Abstract
Detailed amino acid sequence analyses of A1 and A2a adenosine receptors were assembled by analogy to other G-protein-coupled receptors and correlated with pharmacological observations. Sites for phosphorylation, palmitoylation, and sodium binding have been proposed. Striatal A2a receptors from human and other species were photoaffinity-labeled using the selective, radioiodinated agonist PAPA-APEC. Selective chemical affinity labels for A1 and A2a receptors have been introduced. For example, an isothiocyanate, p-DITC-APEC (100 nM), irreversibly diminished the Bmax for [3H]CGS 21680 (2-[4-[(2-carboxyethyl) phenyl] ethylamino]-5’-N-ethylcarboxamidoadenosine) binding in rabbit striatal membranes by 71% (Kd unaffected), suggesting a direct modification of the ligand binding site. Novel trifunctional affinity labels have been designed. Rabbit and human A2a receptors were characterized using [3H]XAC binding in the presence of 50 or 25 nM CPX (8-cyclopentyl-l,3-dipropylxanthine), respectively. The inhibition of A2 radioligand binding by the histidyl-modifying reagent diethylpyrocarbonate suggested the involvement of His residues in interactions with adenosine agonists and antagonists. Properties of transiently expressed mutants of bovine A1 receptors in which either His251 or His278 residues have been substituted with Leu suggest that both histidines are important in binding.
Keywords: affinity labeling, sequence analysis, xanthines, chemical modification, mutagenesis
INTRODUCTION
Adenosine acts as a neuromodulator through at least two receptor subtypes [reviewed in Jacobson et al., 1992a; van Galen et al., 1992; Stiles, 1992; Linden, 19911, A1 and A2. A2 receptors have been further divided into A2a (high agonist affinity, in striatum) and A2b (low agonist affinity, in fibroblasts) receptors. A number of effector mechanisms (cAMP, inositol phosphates, ion channels) are activated by adenosine, the best known being adenylate cyclase (inhibited by A1 and activated by A2). Several developments within the past few years have enabled a rigorous examination of the molecular structure and regulation of adenosine receptors: (1) the synthesis of adenosine derivatives, as agonists, and xanthine and non-xanthine antagonists with receptor subtype selectivity; and (2) the cloning of both A1 and A2a adenosine receptors [Maenhaut et al., 1990; Libert et al., 1991; Mahan et al., 1991; Reppert et al., 1991; Olah et al., 1992]. The cloning of both A1 and A2 adenosine receptors was a fortuitous achievement. The identity of the sequences RDC7 and RDC8, cloned from a dog thyroid cDNA library using the PCR technique through homology to other G-protein-linked receptors, was initially unknown and later found to resemble A1 and A2a receptors, respectively, in radioligand binding and effects on adenylate cyclase. Canine A1 and A2a receptors bear a 51% degree of homology to one another within transmembrane domains. Our goal is to define the parameters of the binding and receptor activation by purines using a variety of means, including synthetic, genetic, spectroscopic, and computational methodology.
Molecular Probes for Adenosine Receptors
We have synthesized new adenosine receptor ligands using a “functionalized congener approach” [Jacobson and Daly, 1991], by which potential positions for attachment of chains on a pharmacophore are empirically probed. The site of attachment must correspond to a region of relaxed steric requirements at or near the receptor binding site. This strategy has allowed us to target accessory sites of favorable interaction on the receptor, and actually enhance the affinity of the ligands. We have used such congeners to make fluorescent probes, superpotent lipid conjugates, affinity labels, etc. Molecular weights in the range of 34,000–40,000 Daltons have been measured for A1 receptors [Stiles, 1992; Linden, 1991]. A2a receptors have been labeled using the iodinated agonist PAPA-APEC (p-aminophenylacetyl conjugate of 2-[(2-aminoethylamino)carbonylethylphenylethylamino]-5’-N-ethylcarboxamidoadenosine) (Fig. 1) [Barrington et al., 1990; Nanoff et al., 1991], suggesting a molecular weight of 45,000 Daltons (bovine striatum). The measured molecular weights of the receptor proteins are in good agreement with the values calculated from sequences (36 K and 45 K for A1- and A2a - receptors, respectively). The rabbit (striatum), rat (PC12 cells), and frog (erythrocyte) A2a receptors are of molecular weight 44–47K, and hamster (DDT1-MF2 cells) A2a receptors appear to be 40K. In membranes containing the rabbit A2a receptors, cleavage of a 7,000 molecular weight fragment was observed in the absence of inhibitors of enzymatic proteolysis. This segment most likely corresponds to the long C-terminal tail found in the A2a but not in the A1 receptor. The receptor 38K fragment still binds ligands appropriately; however, the ability of guanine nucleotides to modulate the coupling to G-protein is enhanced following cleavage.
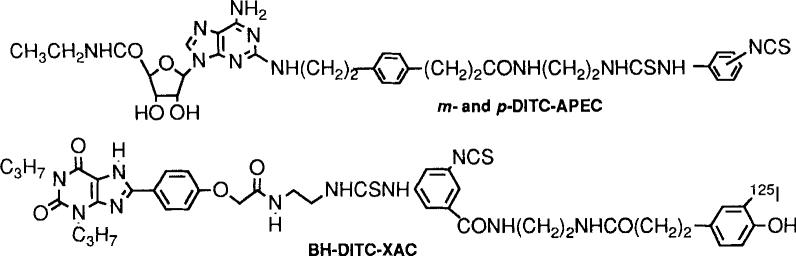
Figure 1.
Structures of isothiocyanate-bearing affinity labels for adenosine receptors. BH-DITC-XAC and the DITC-APEC isomers are selective for A1- and A2-receptors, respectively.
We have characterized the human A2a receptor through radioligand binding and photoaffinity labeling [Ji et al., 1992]. A truly selective A2a antagonist radioligand is lacking; however, one may use [3H] XAC (8-[4-[[[[(2-aminoethyl)amino]carbonyl]methyl]-oxy]phenyl]-1,3-dipropylxanthine) for certain species, such as primates and rabbit in which the A2a receptors have particularly high affinity for 8-phenyl xanthines. Although A1-selective in the rat brain, [3H]XAC binds to human striatal A2a receptors with high affinity (Kd = 2.98 nM). Twenty-five nM CPX (8-cyclopentyl-1,3-dipropylxanthine), an A1-selective antagonist, in the incubation medium effectively eliminated 91% of [3H]XAC (1 nM) binding to human A1 receptors, yet preserved 90% of binding to A2 receptors. [3H]XAC exhibited saturable, binding to A2a sites with a Kd of 2.98 nM and a Bmax of 0.71 pmol/mg protein (25°C, nonspecific binding defined with 0.1 mM NECA (5’-N-ethylcarboxamidoadenosine)). The potency order for antagonists against 1 nM [3H]XAC was CGS15943A > XAC ≈ PD115,199 > PAPA-XAC > CPX > HTQZ ≈ XCC ≈ CP-66,713 > theophylline ≈ caffeine, indicative of A2a receptors. A2a adenosine receptors were detected in the human cortex, albeit at a much lower density (~5%) than in the striaturn. Photoaffinity labeling using 125I-PAPA-APEC revealed a molecular weight of 45K with 43K and 37K proteolytic fragments also observed. In the absence of proteolytic inhibitors, the 37K fragment, which still bound 125I-PAPA-APEC, was predominant.
Isothiocyanate derivatives of adenosine have been developed as selective affinity labels for A1 and A2 adenosine receptors [Jacobson et al., 1992b]. An amine functionalized congener, APEC (2-[(2-aminoethylamino) carbonylethylphenylethylamino]-5’-N-ethylcarboxamido-adenosine), is a selective agonist at A2a adenosine receptors [Hide et al., 1992]. As a means of covalently inhibiting this receptor binding site, APEC was coupled to m- and p-phenylene-di-isothiocyanate (DITC). The resulting isothiocyanate derivatives in preincubation with rabbit or bovine striatal membranes irreversibly inhibited radioligand binding at A2a but not A1 receptors. Inhibition was prevented by theophylline (antagonist) or NECA (agonist). p-DITC-APEC (100 nM) diminished the Bmax for [3H]CGS 21680 binding by 71% (Kd unaffected), suggesting a direct modification of the ligand binding site. Bromoacetyl-APEC was ineffective as an affinity label. Selective inhibitors are potentially of interest in studies of the physiological role of adenosine receptors.
A new radioiodinated xanthine (adenosine antagonist) that binds covalently to A1 adenosine receptors was prepared via a general “trifunctional approach” to receptor ligands and used as a receptor probe [Jacobson et al., 1992c]. BH-DITC-XAC was synthesized via reaction of XAC with a trifunctional aryl diisothiocyanate crosslinker, containing the p-hydroxyphenylpropionyl group (“BH”, Bolton-Hunter reagent) for radioiodination. Following reaction, a remaining isothiocyanate group reacts with the receptor protein. 125l-BH-DlTC-XAC, prepared directly by the chloramine T method and purified by HPLC, bound specifically to A1 receptors. This binding was inhibited in the presence of the adenosine agonists R-PIA, S-PIA, and NECA in a dose-dependent manner and with the order of potency characteristic of bovine A1 receptors. Incubation of affinity-purified bovine A1 receptors (prepared using a XAC-Sepharose affinity column) with 1251-BH-DITC-XAC (0.8 nM) for 2 hours resulted in the specific and clean labeling of a polypeptide band corresponding to MW 36,000, identical to that previously found for the A1 receptor.
Sequence Analysis of Adenosine Receptors and Correlation With Pharmacological Observations
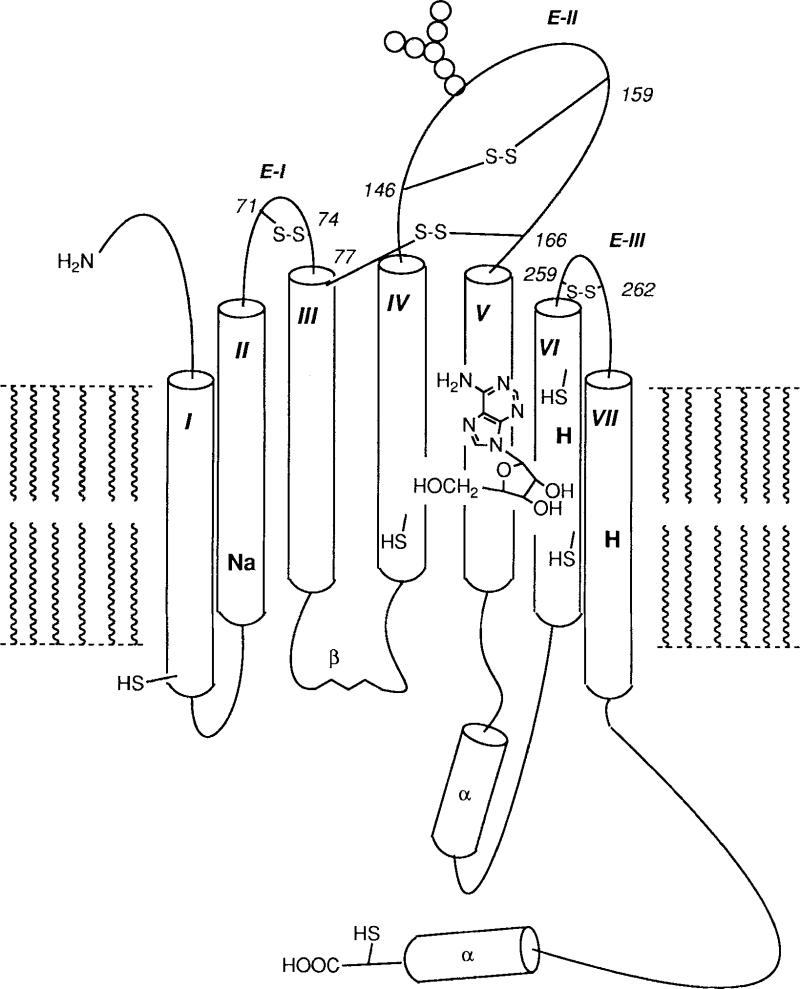
Detailed models of A1 and A2a receptors (Fig. 2) consistent with labeling experiments have been assembled [van Galen et al., 1992] from an amino acid sequence analysis and by analogy to other G-protein-coupled receptors. These models conform with the seven transmembrane domain topology commonly found for receptors linked to G-proteins.
Figure 2.
A proposed model for canine A2a receptors deduced from the primary sequence, including seven transmembrane helices (I–VII), three extracellular loops (E-I–III), two histidinyl residues possibly involved in ligand binding (H), a sodium binding site (Na), and hypothetical disulfide bonds (S-S, residue numbers in italics) and thiol groups. Thiol of Cys82 is not shown.
Glycosy lation
Rat A1 receptors are N-glycosylated with a complex carbohydrate chain, and glycosylation of the A2 receptor is heterogeneous in nature, containing carbohydrate chains of both the complex and the high-mannose types [Barrington et al., 1990]. Asparagine residues that are potential glycosylation sites are located on E-II in both the A1 and A2 sequences. Asn159 is a potential substrate for glycosylation in the A1 receptor. For A2 receptors, two extracellular glycosylation consensus sites were identified (Asn145 and Asn154 on E-II), but glycosylation likely occurs only at Asn154.
Receptor phosphorylation
Desensitization of a number of G-protein-linked receptors is associated with phosphorylation by: (1) second-messenger-activated kinases like protein kinase A (CAMP-dependent protein kinase; PKA) and protein kinase C (PKC) (leading to heterologous desensitization) or (2) receptor-“specific” kinases, such as β-adrenoceptor receptor kinase (βARK) and rhodopsin kinase, that phosphorylate their substrates only in the agonist-occupied state of the receptor (implicated in homologous desensitization). The A1 receptor in DDT1MF-2 cells is phosphorylated in response to agonist [Ramkumar et al., 1991], but A2 phosphorylation has not yet been studied.
The A1 and A2 sequences display a number of consensus patterns for phosphorylation by PKA, PKC or by casein kinase II (CK2), that has so far not been implicated in the phosphorylation of G-protein-coupled receptors. The carboxyl terminus of the A2 receptor is rich in serine and threonine, suggestive of phosphorylation by βARK.
Acylation
Many G-protein-linked receptors contain a cysteine residue in the C-terminal tail close to H-VII, that is palmitoylated and essential for G-protein coupling in adrenergic receptors and in rhodopsin, but not the m2-muscarinic receptor [Van Koppen and Nathanson, 1991]. The palmitoyl group would, in effect, create a fourth cytoplasmic loop by anchoring the cytoplasmic tail to the membrane. The role of an analogous cysteine residue present in the carboxy tail of the A1 receptor (Cys309), but not the A2a receptor, is yet to be determined.
Disulfide bonds
Disulfide bonds between extracellular cysteines are probably involved in maintaining the integrity of the ligand binding site of muscarinic [Savarese et al., 1992] and other G-protein-linked receptors, although these cysteines may not be part of the binding site itself. This is suggested by the susceptibility of ligand binding to dithiothreitol (DTT), a disulfide reducing agent, and by site-directed mutagenesis studies. The analogous cysteines in the adenosine receptor sequences are Cys80 - Cys169 (A1) and Cys77 - Cys166 (A2). Non-intracellular cysteines are abundant in A1 and especially A2 receptors (7 and 11 cysteines, respectively). Other potential candidates for the formation of disulfide bonds include Cys260-Cys263 (A1) and Cys71 - Cys74, Cys146 - Cys159 and Cys259 - Cys262, or any combination thereof (A2). A disulfide bridge between two cysteines four residues apart would likely stabilize the β-turns predicted for E-II (A2), E-III (A1 and A2) and which might also occur in E-I(A2).
We have shown that [3H]CGS 21680 binding to A2 receptors is disrupted following treatment with the disulfide-reactive reagents mercaptoethanol (> 50 mM) or DTT or dithionite (> 10 mM) [Jacobson et al., 1992b]. This suggests that disulfide linkages indeed are involved in maintaining the structural integrity of the A2 receptor.
Sodium regulatory site
The sequence [SN]-L-A-x-[AT]-D occurs near the cytoplasmic end of H-II in both A1 and A2 receptors [Van Galen et al., 1992] and in many other G-protein-linked receptors. In α2-adrenergic receptors, the Asp residue of the analogous sequence is important in the allosteric modulation of agonist binding by Na+. Since agonist binding to both adenosine receptors is similarly regulated by Na+, the Asp55 (A1) and Asp52 (A2) may constitute the site of sodium binding.
Ligand binding site
The binding sites for structurally diverse nonpeptidic ligands, such as rhodopsin, norepinephrine, and acetylcholine, that bind to G-protein-coupled receptors is thought to occur roughly midway along the transmembrane helices, near the center of the cavity. A negatively charged aspartate residue on H-III, that is conserved in all receptors that are activated by biogenic amines, is thought to act as the counter-ion for the positively charged amine of these ligands. Unlike the biogenic amines, adenosine is uncharged at physiological pH, and an anionic residue at this position would offer no electrostatic advantage in binding. The specific aspartate residue corresponds to an uncharged (Val87) residue in A1 receptors.
The inhibitory effects of diethylpyrocarbonate (DEP, a His-modifying reagent) in pharmacological studies with wild type receptors have implicated two histidines in interactions with adenosine agonists and antagonists (shown for both A1 [Klotz et al., 1988] and A2a [Jacobson et al., 1992b] receptors). Exposure of membranes to DEP (2.5 mM) followed by washing was found to inhibit the binding of [3H]CGS 21680 and [3H]XAC to A2a receptors, by 86% and 30%, respectively. NECA protected against inhibition of [3H]CGS 21680 binding better than did theophylline, and the converse was found for [3H]XAC binding. There are two conserved histidine residues in transmembrane domains VI and VII (His251 and His278 in A1 receptors) that are likely the putative histidine residues affected by DEP.
These two histidyl residues have each been replaced with leucyl residues by site-specific mutation of bovine A1 receptors [Olah et al., 1992] and expression in COS7 cells. Leu278 decreased both agonist and antagonist binding by > 90%. In contrast, Leu251 decreased antagonist but not agonist affinity, and a decreased receptor number was found for both agonist and antagonist binding.
In collaboration with Dr. Ad IJzerman, we have recently devised a preliminary molecular model for the binding of adenosine to the A1 receptor [IJzerman et al., 1992], that is consistent with the observed pharmacology, structure activity relationships, and calculated ligand conformation. This model may be used to predict sites for interaction between specific amino acid residues of the receptor and its ligands, and may be tested in studies of mutagenesis of the receptor. Eventually the objective will be to synthesize improved ligands, including chemical affinity labels and possibly ligands of high selectivity for other subtypes (e.g., A2b) [Stehle et al., 1992], consistent with the model.
REFERENCES
- Barrington WW, Jacobson KA, Stiles GL. The glycoprotein nature of the A2 adenosine receptor binding subunit. Mol Pharmacol. 1990;38:177–183. [PMC free article] [PubMed] [Google Scholar]
- Hide I, Padgett WL, Jacobson KA, Daly JW. A2a-Adenosine receptors from rat striatum and rat pheochromocytoma PC12 cells: Characterization with radioligand binding and by activation of adenylate cyclase. Mol Pharmacol. 1992;41:352–359. [PMC free article] [PubMed] [Google Scholar]
- IJzerman AP, van Galen PJM, Jacobson KA. Molecular modeling of adenosine receptors. I. The ligand binding site on the A1 receptor. Drug Design and Discovery. 1992;9:49–67. [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Daly JW. Purine functionalized congeners as molecular probes for adenosine receptors. Nucleosides and Nucleotides. 1991;10:1029–1038. [Google Scholar]
- Jacobson KA, van Galen PJM, Williams M. Perspective, adenosine receptors: Pharmacology, structure activity relationships and therapeutic potential. J Med Chem. 1992a;35:407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Stiles GL, Ji X-D. Chemical modification and irreversible inhibition of striatal A2a-adenosine receptors. Mol Pharmacol. 1992b;42:123–133. [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Olah ME, Stiles GL. Trifunctional ligands: A radioiodinated high affinity acylating antagonist for the A1 adenosine receptor. Pharmacol Comm. 1992c;1:145–154. [PMC free article] [PubMed] [Google Scholar]
- Ji X-D, Stiles GL, van Galen PJM, Jacobson KA. Characterization of human striatal A2-adenosine receptors using radio-ligand binding and photoaffinity labeling. J Receptor Res. 1992;12:149–169. doi: 10.3109/10799899209074789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz K-N, Lohse MJ, Schwabe U. Chemical modification of A1 adenosine receptors in rat brain membranes. Evidence for histidine in different domains of the ligand binding site. J Biol Chem. 1988;263:17522–17526. [PubMed] [Google Scholar]
- Libert F, Schiffmann SN, Lefort A, Parmentier M, Gerard C, Dumont JE, Vanderhaeghen J-J, Vassart G. The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J. 1991;10:1677–1682. doi: 10.1002/j.1460-2075.1991.tb07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J. Structure and Function of A1-Adenosine Receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- Maenhaut C, Van Sande J, Libert F, Abramowicz M, Parmentier M, Dumont JE, Vassart G, Schiffmann S. RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem Biophys Res Comm. 1990;173:1169–1178. doi: 10.1016/s0006-291x(05)80909-x. [DOI] [PubMed] [Google Scholar]
- Mahan LC, McVittie LD, Smyk-Randall EM, Nakata H, Monsma FJ, Gerfen CR, Sibley DR. Cloning and expression of an A1 adenosine receptor from rat brain. Mol Pharmacol. 1991;40:1–7. [PubMed] [Google Scholar]
- Nanoff C, Jacobson KA, Stiles GL. The A2 adenosine receptor: Guanine nucleotide modulation of agonist binding is enhanced by proteolysis. Mol Pharmacol. 1991;39:130–135. [PMC free article] [PubMed] [Google Scholar]
- Olah ME, Ren H, Ostrowski J, Jacobson KA, Stiles GL. Cloning, expression, and characterization of the unique bovine A1-adenosine receptor: Studies on the ligand binding site by site directed mutagenesis. J Biol Chem. 1992;267:10764–10770. [PMC free article] [PubMed] [Google Scholar]
- Ramkumar V, Olah ME, Jacobson KA, Stiles GL. Desensitization of both A1 and A2 adenosine receptors in DDT1 MF-2 smooth muscle cells. Mol Pharniacol. 1991;40:639–647. [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Stehle JH, Rivkees SA. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- Savarese TM, Wang CD, Fraser CM. Site-directed mutagenesis of the Ratml muscarinic acetylcholine receptor: Role of conserved cysteines in receptor function. J Biol Chem. 1992;267:11439–11448. [PubMed] [Google Scholar]
- Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, Reppert SM. Molecular cloning adn expression of the cDNA for a novel A2-adenosine receptor subtype. Mol Endocrinol. 1992;6:384–393. doi: 10.1210/mend.6.3.1584214. [DOI] [PubMed] [Google Scholar]
- Stiles GL. Adenosine receptors. J Biol Chem. 1992;267:6451–6454. [PubMed] [Google Scholar]
- Van Galen PJM, Stiles GL, Michaels G, Jacobson KA. Adenosine A1 and A2 receptors: Structure-function relationships. Medicinal Research Reviews. 1992;12:423–471. doi: 10.1002/med.2610120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Koppen CJ, Nathanson NM. The cysteine residue in the carboxylterminal domain of the m2-muscarinic acetylcholine receptor is not required for receptor-mediated inhibition of adenylate cyclase. J Neurochem. 1991;57:1873. doi: 10.1111/j.1471-4159.1991.tb06397.x. [DOI] [PubMed] [Google Scholar]