tests used in this study are listed in the tables and figures. Parametric methods were used in the analyses where distributional assumptions were met, using the SAS Statistical Package (SAS Institute Inc., V9.1). Where normality, variance, or sample size requirements were not met, comparable non-parametric methods available in SAS, the StatXact Statistical Package,21 or GraphPad Prism software were employed. For all analyses, p-values below 0.05 were considered significant. More information can be found in the Supplementary Materials and methods, which are only available in the online version of the journal. Please find this material with the following direct link to the article: http://www.informahealthcare.com/ (DOI: 10.3109/17482968.2011.585163).
Results
Anti-SODox IgG antibodies are elevated in SALS
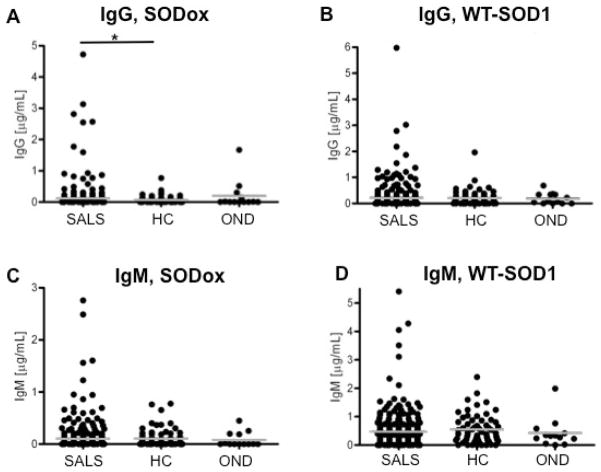
The first ELISA screen quantified anti-SOD1 antibodies against the normal WT-SOD1 and aberrantly oxidized SOD1 (SODox)8;9 antigens in sera obtained through the MGH Clinical Trial Unit (Table 1 and Methods). WT-SOD1 and SODox were used to probe levels of IgM antibodies, which are generated during the primary antibody response upon acute exposure to antigens, as well as IgG antibodies that are produced during the secondary antibody response.22 Anti-SOD1 antibodies were detected in all three cohorts (Figure 1, Table 2), and those cases with the highest concentrations of anti-SOD1 antibodies tested were consistently within the SALS cohort (Figure 1). Mean levels of anti-SODox IgG antibodies are significantly higher in SALS patient sera (0.11 μg/ml) compared to healthy controls (0.07 μg/ml; p = 0.03), with approximately 5–10% of subjects from the SALS cohort exhibiting anti-SODox IgG levels above those detected in the HC and OND cohorts.
Figure 1.
Serum antibody levels are elevated in sporadic amyotrophic lateral sclerosis (SALS) cases compared to healthy controls (HC) and subjects with other neurological disorders (OND). Sera samples for all subjects reported in this figure were obtained through the Clinical Trials Unit at Massachusetts General Hospital (Methods). Sera concentrations (μg/ml) of IgG antibodies against the SODox antigen (A) and WT-SOD1 antigen (B), and concentrations of IgM antibodies against the SODox antigen (C) and WT-SOD1 antigen (D), are shown in a dot-graph representation for the SALS, HC and OND cohorts. Each dot denotes the antibody concentration for a single subject; multiple subjects with the same concentration are represented by horizontal rows of dots. Mean antibody concentrations (Table 2) for the specified cohort are represented by gray bars. *Mean levels of anti-SODox IgG antibodies are significantly higher in SALS patient sera (0.11 μg/ml) compared to controls (0.07 μg/ml, p = 0.03).
Table II.
Mean serum anti-SOD1 antibody concentrations for SALS, healthy control and other neurological disease cohorts.
| Cohorta | IgG, SODox | IgG, WT-SOD1 | IgM, SODox | IgM, WT-SOD1 | ||||
|---|---|---|---|---|---|---|---|---|
| μg/ml ± SE | Number | μg/ml ± SE | Number | μg/ml ± SE | Number | μg/ml ± SE | Number | |
| SALS | 0.11 ± 0.03b | 298 | 0.23 ± 0.03 | 298 | 0.11 ± 0.02 | 298 | 0.48 ± 0.04 | 298 |
| Healthy controls | 0.07 ± 0.02 | 61 | 0.22 ± 0.04 | 61 | 0.11 ± 0.02 | 61 | 0.55 ± 0.07 | 61 |
| Other neurological disorders | 0.20 ± 0.13 | 13 | 0.19 ± 0.06 | 13 | 0.08 ± 0.04 | 13 | 0.43 ± 0.14 | 13 |
Sera samples for all subjects reported in this table were obtained through the Clinical Trials Unit at Massachusetts General Hospital (Methods).
The difference in mean levels of anti-SODox IgG antibodies between SALS and healthy control cohorts is statistically significant (p=0.03), which is indicated by the bold font, as determined by the Wilcoxon Z-test; the Bonferroni correction requires an α=0.05/3=0.0167 for statistical significance for this comparison.
We were able to detect SOD1 antigens by a western blot analysis, but only under native (non-denaturing) conditions (data not shown). Under native conditions the specific three-dimensional segment of the SOD1 antigen that is recognized by the antibody (conformational epitope) is conserved, as opposed to a denaturing western blot analysis that disrupts this conformation and only allows recognition of linear sequences. Thus, our findings indicate that human anti-SOD1 antibodies in these cases are reactive for a conformational epitope of SOD1. Native western blot analysis are inherently insensitive and the biological samples employed here were limited, and thus only those serum samples with the highest levels of anti-SOD1 antibodies were able to detect SOD1 antigens by a native western blot analysis (data not shown).
We subjected a subset (n = 7) of cerebrospinal fluid (CSF) samples to our ELISA, and included CSF from individuals who exhibited relatively high levels of serum anti-SOD1 antibodies. Anti-SOD1 antibodies were not detected in any of our CSF samples (data not shown). Since antibody levels in CSF are approximately 1000-fold lower than in human serum,23 it is likely that potential anti-SOD1 antibodies in our CSF samples are below the limit of detection of our ELISA.
Demographic analyses confirmed that race and gender are not confounders for the mean anti-SOD1 antibody levels (Table 1, Supplementary Tables 1, 2 – only available in the online version of the journal. Please find this material with the following direct link to the article: http://www.informahealthcare.com/ (DOI: 10.3109/17482968.2011.585163). Although the mean age for sera-sample collection is higher for the SALS compared to HC cohort (Table 1), this difference is not a confounder for the elevated levels of anti-SODox IgG detected in SALS because these antibody levels do not correlate with age of collection (Supplementary Table 2 – only available in the online version of the journal. Please find this material with the following direct link to the article: http://www.informahealthcare.com/ (DOI: 10.3109/17482968.2011.585163).
Moreover, in the healthy controls cohort, anti-SOD1 antibody levels do not correlate with age of collection. For the SALS cohort, we have also analyzed the interval between age of collection and onset of symptoms. The mean interval between these time-points was 2.1 years (± 0.11) and no correlation with the antibody level was detected.
Anti-SOD antibodies levels predict survival
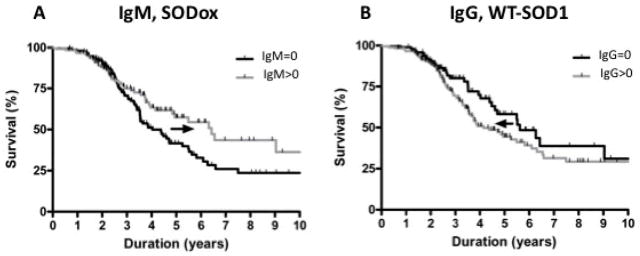
For those SALS subjects with detectable levels of anti-SOD1 antibodies, we sought to determine whether their antibody levels have an impact on the SALS disease course. First, we investigated whether anti-SOD1 antibody levels correlate with SALS disease duration (survival). The 153 (55%) SALS subjects that expressed SODox IgM antibodies (IgM > 0) were compared to the 127 (45%) SALS subjects without SODox IgM antibodies (IgM = 0) for which survival data were available, and the survival analysis (Figure 2A) revealed that subjects expressing SODox IgM antibodies have a longer survival of 6.4 years (CI 4.1–12.5) compared to those SALS subjects without SODox IgM antibodies, who have a mean survival of only 4.0 years (CI 3.5–5.5). This 2.4-year difference results in a hazard ratio of 1.3, and indicates that higher IgM SODox antibody levels may confer a survival advantage.
Figure 2.
Anti-SOD1 antibody levels predict disease duration in the SALS cohort. Sera samples for all subjects reported in this figure were obtained through the Clinical Trials Unit at Massachusetts General Hospital (Methods). (A) SALS subjects with SODox IgM antibodies (IgM >0; n = 153) exhibit significantly longer median survival (6.4 years; CI 4.1–12.5; gray curve) compared to subjects without SODox IgM antibodies (IgM = 0; n = 127) (median survival 4.0 years; CI 3.5–5.5; black curve). The parameter corresponding with IgM rank in a Cox proportional hazards model of survival had a p-value of 0.05. (B) SALS subjects with WT-SOD1 IgG antibodies (IgG >0; n = 182) exhibit significantly shorter median survival (4.1 years; CI 3.6–5.8; gray curve) compared to subjects without WT-SOD1 IgG antibodies (IgG = 0; n = 97) (median survival 5.6 years; CI 4.5–16; black curve). The parameter corresponding with IgG rank in a Cox proportional hazards model of survival had a p-value of 0.04. The p-value remained significant when age was included in the model. (A,B) Black arrows indicate the impact of elevated anti-WT SOD1 IgG or anti-SODox IgM antibody levels on survival.
In contrast, there is an inverse correlation between survival and WT-SOD1 IgG antibody levels. The 182 (65%) SALS subjects expressing WT-SOD1 IgG antibodies (IgG > 0) exhibit significantly shorter survivals than the 97 (35%) SALS subjects who lack WT-SOD1 IgG antibodies (IgG = 0). The median survival for SALS patients without WT-SOD1 IgG antibodies is 5.6 years (CI 4.5–16.0), whereas survival is only 4.1 years (CI 3.6–5.8) for subjects with WT-SOD1 IgG antibodies (Figure 2B). This 1.5-year difference in the mean survival corresponds to a hazard ratio of 0.6, and suggests that antibodies against the normal, WT-SOD1 protein may be disadvantageous to individuals with SALS.
We note the difference in antibody isotype (IgM vs. lgG) for the aforementioned antibodies. An analysis of IgM versus IgG antibody levels revealed an inverse correlation between anti-SODox IgM and IgG antibodies (Spearman’s rank = −0.46, p < 0.0001), and between WT-SOD1 IgM and IgG antibodies (Spearman’s rank = −0.13, p = 0.02). Thus, SALS individuals express elevated levels either of anti-SOD1 IgM or IgG antibodies, but rarely elevated levels of both antibody isotypes. The differential expression of anti-SOD1 IgM and IgG antibodies suggests that the immune repertoires of these SALS patients are bimodal, exhibiting either predominantly primary (IgM) or secondary (IgG) responses to SOD1 antigens.
Correlation of anti-SOD1 antibodies with age and site of SALS onset
Subsequently, we evaluated additional clinical features, including age of disease onset and site of onset, for SALS individuals expressing anti-SOD1 antibodies (IgG, IgM > 0) compared to individuals lacking these antibodies (IgG, IgM = 0). There was no significant difference in age of onset or site of onset for SALS individuals expressing anti-SODox and WT-SOD1 IgM antibodies compared to individuals lacking these antibodies (Table 3).
Table III.
Clinical parameters for the SALS cohort as a function of anti-SOD1 antibody levels.
| Antibody | Mean age of onsetb | Site of onsetc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ab>0 | Ab = 0 | Ab>0 | Ab = 0 | ||||||
| years | n | years | n | %limb/% bulbar | n | %limb/% bulbar | n | ||
| SALSa | IgG, SODox | 55.8 ± 1.0 (0.01) | 189 | 52.1 ± 1.1 | 106 | 72/28 | 86 | 81/19 | 204 |
| IgG, WT-SOD1 | 56.2 ± 0.9 (0.001) | 196 | 51.1 ± 1.3 | 99 | 78/22 | 194 | 76/20 | 96 | |
| IgM, SODox | 53.4 ± 1.1 | 136 | 55.4 ± 1.0 | 159 | 82/18 | 131 | 75/24 | 159 | |
| IgM, WT-SOD1 | 54.1 ± 0.7 | 272 | 59.1 ± 3.4 | 23 | 79/21 | 267 | 70/30 | 23 | |
Sera samples for all subjects reported in this table were obtained through the Clinical Trials Unit at Massachusetts General Hospital (Methods).
The mean age of onset is compared between SALS patients expressing the indicated antibody and SALS-patients without detectable antibodies.
The ratio of limb/bulbar onset is compared between SALS-patients with- and without detectable antibodies.
The Wilcoxon Z-test was used to determine statistically significant differences in mean age; for differences in site of onset a Fisher’s Exact Test was used. Significant comparisons are highlighted in bold, and the corresponding p-values are shown in parentheses within the ‘Ab>0’ column.
Sera samples containing anti-SODox and WT-SOD1 IgG antibodies corresponded to a mean age of onset of 55.8 ± 1.0 and 56.2 ± 0.9 years, respectively, whereas the respective mean age of onset for individuals lacking these antibodies was 52.1 ± 1.1 and 51.1 ± 1.3 years (Table 3). The age of onset for these four groups is similar to the mean age of onset (55 years) for this disease,24 and thus it is unlikely that the age differences alone could make a significant impact on the disease course. There were no significant differences for site of onset between groups expressing anti-SOD1 IgG antibodies and those that do not (Table 3).
Anti-SOD1 antibodies do not correlate with disease severity for OND
The small sample size and heterogeneous nature of the initial OND cohort precluded us from evaluating whether anti-SOD1 antibodies are significantly elevated in non-ALS cases. Therefore, a second ELISA screen was performed with sera from 50 AD, 50 PD, and 50 HC cohorts, obtained through the HBS (Methods and Table 4). A separate HC cohort was screened to account for potential differences in sample handling/storage between the MGH and HBS facilities. Anti-SOD1 antibodies were detected in all HBS cohorts (Table 5 and Supplementary Figure 1) as observed for the MGH cohorts (Figure 1, Table 2). The mean level of anti-SODox IgG antibodies is higher in PD patient sera (0.09 μg/ml) compared to HC (0.04 μg/ml, p = 0.02; Table 5). However, this statistical significance is dependent upon one case (IgG = 3.8 μg/ml; Supplementary Figure 1) and is not statistically significant after a Bonferroni correction (Table 5).
Table IV.
Demographic characteristics of the study subjects from the Harvard NeuroDiscovery Center
| Alzheimer’s disease | Parkinson’s disease | Healthy controls | |
|---|---|---|---|
| Number of subjectsa | 50 | 50 | 50 |
| Ratio male/female (%) | 54/46 | 54/46 | 54/46 |
| Age of collection (mean years, ± SE) | 74.8 ± 1.1 | 74.6 ± 1.1 | 74.6 ± 1.1 |
Sera samples for all subjects reported in this table were obtained through the Harvard NeuroDiscovery Center (Methods). There is no significant difference in male/female ratio or mean age of serum sample collection (years) between subjects with Alzheimer’s disease, Parkinson’s disease or healthy controls, as determined by Chi-square test and the Wilcoxon Z-test respectively.
When we combined data from both ELISA screens, we observed that cases with the highest (top 10%) anti-SOD1 antibodies are consistently within the SALS cohort (n = 298) relative to all other cohorts (n = 224) (Supplementary Figure 2 and Supplementary Table 3 – only available in the online version of the journal. Please find this material with the following direct link to the article: http://www.informahealthcare.com/ (DOI: 10.3109/17482968.2011.585163).
In contrast to the correlations observed between SALS survival and anti-SOD1 antibodies (Figure 2), there are no statistically significant correlations between anti-SOD1 antibodies and AD or PD disease severity, as measured by the Mini Mental State Exam (MMSE) and the lower Unified Parkinson’s Disease Rating Scale (UPDRS), respectively. Therefore, the clinical impact of anti-SOD1 antibodies is more relevant to SALS survival than to the disease severity measures of other neurodegenerative diseases.
Discussion
Aberrant WT-SOD1 is associated with SALS
SOD1 mutations acquired through Mendelian inheritance play an unequivocal role in FALS pathogenesis, and recent lines of evidence suggest an analogous role for WT-SOD1 in the more common SALS. In vitro studies document that aberrant post-translational modifications to the WT-SOD1 proteins, including oxidation,8;9;25 de-metallation,26;27 disulfide reduction,28 and loss of dimer-subunit integrity29 can induce the WT-SOD1 protein to acquire mutant-like properties. Moreover, misfolded WT-SOD1 has been detected in post mortem human SALS spinal cord tissues by immunohistochemistry.5;9 Vaccination against WT-apo SOD1 has been shown to reduce the load and toxic effects of FALS-linked SOD1-G93A in the respective transgenic ALS mouse model,12 further demonstrating that modified WT- and mutant-SOD1 proteins share a similar ‘toxic’ conformation.
Our ELISA screen revealed that the mean levels of anti-SODox IgG antibodies are significantly higher in SALS patient sera (Table 2). We note that while the significance of this result is diminished with a Bonferonni correction, the correlations between anti-SODox antibodies and SALS both in Figure 1 and the survival analysis (discussed below) provide cumulative evidence that implicate modified WT-SOD1 species in SALS pathogenesis. That anti-SOD1 antibodies are not detected in all SALS samples suggests that modified WT-SOD1 may be involved in a subset of SALS cases, consistent with previous immunohistochemical results.9
Anti-SOD1 antibody production and antibody isotypes
Since SOD1 is intracellular, it is somewhat surprising a priori that the immune system should mount a response to this protein. At least three factors may be important in this regard. First, it is conceivable that the process of cell death simply releases the SOD1 protein within the CNS, which would account for the presence of anti-SOD1 antibodies in healthy control cohorts, as well as in the disease (SALS and OND) cohorts. Secondly, recent data indicate that SOD1 is actively secreted.30;31 The neurosecretory chromogranin proteins promote the selective secretion of mutant SOD1 over WT-SOD1 in cultured cells.3 In light of the observation that SODox mimics FALS-linked SOD1 mutants,8;9 enhanced secretion of SODox or other modified WT-SOD1 species by chromogranins may explain the elevated levels of anti-SODox IgG antibodies detected in our SALS cohort (Table 2). Finally, a third factor that may contribute to the genesis of anti-SOD1 antibodies is neurodegenerative disease-dependent loss of integrity of the blood-brain and blood-spinal cord barriers, which has been documented in ALS mouse models.32
Given that there appears to be a robust humoral immune response to SOD1 in ALS, can we understand the patterns of immunoglobulin isotypes that we detect? Our ELISAs reveal that SALS subjects differentially express IgG and IgM anti-SOD1 antibodies, indicative of an isotype class-switch. In addition, younger SALS subjects express higher levels of anti-SOD1 IgM antibodies, whereas older subjects express higher levels of anti-SOD1 IgG antibodies (Supplementary Table 2 – only available in the online version of the journal. Please find this material with the following direct link to the article: http://www.informahealthcare.com/ (DOI:10.3109/17482968.2011.585163).
A longitudinal study, like that reported by Zhang et al.,33 is needed to further address the possibility that the differential expression of IgM and IgG antibodies reflects early and late stages, respectively, of a SALS immune response to SOD1.
The impact of anti-SOD1 antibodies on disease course
Anti-SOD1 antibodies can potentially play a therapeutic or pathogenic role in SALS. Both active and passive immunization with the recombinant SOD1 antigens have delayed disease onset and/or prolonged survival in ALS mouse models.7;12 Intracerebroventricular infusion of monoclonal anti-SOD1 antibodies also prolongs the lifespan of ALS mice.11 These studies support a therapeutic role for anti-SOD1 antibodies. By contrast, several reports have described aberrant accumulation34;35 and toxicity of IgG isolated from SALS samples. For instance, purified human ALS IgG is toxic to neural cells in culture,36;37 and induces profound ultrastructural changes to spinal motor neurons when injected into mice.38
Our results are consistent with the view that some categories of anti-SOD1 antibodies may attenuate the rate of progression of ALS. Specifically, SALS subjects with anti-SODox IgM antibodies survive longer than subjects lacking these antibodies (Figure 2A). A possible mechanism for this beneficial influence is that anti-SODox IgM antibodies reduce SODox levels in the CNS, which in turn permits longer survival, reminiscent of the studies by Urushutani et al..7 Therefore, the correlation between anti-SODox antibodies and prolonged survival further supports the hypothesis that aberrant WT-SOD1 proteins exhibit toxicity in SALS pathogenesis. In contrast, WT-SOD1 IgG antibody levels correlate with reduced survival for the SALS cohort (Figure 2B). By analogy, it may be disadvantageous to reduce levels of WT-SOD1, which may play a role in ameliorating the high levels of oxidative stress associated with SALS.39
The role of anti-SOD1 antibodies: SALS versus non-SALS
An important issue in understanding the biological significance of the anti-SOD1 antibodies we have detected is their specificity. Our data clearly show that anti-SOD1 antibodies are present in subjects with other neurological diseases, and to a lesser extent in healthy control subjects. While anti-SOD1 antibodies correlate with SALS survival, they do not appear to impact disease severity measures associated with AD and PD. The results of our study indicate that the influence of anti-SOD1 antibodies may be more significant to ALS than other neurodegenerative diseases.
Conclusion
We found that auto-antibodies against an aberrant form of SODox are associated with SALS, and that levels of anti-SOD1 antibodies correlate with ALS disease duration. These findings have important therapeutic implications in light of the observations that aberrant WT-SOD1 proteins are associated with SALS pathogenesis, and that immunotherapeutic approaches against aberrant SOD1 proteins exhibit beneficial effects in mice. Therefore, it may be possible to implement immunotherapeutic strategies against misfolded SOD1 for both SALS and FALS.
Supplementary Material
Acknowledgments
We thank Anne Hunt and the Eunice Kennedy Shriver Center at the University of Massachusetts Medical School (UMMS) for statistical analyses, the UMMS Proteomic and Mass spectrometry Core facility at UMMS, the Northeast ALS Consortium and the Neurology Clinical Trial Unit for contributing sera samples. We acknowledge financial support from the ALS Therapy Alliance-CVS Pharmacy (D.A.B.), the ALS Association (D.A.B., and R.H.B. Jr), the US National Institutes of Health (D.A.B. (National Institute on Neurological Disorders and Stroke)), R.H.B. Jr (National Institute on Neurological Disorders and Stroke), the Angel Fund (R.H.B. Jr) and Project ALS (R.H.B. Jr).
References
- 1.Bruijn LI, Houseweart MK, Kato S, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998 Sep 18;281(5384):1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 2.Chattopadhyay M, Valentine JS. Aggregation of Copper-Zinc Superoxide Dismutase in Familial and Sporadic ALS. Antioxid Redox Signal. 2009;11:1603–1614. doi: 10.1089/ars.2009.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 4.Magrane J, Hervias I, Henning MS, Damiano M, Kawamata H, Manfredi G. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18:4552–4564. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsberg K, Jonsson PA, Andersen PM, et al. Novel antibodies reveal inclusions containing non-native SOD1 in sporadic ALS patients. PLoS One. 2010;5:e11552. doi: 10.1371/journal.pone.0011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruzman A, Wood WL, Alpert E, et al. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2007;104:12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urushitani M, Ezzi SA, Julien JP. Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2007;104:2495–2500. doi: 10.1073/pnas.0606201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezzi SA, Urushitani M, Julien JP. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J Neurochem. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- 9.Bosco DA, Morfini G, Karabacak NM, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morfini GA, Burns M, Binder LI, et al. Axonal transport defects in neurodegenerative diseases. J Neurosc. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gros-Louis F, Soucy G, Lariviere R, Julien JP. Intracerebroventricular infusion of monoclonal antibody or its derived Fab fragment against misfolded forms of SOD1 mutant delays mortality in a mouse model of ALS. J Neurochem. 2010;113:1188–1199. doi: 10.1111/j.1471-4159.2010.06683.x. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi S, Fujiwara N, Ido A, et al. Induction of protective immunity by vaccination with wild-type apo superoxide dismutase 1 in mutant SOD1 transgenic mice. J Neuropathol Exp Neurol. 2010;69:1044–1056. doi: 10.1097/NEN.0b013e3181f4a90a. [DOI] [PubMed] [Google Scholar]
- 13.Ray SS, Nowak RJ, Strokovich K, Brown RH, Jr, Walz T, Lansbury PT., Jr An intersubunit disulfide bond prevents in vitro aggregation of a superoxide dismutase-1 mutant linked to familial amytrophic lateral sclerosis. Biochemistry. 2004;43:4899–4905. doi: 10.1021/bi030246r. [DOI] [PubMed] [Google Scholar]
- 14.Hayward LJ, Rodriguez JA, Kim JW, et al. Decreased metallation and activity in subsets of mutant superoxide dismutases associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2002;277:15923–15931. doi: 10.1074/jbc.M112087200. [DOI] [PubMed] [Google Scholar]
- 15.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972;109:129–135. [PubMed] [Google Scholar]
- 16.Steinitz M. Quantitation of the blocking effect of tween 20 and bovine serum albumin in ELISA microwells. Anal Biochem. 2000;282:232–238. doi: 10.1006/abio.2000.4602. [DOI] [PubMed] [Google Scholar]
- 17.McQuillen DP, Gulati S, Ram S, et al. Complement processing and immunoglobulin binding to Neisseria gonorrhoeae determined in vitro simulates in vivo effects. J Infect Dis. 1999;179:124–135. doi: 10.1086/314545. [DOI] [PubMed] [Google Scholar]
- 18.Ngampasutadol J, Rice PA, Walsh MT, Gulati S. Characterization of a peptide vaccine candidate mimicking an oligosaccharide epitope of Neisseria gonorrhoeae and resultant immune responses and function. Vaccine. 2006;24:157–170. doi: 10.1016/j.vaccine.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 19.Gnehm HE, Pelton SI, Gulati S, Rice PA. Characterization of antigens from nontypable Haemophilus influenzae recognized by human bactericidal antibodies. Role of Haemophilus outer membrane proteins. J Clin Invest. 1985;75:1645–1658. doi: 10.1172/JCI111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice PA, Vayo HE, Tam MR, Blake MS. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986;164:1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta C, Patel N. Software CS. StatXact8. Cambridge, MA: 2008. [Google Scholar]
- 22.Rakhit R, Cunningham P, Furtos-Matei A, et al. Oxidation-induced misfolding and aggregation of superoxide dismutase and its implications for amyotrophic lateral sclerosis. J Biol Chem. 2002;277:47551–47556. doi: 10.1074/jbc.M207356200. [DOI] [PubMed] [Google Scholar]
- 23.Durazo A, Shaw BF, Chattopadhyay M, et al. Metal-free superoxide dismutase-1 and three different ALS variants share a similar partially unfolded {beta}-barrel at physiological temperature. J Biol Chem. 2009;284:34382–34389. doi: 10.1074/jbc.M109.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estevez AG, Crow JP, Sampson JB, et al. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 25.Lindberg MJ, Normark J, Holmgren A, Oliveberg M. Folding of human superoxide dismutase: disulfide reduction prevents dimerization and produces marginally stable monomers. Proc Natl Acad Sci U S A. 2004;101:15893–15898. doi: 10.1073/pnas.0403979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A. Monomeric Cu, Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J Biol Chem. 2004;279:15499–15504. doi: 10.1074/jbc.M313295200. [DOI] [PubMed] [Google Scholar]
- 27.Cimini V, Ruggiero G, Buonomo T, et al. CuZn-superoxide dismutase in human thymus: immunocytochemical localisation and secretion in thymus-derived epithelial and fibroblast cell lines. Histochem Cell Biol. 2002;118:163–169. doi: 10.1007/s00418-002-0429-8. [DOI] [PubMed] [Google Scholar]
- 28.Mondola P, Ruggiero G, Seru R, et al. The Cu, Zn superoxide dismutase in neuroblastoma SK-N-BE cells is exported by a microvesicles dependent pathway. Brain Res Mol Brain Res. 2003;110:45–51. doi: 10.1016/s0169-328x(02)00583-1. [DOI] [PubMed] [Google Scholar]
- 29.Garbuzova-Davis S, Saporta S, Haller E, et al. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS One. 2007;2:e1205. doi: 10.1371/journal.pone.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang R, Gascon R, Miller RG, et al. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS) J Neuroimmunol. 2005;159:215–224. doi: 10.1016/j.jneuroim.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Donnenfeld H, Kascsak RJ, Bartfeld H. Deposits of IgG and C3 in the spinal cord and motor cortex of ALS patients. J Neuroimmunol. 1984;6:51–57. doi: 10.1016/0165-5728(84)90042-0. [DOI] [PubMed] [Google Scholar]
- 32.Engelhardt JI, Appel SH. IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol. 1990;47:1210–1216. doi: 10.1001/archneur.1990.00530110068019. [DOI] [PubMed] [Google Scholar]
- 33.Demestre M, Pullen A, Orrell RW, Orth M. ALS-IgG-induced selective motor neurone apoptosis in rat mixed primary spinal cord cultures. J Neurochem. 2005;94:268–275. doi: 10.1111/j.1471-4159.2005.03184.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith RG, Alexianu ME, Crawford G, Nyormoi O, Stefani E, Appel SH. Cytotoxicity of immunoglobulins from amyotrophic lateral sclerosis patients on a hybrid motoneuron cell line. Proc Natl Acad Sci U S A. 1994;91:3393–3397. doi: 10.1073/pnas.91.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pullen AH, Humphreys P. Ultrastructural analysis of spinal motoneurones from mice treated with IgG from ALS patients, healthy individuals, or disease controls. J Neurol Sci. 2000;180:35–45. doi: 10.1016/s0022-510x(00)00427-5. [DOI] [PubMed] [Google Scholar]
- 36.Fridovich I. Superoxide anion radical (O2−), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.