Abstract
Most studies linking obesity and periodontal disease have been cross-sectional in design. We examined whether gains in body weight, waist circumference, and arm fat area are associated with periodontitis progression in 893 non-diabetic men followed for up to four decades in the prospective VA Dental Longitudinal Study. Probing pocket depth (PPD) was measured by calibrated examiners. Repeated-measures generalized linear models estimated the mean cumulative numbers of teeth with PPD events (PPD > 3 mm) at each dental examination and the slopes associated with increasing numbers of affected teeth over time. Means were adjusted for baseline PPD, education, and cigarette pack-years, and time-dependent values of age, mean plaque score, cigarette packs/day, brushing, and flossing. Men who were overweight at baseline and gained weight most rapidly (> 0.19 kg/yr or ~15 lb during follow-up) had significantly more PPD events than men in the lowest tertile of weight gain (≤ -0.05 kg/yr). Overweight men whose waist circumference increased > 0.14–0.39 or > 0.39 cm/yr experienced more PPD events than men in the lowest tertile (≤ 0.14 cm/yr). Increase in arm fat area was associated with disease progression in normal-weight men. These results suggest that tracking adiposity changes with easily obtained anthropometric measures may help predict risk of periodontitis progression.
Keywords: overweight, obesity, body mass index, waist circumference, adiposity, periodontal disease
Introduction
Chronic periodontal disease affects approximately 35% of US adults over age 30 yrs and is a primary cause of tooth loss. Risk assessment studies have identified the inclusion of age, male gender, smoking, and diabetes mellitus as several subject-level characteristics associated with periodontal disease severity and progression (Al-Shammari et al., 2005). A recent meta-analysis concluded that increased body mass index (BMI), waist circumference (WC), and other obesity indices are significantly associated with higher prevalence of periodontal disease (Suvan et al., 2011).
To date, the association of obesity and periodontal disease has been examined in largely cross-sectional studies. Our previous paper (Gorman et al., 2012) used a prospective design to demonstrate a potentially causal relationship between obesity status and future risk of periodontitis. Risk of moderate to severe periodontitis over a 27-year period, as measured by probing depth, clinical attachment loss, or alveolar bone loss, was 40 to 72% greater in obese men compared with normal-weight men. Furthermore, a high waist-circumference-to-height ratio (> 50%) modestly increased periodontitis risk even among normal-weight and overweight men. It is not known how changes in adiposity, regardless of initial weight status, affect periodontitis incidence and progression. The primary aim of this study was to examine whether a relationship exists between periodontitis and changes in body composition in the Dental Longitudinal Study (DLS) participants.
Materials & Methods
Participants
Participants were 893 white males in the Department of Veterans Affairs (VA) Dental Longitudinal Study, a closed-panel, observational cohort study of aging and oral health among healthy men (Kapur et al., 1972). Oral health, weight, medical health, and lifestyle were assessed at triennial dental and medical examinations. Data used in the present study were collected between 1968 and 1998. While most participants are veterans, they are not patients in the VA healthcare system and receive dental and medical care from the private sector. Of the 1,231 men initially enrolled, 338 were excluded from the current analyses for the following reasons: edentate at baseline (n = 73), no follow-up examinations (n = 109), missing all anthropometric and covariate data (n = 11), and developed cancer (other than non-melanoma skin cancer, n = 76) or diabetes (n = 69) during follow-up. The institutional review boards of the VA Boston Healthcare System and Boston University Medical Campus approved the protocol. All participants gave informed consent on approved forms before each examination.
Dental Examinations
Probing pocket depth (PPD) was measured by calibrated examiners (Gorman et al., 2012). At the first 6 examinations, only the maximum PPD per tooth was recorded on a 4-point interval scale (0 = ≤ 2 mm; 1 = > 2- ≤ 3 mm; 2 = > 3- < 5 mm; 3 = ≥ 5 mm). At subsequent examinations, all values were recorded in mm but were categorized according to the earlier scheme for data analysis. During the entire follow-up, three calibrated periodontists serially served as examiners (see Appendix 1). Interexaminer agreement of PPD was 41% and kappa was 0.26, based on repeat assessments on 25 participants (Feldman et al., 1982, 1985; Fleiss and Chilton, 1983; Alman et al., 1986). After participants rinsed with a disclosing solution, presence or absence of supragingival plaque was determined on 4 sites (Feldman et al., 1986), and the worst site was recorded. Third molars were excluded from all analyses.
Presence of periodontitis at baseline was defined as 4 or more teeth with PPD > 3 mm (Burt, 2005). PPD progression was described by the cumulative number of teeth at each examination that had ever experienced a PPD event. To avoid underestimating PPD progression, we counted 2 distinct outcomes as PPD events: PPD > 3 mm, or tooth loss, provided the tooth showed periodontal involvement (PPD > 2 mm but ≤ 3 mm) prior to loss. Seventeen percent of teeth with PPD > 3 mm at any examination reverted to a lower score and remained lower until the last available examination (i.e., would never have been counted as an event).
Radiographic alveolar bone loss (ABL) was measured as described in Appendix 1.
Anthropometry
At each medical examination, height (inches) was measured on a stadiometer and weight (pounds) on a balance beam scale, and converted to metric units for computation of BMI (kg/m2). Waist circumference (WC, cm) was measured with an inelastic tape measure at the narrowest part of the torso at the end of a normal expiration as the participant stood erect, relaxed, with arms at the sides and feet together. Triceps fatfold (TSF, mm) measurements were measured twice, and the average was recorded. Midarm circumference (MAC, mm) was measured at the same site as TSF. Arm fat area (AFA, cm2) was computed as (Heymsfield et al., 1999).
BMI was categorized according to accepted cut-off values: normal-weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (> 30 kg/m2). Two men whose BMI was < 18.1 kg/m2 at baseline were excluded from further analyses. We computed annual rates of change in body weight, WC, and AFA by subtracting the last available measurement from the baseline and dividing by the number of intervening yrs. These rates were then categorized into tertiles.
Other Information
Participants completed questionnaires concerning oral hygiene and smoking habits. Baseline pack-years of cigarette exposure were computed as average packs/day times number of yrs smoked. Educational status was collected at baseline.
Statistical Methods
One-way ANOVA with a post hoc Tukey test and the Chi-square statistic were used to determine differences in periodontitis measures, adiposity indices, and participant characteristics among baseline BMI categories. Repeated-measures generalized linear models estimated the adjusted mean cumulative number of teeth with PPD events at each dental examination. Means were adjusted for baseline periodontal disease (< or ≥ 4 teeth with PPD > 3 mm), education (high school or less, some college, college graduate), baseline pack-years of cigarette exposure, age, whole-mouth mean plaque score, brushing frequency (never, once/day, 2+ times/day), flossing frequency (< once/wk, once/wk or more), and current cigarette packs/day (none, ½, 1+); the latter 5 variables were treated as time-varying covariates. An auto-regressive working correlation matrix was specified to account for the correlation of observations within individuals over time. Because interaction terms for baseline BMI and rates of change in body weight, WC, and AFA were statistically significant, all models were stratified by baseline BMI category. Differences in beta coefficients (slopes) of PPD progression were tested with the F statistic.
Several sensitivity analyses were performed. The models were repeated in a subset of participants who attended all 9 examinations (n = 216), and it was found that patterns of PPD progression and differences in overall means were no different than when drop-outs were included in the analyses (Appendix 2). Alternate definitions of baseline periodontitis and PPD progression were also examined, with no appreciable differences in the findings (Appendix 3). All analyses were conducted with SAS 9.1 (Cary, NC, USA).
Analyses were repeated with ABL progression as the outcome (Appendix 1).
Results
Baseline Data
Baseline characteristics of participants stratified by baseline BMI category are summarized in the Table. WC and AFA trended significantly higher, while brushing and flossing frequencies were lower, in the overweight and obese groups. WC, number of teeth, and number of teeth with PPD > 3 mm were significantly different between the normal-weight and obese categories.
Table.
Characteristics of 893 Dental Longitudinal Study Participants by Baseline BMI Category
| Variable | Normal-weight (BMI 18.5-24.9 kg/m2) | Overweight (BMI 25-29.9 kg/m2) | Obese (BMI ≥ 30 kg/m2) |
|---|---|---|---|
| N (%) | 326 (36%) | 500 (56%) | 67 (8%) |
| Age (yrs) | 47.2 ± 9.4 | 47.4 ± 8.5 | 48.3 ± 8.9 |
| Waist circumference (cm) | 85.8 ± 4.4 | 93.9 ± 4.8** | 103.3 ± 5.1** |
| Waist-height ratio (%) | 49.0 ± 2.6 | 53.7 ± 2.7** | 59.0 ± 2.7** |
| Number of teeth | 22.7 ± 5.7 | 22.6 ± 5.2** | 20.8 ± 6.1** |
| Number of teeth with probing pocket depth > 3 mm | 3.1 ± 3.9 | 3.8 ± 4.4** | 4.3 ± 3.8** |
| % Men with ≥ 4 teeth with probing pocket depth > 3 mm | 31% | 36% | 49%† |
| Whole-mouth mean plaque scorea | 1.5 (1.2, 1.9) | 1.6 (1.3, 1.9) | 1.5 (1.2, 2.0) |
| Weight change (kg/yr) | 0.09 ± 0.38 | 0.05 ± 0.41 | 0.05 ± 0.75 |
| Arm Fat Area change (cm2/yr) | 0.41 ± 0.39 | 0.37 ± 0.45 | 0.43 ± 0.73 |
| Waist circumference change (cm/yr) | 0.29 ± 0.35 | 0.25 ± 0.36 | 0.24 ± 0.57 |
| % Cigarette use | |||
| None | 73% | 79% | 70% |
| ½ pack/day | 15% | 13% | 19% |
| ≥ 1 pack/day | 12% | 8% | 11% |
| % Men with education beyond high school | 70% | 68% | 61% |
| % Brush twice or more/day† | 48% | 39% | 39% |
| % Floss once/week or more† | 40% | 32% | 34% |
Mean ± SD shown unless otherwise indicated.
Median with 25th and 75th percentiles in parentheses.
Significantly different from normal-weight category, p < 0.05, from ANOVA.
Significant difference among BMI categories, p < 0.05, Chi-square statistic.
Prospective Data
Among participants who were normal-weight at baseline (Fig. 1), the overall mean (across all examinations) number of teeth with PPD events was higher, with the highest rate of weight gain (tertile 3) relative to the lowest (tertile 1), p < 0.05. Findings were similar for tertiles of change in WC and AFA. There were no significant differences in the slopes for any of the obesity indicators.
Figure 1.
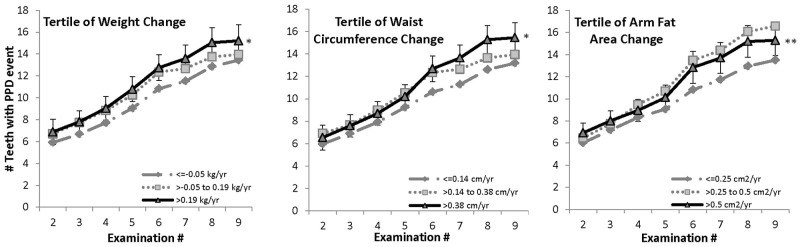
Mean cumulative number of teeth with PPD events in men who were normal-weight at baseline (BMI 18.5-24.9 kg/m2). Means were adjusted for baseline periodontitis (< or ≥ 4 teeth with PPD > 3 mm), education (high school or less, some college, college graduate), baseline pack-years of cigarette exposure, age, whole-mouth mean plaque score, brushing frequency (never, once/day, 2+ times/day), flossing frequency (< once/wk, once/wk or more), and current cigarette packs/day (none, ½, 1 or more). The 95% CI is shown for largest gain tertile. Total Ns at examinations 2-9 were 318, 287, 246, 222, 201, 174, 147, and 115. aPPD event defined as PPD > 3 mm, or tooth loss, provided the tooth showed periodontal involvement (PPD > 2 mm but ≤ 3 mm) prior to loss. *Overall mean in tertile 3 significantly different from that in tertile 1, p < 0.05. **Overall mean in tertile 3 significantly different from that in tertile 1, p < 0.01.
Among overweight participants (Fig. 2), those with either moderate (tertile 2) or large (tertile 3) increases in body weight had higher overall mean numbers of teeth affected with PPD (p < 0.05 and p < 0.01, respectively) as compared with those with minimal weight gain (tertile 1), and the slopes in the highest and lowest tertiles were significantly different. The highest tertile of waist circumference change had a higher overall mean number of PPD events relative to tertile 1 (p < 0.01), but no difference in the slopes. There was no significant difference among AFA tertiles for overweight men.
Figure 2.
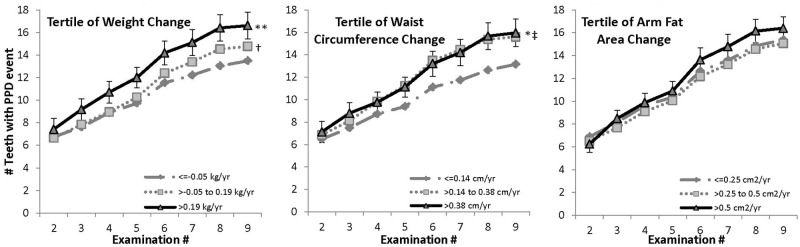
Mean cumulative number of teeth with PPD events in men who were overweight at baseline (BMI 25-29.9 kg/m2). Means were adjusted for baseline periodontitis (< or ≥ 4 teeth with PPD > 3 mm), education (high school or less, some college, college graduate), baseline pack-years of cigarette exposure, age, whole-mouth mean plaque score, brushing frequency (never, once/day, 2+ times/day), flossing frequency (< once/wk, once/wk or more), and current cigarette packs/day (none, ½, 1 or more). The 95% CI is shown for the largest gain tertile. Total Ns at examinations 2-9 were 492, 434, 381, 346, 314, 260, 227, and 167. a PPD event defined as PPD > 3 mm, or tooth loss, provided the tooth showed periodontal involvement (PPD > 2 mm but ≤ 3 mm) prior to loss. * Overall mean in tertile 3 significantly different from that in tertile 1, p < 0.05. ** Overall mean in tertile 3 significantly different from that in tertile 1, p < 0.01. † Overall mean in tertile 2 significantly different from that in tertile 1, p < 0.01. ‡ Beta coefficient for interaction with examination number (slope) in tertile 3 significantly different from that in tertile 1, p < 0.01.
There were no significant differences in overall means by tertile for change in weight, waist circumference, or arm fat area among men who were obese at baseline (Fig. 3).
Figure 3.
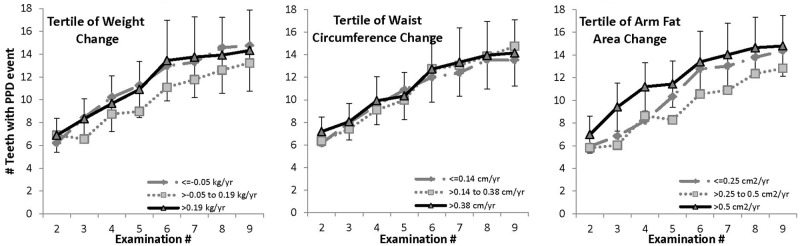
Mean cumulative number of teeth with PPD events in men who were obese at baseline (BMI ≥ 30 kg/m2). Means were adjusted for baseline periodontitis (< or ≥ 4 teeth with PPD > 3 mm), education (high school or less, some college, college graduate), baseline pack-years of cigarette exposure, age, whole-mouth mean plaque score, brushing frequency (never, once/day, 2+ times/day), flossing frequency (< once/wk, once/wk or more), and current cigarette packs/day (none, ½, 1 or more). The 95% CI is shown for the largest gain tertile. Total Ns at examinations 2-9 were 65, 54, 46, 40, 33, 26, 22, and 17. a PPD event defined as PPD > 3 mm, or tooth loss, provided the tooth showed periodontal involvement (PPD > 2 mm but ≤ 3 mm) prior to loss.
Among men who were overweight at baseline, we estimate that those in the highest tertile of weight gain or WC gain developed at least 4 more teeth with deep pockets (PPD > 3 mm) during follow-up than those in the lowest tertile. Among men who were normal weight at baseline, the corresponding differences were 1 tooth with PPD > 3 mm between extreme tertiles of weight gain, and 0.4 teeth with PPD > 3 mm between extreme tertiles of WC change.
Discussion
Analysis of our longitudinal data spanning almost 30 yrs of follow-up indicates that periodontitis is more extensive in men who gain adiposity at moderate to rapid rates, and that large gains may accelerate the rate of PPD progression, especially in normal-weight and overweight men. The findings were less clear for those participants already obese at enrollment. This may be due to the low prevalence of obesity (8%) and the large number of overweight men in the 1960s and 1970s, resulting in reduced statistical power in the former group compared with the latter. It is also plausible that other health-compromising behaviors were occurring within the obese group to worsen periodontitis.
These results support and extend the findings of cross- sectional studies (Suvan et al., 2011) and a limited number of prospective studies (T Morita et al., 2010; Jimenez et al., 2012; I Morita et al., 2011). Jimenez et al. (2012) used self-reported anthropometric and periodontal disease data to evaluate the risk of periodontal disease in relation to obesity, waist circumference, and waist-hip ratio in the all-male Health Professionals Follow-Up Study. Each of the adiposity indices was associated with an approximately 30% increase in risk of periodontitis. Two studies of employed adults in Japan followed participants for up to 5 yrs to track development of periodontitis (PPD ≥ 4 mm on any of 10 representative teeth) (T Morita et al., 2010; I Morita et al., 2011). In men, overweight or obese status at baseline was associated with hazard ratios for periodontitis that were 30% and 44% greater, respectively, than in men with BMI < 22 kg/m2 (Morita et al., 2011). A 4-year study monitored the onset of metabolic syndrome, of which obesity is one element, in relation to baseline periodontal disease status (Morita et al., 2010). The rate of developing obesity was 1.7 times higher in participants with periodontitis than in those without, implying that the relationship may not be one of straightforward cause and effect. Our findings do not rule out a bi-directional association, but are consistent with a dynamic relationship in which increases in adiposity and worsening of probing pocket depth take place concurrently.
It may be important to distinguish among the different body areas where fat is distributed when describing chronic disease risk, including periodontal disease. WC is a marker of intra-abdominal visceral fat and AFA of upper body subcutaneous adiposity, both of which are strongly associated with increased risks of cardiovascular disease, type 2 diabetes, and other diseases (Jensen, 2008; Santosa and Jensen, 2008). Whereas body weight and BMI are easily measured and correlate with overall adiposity, they do not describe body fat distribution. Men in particular are affected by variations in the amount of muscle and weight of bone (Dalla Vecchia et al., 2005), so that overweight BMI values are not necessarily indicative of excessive fat. Several cross-sectional studies concluded that WC or waist-to-hip ratio was as strongly or more strongly related to odds of periodontal disease than BMI (Al-Zahrani et al., 2003; Wood et al., 2003; Kim et al., 2010). Using more than one index of obesity may refine the level of periodontitis risk. Within a given BMI category, males were shown to have higher odds of periodontitis as WC increased (El-Sayed Amin, 2010; Gorman et al., 2012).
Research continues to support the role of overweight and obesity as an independent aggravating risk factor for development of many chronic diseases. Results from our epidemiologic study support the hypothesis that body fat may play a role in the development of periodontal disease, but do not provide information about possible underlying mechanisms. Biological mechanisms linking obesity with other inflammatory diseases, such as coronary artery disease, metabolic syndrome, diabetes mellitus, and osteoarthritis, may involve oxidative stress, adipocytokines, and other related hormones. Pro-inflammatory cytokines produced in response to periodontal disease such IL-1β and interferon-γ, as well as Gram-negative lipopolysaccharides, may also interfere with lipid metabolism and further contribute to obesity and obesity-related co-morbidities.
Major strengths of this study are the prospective design, extended follow-up period, and control for multiple potential confounders. Limitations include the all-male, predominantly white ethnic make-up of the cohort, the fact that participants were self-selected, multiple examiners, and lack of ability to generalize these findings to a larger, diverse population. However, when the DLS participant data are compared with those from the NHANES surveys from 1960 to 2000, the increases in mean WC and proportion with WC > 102 cm among DLS males were similar to those among NHANES males over the same time period (Okosun et al., 2004).
Social determinants, or health behaviors that extend beyond the individual level and are a consequence of the social conditions and environment in which people live, may have a fundamental impact on oral health (Watt and Sheiham, 2012). An additional limitation is that the DLS cohort has a relatively narrow social gradient with respect to social factors such as socio-economic status, employment status, and access to medical and dental care compared with the general population. Many participants were employed in professional and managerial positions, and few were exposed to hazardous environmental conditions. More than two-thirds reported regular dental prophylaxis at any given examination. We initially examined effects of many other predictors, such as socio-economic status, alcohol, and physical activity, but found that they did not significantly add to the explanation of periodontitis progression in our cohort and so were not considered further. The final models were controlled for education, smoking, and oral hygiene, but these predictors may not adequately capture the full range of social determinants of oral health.
In conclusion, normal-weight and overweight, non-diabetic, white males with rapid rates of weight, WC, and AFA gain had more periodontitis and periodontitis progression compared with those who had smaller gains. Further longitudinal studies are needed to confirm these findings and extend them to more diverse and current populations. Additional longitudinal assessments of changes in weight and adiposity with periodontitis development will also elucidate potential biologic mechanisms.
Footnotes
This work was supported by National Institute of Dental and Craniofacial Research R01 DE019833 and K24 DE00419. The Dental Longitudinal Study and Normative Aging Study are components of the Massachusetts Veterans Epidemiology Research and Information Center, which is supported by the US Department of Veterans Affairs Cooperative Studies Program. Dr. Garcia was a recipient of a Veterans Affairs Career Development Award in Health Services Research from the VA HSRD Service. Views expressed in this paper are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Al-Shammari KF, Al-Khabbaz AK, Al-Ansari JM, Neiva R, Wang HL. (2005). Risk indicators for tooth loss due to periodontal disease. J Periodontol 76:1910-1918 [DOI] [PubMed] [Google Scholar]
- Al-Zahrani MS, Bissada NF, Borawski EA. (2003). Obesity and periodontal disease in young, middle-aged, and older adults. J Periodontol 74:610-615 [DOI] [PubMed] [Google Scholar]
- Alman JE, Garcia RI, Chauncey HH. (1986). Examiner agreement for periodontal variables – a second look. J Dent Res 65(Spec Iss): #295 (abstract) [Google Scholar]
- Burt B. (2005). Position paper: epidemiology of periodontal diseases. J Periodontol 76:1406-1419 [DOI] [PubMed] [Google Scholar]
- Dalla Vecchia CF, Susin C, Rösing CK, Oppermann RV, Albandar JM. (2005). Overweight and obesity as risk indicators for periodontitis in adults. J Periodontol 76:1721-1728 [DOI] [PubMed] [Google Scholar]
- El-Sayed Amin H. (2010). Relationship between overall and abdominal obesity and periodontal disease among young adults. East Mediterr Health J 16:429-433 [PubMed] [Google Scholar]
- Feldman RS, Douglass CW, Loftus ER, Kapur KK, Chauncey HH. (1982). Interexaminer agreement in the measurement of periodontal disease. J Periodontal Res 17:80-89 [DOI] [PubMed] [Google Scholar]
- Feldman RS, Garcia RI, Alman JE, Chauncey HH. (1985). Interexaminer agreement in the measurement of periodontal disease. J Dent Res 64(Spec Iss): #262 (abstract) [DOI] [PubMed] [Google Scholar]
- Feldman RS, Alman JE, Chauncey HM. (1986). Design and analysis considerations for a longitudinal study of periodontal disease. J Clin Periodontol 13:506-510 [DOI] [PubMed] [Google Scholar]
- Fleiss JL, Chilton NW. (1983). The measurement of interexaminer agreement on periodontal disease. J Periodontal Res 18:601-606 [DOI] [PubMed] [Google Scholar]
- Gorman A, Kaye EK, Apovian C, Fung TT, Nunn M, Garcia RI. (2012). Overweight and obesity predict time to periodontal disease progression in men. J Clin Periodontol 39:107-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Baumgartner RN, Pan SF. (1999). Nutrition assessment of malnutrition by anthropometric methods. In: Modern nutrition in health and disease. 9th ed Shils ME, Olson JA, Shike M, Ross AC, editors. Baltimore, MD: Williams & Wilkins, pp; 903-921 [Google Scholar]
- Jensen MD. (2008). Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93(11 Suppl 1):57S-63S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M, Hu FB, Marino M, Li Y, Joshipura KJ. (2012). Prospective associations between measures of adiposity and periodontal disease. Obesity 20:1718-1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur KK, Glass RL, Loftus ER, Alman JE, Feller RP. (1972). The Veterans Administration longitudinal study of oral health and disease. Int J Aging Hum Dev 3:125-137 [Google Scholar]
- Kim EJ, Jin BH, Bae KH. (2010). Periodontitis and obesity: a study of the Fourth Korean National Health and Nutrition Examination Survey. J Periodontol 82:533-542 [DOI] [PubMed] [Google Scholar]
- Morita I, Okamoto Y, Yoshii S, Nakagaki H, Misuno K, Sheiham A, et al. (2011). Five-year incidence of periodontal disease is related to body mass index. J Dent Res 90:199-202 [DOI] [PubMed] [Google Scholar]
- Morita T, Yamazaki Y, Mita A, Takada T, Seto M, Nishinoue N, et al. (2010). A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol 81:512-519 [DOI] [PubMed] [Google Scholar]
- Okosun IS, Chandra KM, Boev A, Boltri JM, Choi ST, Parish DC, et al. (2004). Abdominal adiposity in US adults: prevalence and trends, 1960-2000. Prev Med 39:197-206 [DOI] [PubMed] [Google Scholar]
- Santosa S, Jensen MD. (2008). Why are we shaped differently, and why does it matter? Am J Physiol Endocrinol Metab 295:E531-E535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvan J, D’Aiuto FD, Moles DR, Petrie A, Donos N. (2011). Association between overweight/obesity and periodontitis in adults. A systematic review. Obes Rev 12:e381-e404 [DOI] [PubMed] [Google Scholar]
- Watt RG, Sheiham A. (2012). Integrating the common risk factor approach into a social determinants framework. Community Dent Oral Epidemiol [Epub ahead of print Mar 20, 2012] (in press). [DOI] [PubMed] [Google Scholar]
- Wood N, Johnson RB, Streckfus CF. (2003). Comparison of body composition and periodontal disease using nutritional assessment technique: Third National Health and Nutrition Examination Survey (NHANES III). J Clin Periodontol 30:321-327 [DOI] [PubMed] [Google Scholar]


