Abstract
Background
Liposomes containing pH-sensitive polymers are promising candidates for the treatment of tumors and localized infection. This study aimed to identify parameters influencing the extent of contents release from poly(ethylacrylic acid) (PEAA) vesicles, focusing on the effects of polymer size, lipid composition, vesicle surface charge, and temperature.
Methods
Anchored lipid pH-sensitive PEAA was synthesized using PEAA with a molecular weight of 8.4 kDa. PEAA vesicles were prepared by insertion of the lipid-anchored PEAA into preformed large unilamellar vesicles. The preformed liposomes were manipulated by varying the phosphocholine and cholesterol content, and by adding negative or positive charges to the liposomes. A calcein release assay was used to evaluate the effects of polymer size, liposome composition, surface charge, and temperature on liposomal permeability.
Results
The release efficiency of the calcein-entrapped vesicles was found to be dependent on the PEAA polymer size. PEAA vesicles containing a phosphatidylcholine to cholesterol ratio of 60:40 (mol/mol) released more than 80% of their calcein content when the molecular weight of PEAA was larger than 8.4 kDa. Therefore, the same-sized polymer of 8.4 kDa was used for the rest of study. The calcein release potential was found to decrease as the percentage of cholesterol increased and with an increase in the phosphocholine acyl chain length (DMPC DPPC DSPC). Negatively charged and neutral vesicles released similar amounts of calcein, whereas positively charged liposomes released a significant amount of their contents. pH-sensitive release was dependent on temperature. Dramatic content release was observed at higher temperatures.
Conclusion
The observed synergistic effect of pH and temperature on release of the contents of PEAA vesicles suggests that this pH-sensitive liposome might be a good candidate for intracellular drug delivery in the treatment of tumors or localized infection.
Keywords: liposomes, pH-sensitive, PEAA, liposomal permeability
Introduction
The design of nanoliposomal drug delivery systems able to undergo controlled fusion and release has been the subject of intense research.1–5 Considering that the primary route of liposome uptake in cells is through relatively low pH endosomes, pH-sensitive liposomes may be a favorable approach.6–8 It has been reported that pH-sensitive vesicles with polymers immobilized on their surfaces can effectively mediate this type of release and fusion.9 In previous studies, we constructed pH-sensitive liposomes by either conjugating thiolated poly(ethylacrylic acid) (PEAA) to vesicles containing maleimide10 or inserting lipid-anchored polymers into preformed liposomes.11 The resulting PEAA liposomes are stable under physiological conditions and can fuse with adjacent membranes and release their contents upon acidification. Importantly, it has been demonstrated that the association of lipid-anchored PEAA with vesicles promotes intracellular delivery of vesicular contents in vitro in a cultured cell line.12
This study attempted to identify parameters influencing the extent of release of contents from PEAA vesicles, focusing on the effects of polymer size, lipid composition, vesicle surface charge, and temperature. Such information is important for optimizing liposomal PEAA formulations as intracellular drug delivery carriers that are able to release their contents efficiently in a pH-dependent manner.
Materials and methods
Chemicals
All chemical reagents were commercially available products of high purity and were used without further purification. In order to quantify the polymer, PEAA was fluorescent-labeled with small amounts of pyrene, and synthesized as described previously. Different phospholipids, including egg phosphatidylcholine, 1,2 dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2 dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2 distearoyl-sn-glycero-3-phosphocholine (DSPC), phosphatidylinositol (PI), dioleoyl-1,2-diacyl-3-dimethylammonium-propane (DODAP), and 1,2-distearoylsn-glycero-3-phosphoethanolamine (DSPE) were obtained from Northern Lipids (Vancouver, Canada). N,N-dioleyl-N,N-dimethylammonium chloride (DODAC) was a gift from Helican Biotechnology Inc (Vancouver, Canada). [3H] cholesterylhexadecyl ether (3H-CHE) was obtained from Du Pont (NEN Research Products, Boston, MA). Cholesterol and 1,3-dicyclohexylcarbodiimide were obtained from Aldrich Chemical Corporation (St Louis, MO). T-octylphenoxypolyethoxyethanol (Triton X-100), calcein, and Sepharose CL-6B were acquired from Sigma Chemicals (St Louis, MO).
Synthesis of lipo-PEAA
Anchored PEAA (lipo-PEAA) was synthesized using 8.4 kDa PEAA as described previously.13 Briefly, 100 mg (100 mmol unit, unit molecular weight 100) of PEAA and 3 mmol of 1-decylamine were dissolved in water (pH 7.0). A 20 mg/mL 1,3-dicyclohexylcarbodiimide solution was then slowly added to the reaction mixture until the amine disappeared (monitored by thin layer chromatography, CHCl3:MeOH:triethylamine, 8:2:0.2, visualized by ninhydrin). The resulting derivate was precipitated by adjusting the pH of the solution to pH 2–3. The supernatants were then removed and the pellets were redissolved in 2 M NaOH solution, stirred for 30 minutes, and readjusted to pH 2–3. The suspension was centrifuged and the resulting pellets were washed 3–5 times in water and lyophilized. A typical yield was 60%–70%. In our paper, PEAA-C10 (lipo-PEAA with a decyl chain introduced) was used for post-insertion processing and forming PEAA liposomes.
Preparation of PEAA liposomes
Large unilamellar vesicles (LUVs) were prepared by extrusion as described by Hope et al.14 Appropriate amounts of lipid mixtures with trace amounts of 3H-CHE (1.33 μCi/4 μmol) in chloroform were dried under a stream of nitrogen gas to form a homogeneous lipid film. Any trace solvent was then removed under vacuum overnight. The lipid film was hydrated in HEPES-calcein buffered saline (20 mM HEPES, 100 mM calcein, pH 7.5) by vortex mixing. The resulting multilamellar vesicles were frozen/thawed (liquid nitrogen/55°C) five times and extruded 10 times at 55°C through two stacked 100 nm polycarbonate filters (Nuclepore™, Whatman, Clifton, NJ) using an extrusion device (Vancouver, Canada). Untrapped free calcein was removed by chromatography using a 1.1 × 20 cm Sepharose CL-6B column (Sigma Chemical Corporation) equilibrated with HEPES-buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.5).
PEAA-LUVs were then prepared by incubation of preformed LUVs with lipo-PEAA solution (PEAA-C10, pH 7.4) in an appropriate ratio at room temperature overnight as previously described.13 The mixture was eluted on a Sepharose CL-6B column (1.5 × 20 cm) and the fractions were assessed for lipid and polymer content. Noninserted free polymer was separated from PEAA-LUVs on a Sepharose CL-6B column as described in our previous work.13
The percentage of polymer insertion varied according to the nature of the preformed vesicles, especially for the charged ones. Hydrophobic PEAA segments are unstable in an aqueous environment and are therefore expected to penetrate into the hydrophobic interior of a lipid bilayer through Van der Waals forces.15,16 A high PEAA concentration would lead to solubilization of the lipid membrane, while a low PEAA concentration would probably cause defective lipid packing, resulting in release of the vesicle contents. Therefore, a 10 mol% PEAA composition is used in our research.17
Determination of liposome size
Liposome size was determined by quasi-elastic light scattering using a Nicomp 370 submicron particle sizer (Santa Barbara, CA).
Calcein release
A calcein release assay was used to evaluate liposomal permeability. 18 Calcein, a nonpermeable aqueous fluorescent dye, was used as a marker for determining the stability of the vesicles and the permeability of the different vesicles on acidification and changes in temperature. Calcein was trapped in the liposomes at a self-quenching concentration (100 mM). When released from the vesicles, it was diluted and the fluorescent intensity of the solution increased. Two release assays were used in this study. One assessed the potency of different sized, nonanchored polymers for triggering calcein release. The other measured the calcein release from PEAA-LUVs.
First release assay
For quantification of the vesicle permeability induced by polymers of different size (without a lipid anchor), fluorescent aqueous calcein (100 mM) was encapsulated into LUV (egg phosphatidylcholine to cholesterol ratio 60:40, mol/mol) and free calcein was removed using a gel column as already described for the preparation of PEAA liposomes. An assay solution of 1.0 mg/mL LUV containing calcein was prepared by diluting the stock formulation with HEPES-buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.5). Next, 10 μL of polymer (1 mg/mL, pH 7.5) was added to 1.0 mL of the diluted LUV containing calcein. A 100 μL aliquot of the test solution was diluted to 1.2 mL with HEPES-buffered saline (pH 7.5). The fluorescence intensity of calcein (F0) as the initial background was determined using an Aminco Bowman luminescence spectrofluorometer (SLM-Aminco, Urbana, IL) at 520 nm (emission) and 495 nm (excitation). The test solution was then adjusted to pH 4.5 with 1% HCl and allowed to equilibrate for 20 minutes at room temperature. A 100 μL aliquot was diluted to 1.2 mL with HEPES-buffered saline (pH 7.5) and the fluorescence intensity was measured (Ft). Maximal fluorescence intensity (Fmax), representing complete release of encapsulated calcein, was measured after solubilization of the vesicles (a 100 μL aliquot diluted with 1.2 mL of HEPES-buffered saline) with 10 μL of 10% Triton X-100. The potency of polymer in inducing vesicle permeability was calculated as the percentage of calcein released as shown in equation 1.
| (1) |
Second release assay
The permeability of the polymer-associated PEAA-LUV containing calcein liposomes and of the control samples were acidified to pH 4.5 with 1% HCl. After 20 minutes of equilibration, an 100 μL aliquot was withdrawn and diluted with 1.2 mL of HEPES-buffered saline and the fluorescence intensity of the diluted solution was measured using an Aminco Bowman Series 2 luminescence spectrofluorometer at 530 nm (slit width 4 nm) under steady-state excitation at 495 nm (slit width 4 nm). The initial and final fluorescence intensities were determined using the method described above and the percentage release was calculated as in equation 1.
To determine the kinetics of calcein release at different temperatures, the test sample was incubated in a temperature-controlled water bath at a predetermined temperature. The initial pH of 7.5 was maintained for a period of 10 minutes before acidifying the solution to pH 4.5. At different time points, a 100 μL aliquot was withdrawn, diluted with 1.2 mL of HEPES-buffered saline, and the fluorescence intensity of the solution was measured (Ft is the fluorescence intensity at time t). The maximum fluorescence intensity (Fmax), representing complete release of the encapsulated calcein, was determined following solubilization of the vesicles with Triton X-100 (10% of lipid concentration). The percentage of calcein released was calculated using equation 1.
Results
Effect of polymer size on vesicle permeability
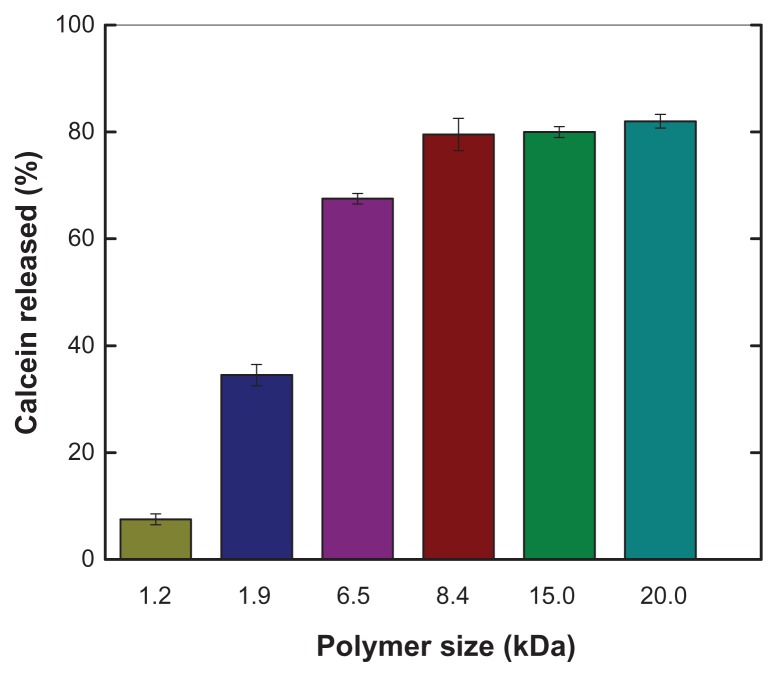
It was expected that the ability of PEAA to trigger release of vesicular contents would be dependent on polymer size, but the polymer size cutoff for inducing efficient release of commonly used liposomes was unknown. Therefore, using an in vitro calcein release assay, we quantitatively measured content release from vesicles composed of egg phosphatidylcholine and cholesterol (ratio 60:40) with different-sized PEAA. Figure 1 shows that the tendency of calcein to be released under acidic conditions increased as the polymer size increased. The extent of calcein release reached a plateau when PEAA was larger than 8.4 kDa. In order to evaluate the effects of lipid composition, charge, and temperature on the permeability of pH-sensitive vesicles containing lipo-PEAA, we used the same-sized polymer of 8.4 kDa for the rest of study.
Figure 1.
Effect of polymer size on pH-sensitive calcein release from large unilamellar vesicles.
Notes: Calcein release was measured by a calcein release assay, with calcein entrapped in large unilamellar vesicles and free polymers as described in the text. Liposomes were composed of egg phosphatidylcholine and cholesterol in a ratio of 60:40 (mol/mol) and calcein 100 mM. Free PEAA with a designed size was mixed with large unilamellar vesicles containing calcein at a polymer-to-lipid ratio of 10 μg/mg. The fluorescence intensity of the vesicles was measured after 20 minutes of incubation after acidification to pH 4.5.
Abbreviation: PEAA, poly(ethylacrylic acid).
Formulation and characterization of pH-sensitive vesicles containing lipo-PEAA
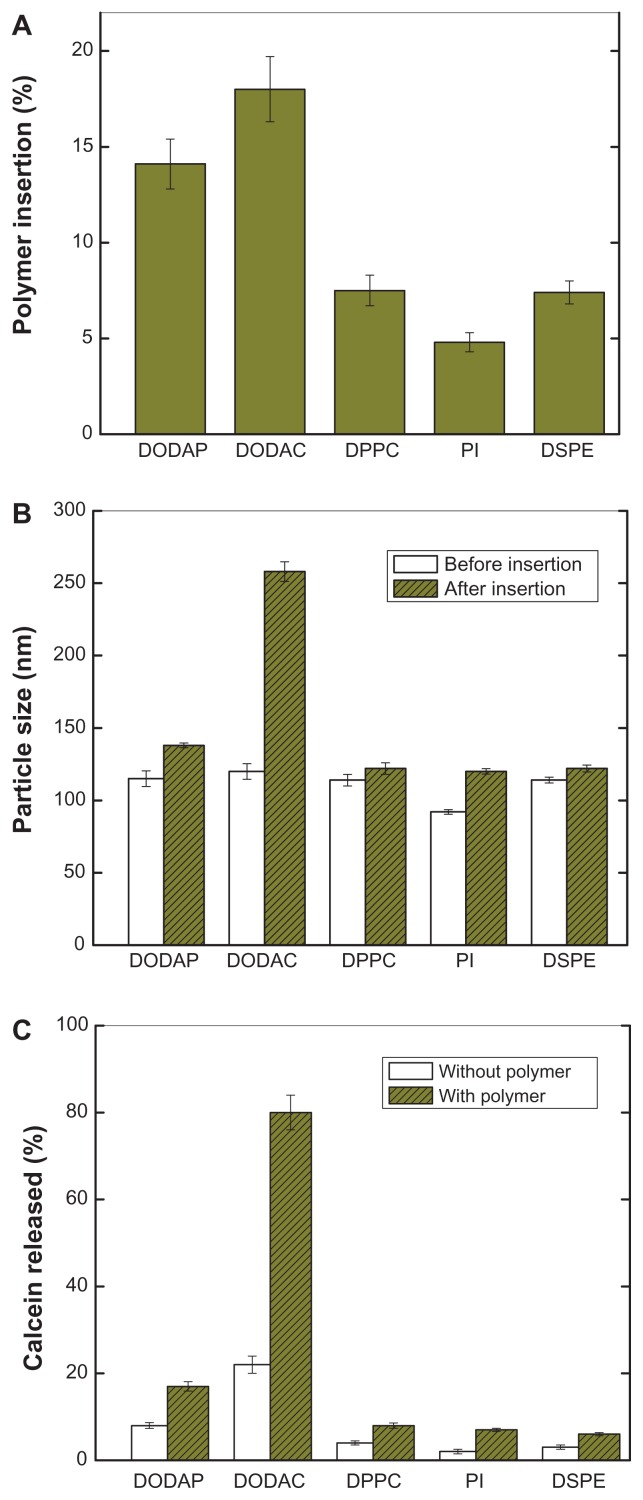
Because the pH-sensitive PEAA vesicles were intended for pharmaceutical use, it was important to identify parameters influencing formulation of characteristic PEAA vesicles. These parameters included the efficiency of polymer association, formulation stability, size change during polymer insertion, and efficient content release under acidic conditions. Different vesicles with either a positive, neutral, or negative net surface charge were used for our post-insertion study. As indicated in Figure 2A, positively charged liposomes with either a permanent charge (DODAC) or protonable amine (DODAP) resulted in a much higher amount of associated polymers, while neutrally (DPPC) and negatively (PI or DSPE) charged vesicles contained smaller amounts of inserted polymers. The different phosphocholine acyl chain lengths, eg, DPPC, PI, or DSPE, did not affect the efficiency of polymer insertion.
Figure 2.
Characteristics of pH-sensitive PEAA vesicles with differing lipid composition by the post-insertion method. LUVs with encapsulated calcein 100 mM were prepared using a hydration extrusion method. PEAA-LUVs were then prepared by incubating lipo-PEAA with preformed LUVs at a polymer-to-lipid ratio of 20 μg/mg. (A) Percentage polymer insertion when different phospholipids were used to prepare the PEAA-liposomes. The noninserted polymer was removed, the liposomes were determined by radioactivity, and the polymer was quantified by fluorescence intensity. (B) Particle size of PEAA-LUVs. (C) Calcein released from PEAA-LUVs overnight at room temperature with incubation at neutral conditions (pH 7.4). Calcein release was measured as described in the text.
Abbreviations: DODAP, dioleoyl-1,2-diacyl-3-dimethylammonium-propane; DODAC, N,N-dioleyl-N,N-dimethylammonium chloride; DPPC, 1,2 dipalmitoyl-sn-glycero-3-phosphocholine; PI, phosphatidylinositol; DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
Upon mixing DODAC-containing liposomes and lipo-PEAA, extensive aggregation took place (Figure 2B), anticipated because of the strong electron interaction between the multiple oppositely charged DODAC-containing liposomes and lipo-PEAA. Surprisingly, the size increase of DODAP-containing liposomes after polymer insertion was much smaller than that for DODAC, although the value was still higher than that of both the neutrally and negatively charged liposomes. Because the amine of DODAP is protonable and its charged form is environmentally dependent, it may exist in a partially charged form in the bilayer. A much lower pKa for a similar amino lipid, AL1, in the membrane bilayer has been reported by Bailey and Cullis.19 This may also be the case for DODAP. The net amount of charge would be lower for DODAP near the membrane surface and the interaction with the negatively charged PEAA would be subsequently reduced. Other factors, such as the different orientation of DODAC and DODAP in the lipid bilayer, could also contribute to the different interactions between these two amino lipids and negatively charged PEAA but, at present, we do not have evidence to support or rule this out.
In all cases, except for the DODAC-containing vesicles, only small amounts of calcein leaked out after insertion of the polymer, indicating that polymer insertion did not affect the stability of most of the liposomes. As demonstrated in Figure 2C, the encapsulated calcein was completed released from DODAC vesicles upon incubation with lipo-PEAA. The interaction between PEAA and DODAC was so strong that, even without a pH change, the lipid bilayer was already greatly disrupted, causing calcein release and vesicular aggregation. Due to failure to prepare a satisfactory formulation, we did not study DODAC-containing liposomes in a further experiment. The formulations used in the rest of the study were all purified using the gel filtration column to remove the noninserted polymers and trace amounts of released calcein. In summary, PEAA liposomes with different lipid compositions were successfully prepared, with good polymer association, a narrow particle size distribution, and their contents encapsulated using a post-insertion method.
Effect of cholesterol content on vesicle permeability
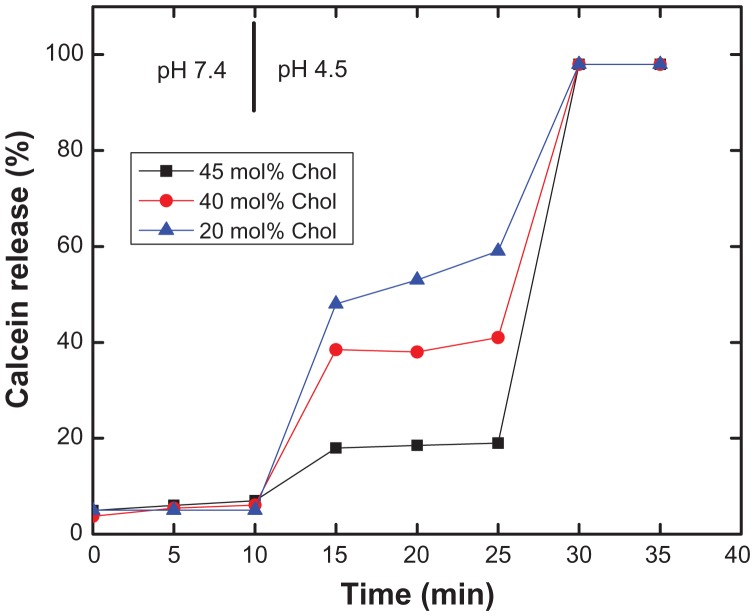
It has previously been shown that cholesterol increases the stability of liposomes when added as a component.20,21 This prompted us to insert lipo-PEAA without concern about loss of vesicular content. An increase in cholesterol content may also reduce or prevent release of contents when PEAA-LUV is acidified, because the rigidity and fluidity of the liposomal membrane is changed. Mills et al found that cholesterol reduced the potency of PEAA with regard to pH-induced liposomal permeability in an experiment using free PEAA and liposomes with encapsulated fluorescent dye.22,23 The critical pH, ie, the point at which the polymer is hydrophobic enough upon partial acidification to interact with the liposomal bilayer and trigger release of the contents, was shifted from pH 6.7 for the pure SOPC formulation to pH 6.1 for liposomes containing 60% cholesterol. Therefore, in the present study, the effect of cholesterol on the permeability of PEAA vesicles comprising DSPC and cholesterol, as shown in the release kinetics study in Figure 3, was examined using a calcein release assay. The higher the cholesterol content, the lower the permeability of the PEAA liposomes; 18%, 38.5%, and 48% of calcein leaked out from DSPC vesicles containing 20, 40, and 45 mol%, respectively, of cholesterol 15 minutes after acidification (pH 4.5). Vesicular stability resulting from addition of cholesterol was also observed for liposomes containing DMPC and DPPC. Formulations with cholesterol were stable at neutral conditions (pH 7.4), while the calcein was almost completely released upon acidification. Although a very stable liposome (DSPC and cholesterol) was used in the experiment (Figure 3), a significant amount of calcein was still released under acidic conditions, indicating that PEAA has a very efficient pH-dependent membrane permeability potential.
Figure 3.
Effect of cholesterol content on pH-induced calcein release of PEAA-LUVs.
Notes: Preformed LUVs with DSPC and differing amounts of cholesterol were prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-to-lipid ratio of 20 μg/mg. Calcein released from PEAA-LUVs was measured at room temperature (25°C) as described in the text.
Abbreviations: Chol, cholesterol; DSPC, 1,2 distearoyl-sn-glycero-3-phosphocholine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
Effect of different lipid chain lengths on vesicle permeability
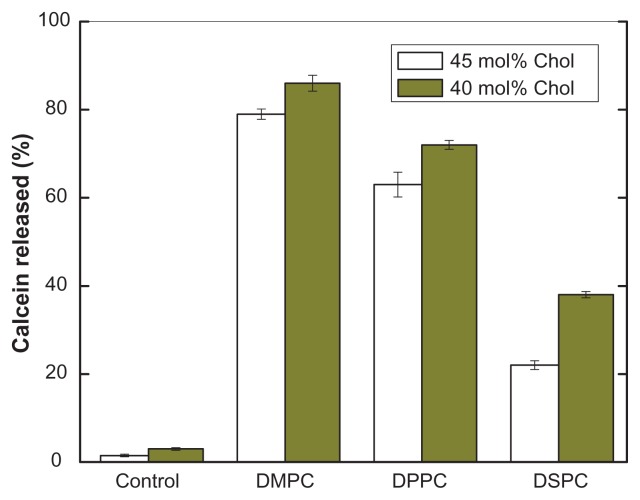
pH-sensitive calcein release from PEAA-LUV was also dependent on the acyl chain length of phosphatidylcholine. Figure 4 shows that the amount of calcein released was proportional to the chain length of phosphocholine, regardless of whether 40 mol% or 45 mol% cholesterol was included in the liposomes. It is known that membrane permeability is higher in a liquid phase state (temperature higher than the gel-solid transition temperature). The calcein release observed was induced by pH-sensitive PEAA, not by temperature-controlled slow release. This was confirmed by a control experiment in which liposomal vesicles with no polymer content did not release significant amounts of calcein when the test solution was acidified to pH 4.5.
Figure 4.
Effect of lipid chain length on pH-induced calcein release from PEAA-LUVs.
Notes: Preformed LUVs with differing cholesterol content (45 mol%, 40 mol%) were prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-tolipid ratio of 20 μg/mg. Liposomal vesicles without PEAA inserted served as controls. The amount of calcein released from PEAA-LUVs was measured after 20 minutes of incubation at room temperature (25°C) as described in the text.
Abbreviations: Chol, cholesterol; DMPC, 1,2 dimyristoyl-sn-glycero-3-phosphocholine; DSPC, 1,2 distearoyl-sn-glycero-3-phosphocholine; DPPC, 1,2 dipalmitoyl-sn-glycero-3-phosphocholine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
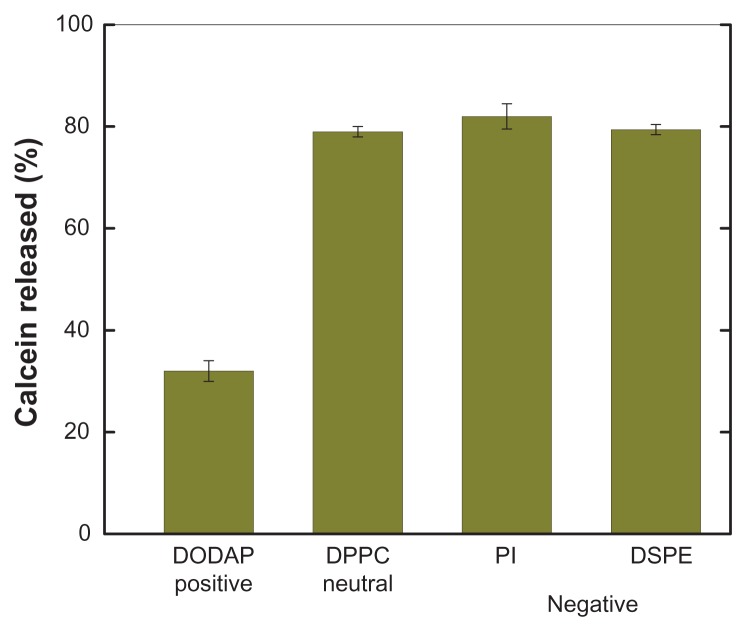
Effect of charge on vesicle permeability
pH-induced calcein release from PEAA-LUV was strongly influenced by a positive surface charge, but not a negative charge (Figure 5). The effect of charge on lipid behavior is governed by the overall surface charge density of the liposome, the lipid head group,14 and the polymer-liposome interaction before and after acidification. We selected negatively and positively charged lipids to compare the effect of different charges on PEAA vesicle formulation and their pH-induced permeability. It seemed that there were more problems with the positively charged preformed vesicles. As mentioned earlier, we were unable to prepare DODAC-containing PEAA-LUV due to aggregation and content release, and vesicles containing DODAP released much lower amounts of calcein than either neutral or negatively charged vesicles. It was considered that negatively charged PEAA could form a stable structure by partial neutralization of the positive charge of DODAP, reducing its sensitivity to protonation, and losing polymer fusion activity and permeability.
Figure 5.
Effect of charged lipids on pH-induced release of calcein from PEAA-LUVs.
Notes: Preformed neutral LUVs (40 mol% cholesterol) or charged LUVs (40 mol% cholesterol, and 10 mol% charged lipid introduced) were prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-to-lipid ratio of 20 μg/mg. The calcein released from the PEAA-LUVs was measured after 20 minutes of incubation at room temperature (25°C) as described in the text.
Abbreviations: DODAP, dioleoyl-1,2-diacyl-3-dimethylammonium-propane; DPPC, 1,2 dipalmitoyl-sn-glycero-3-phosphocholine; PI, phosphatidylinositol; DSPE, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
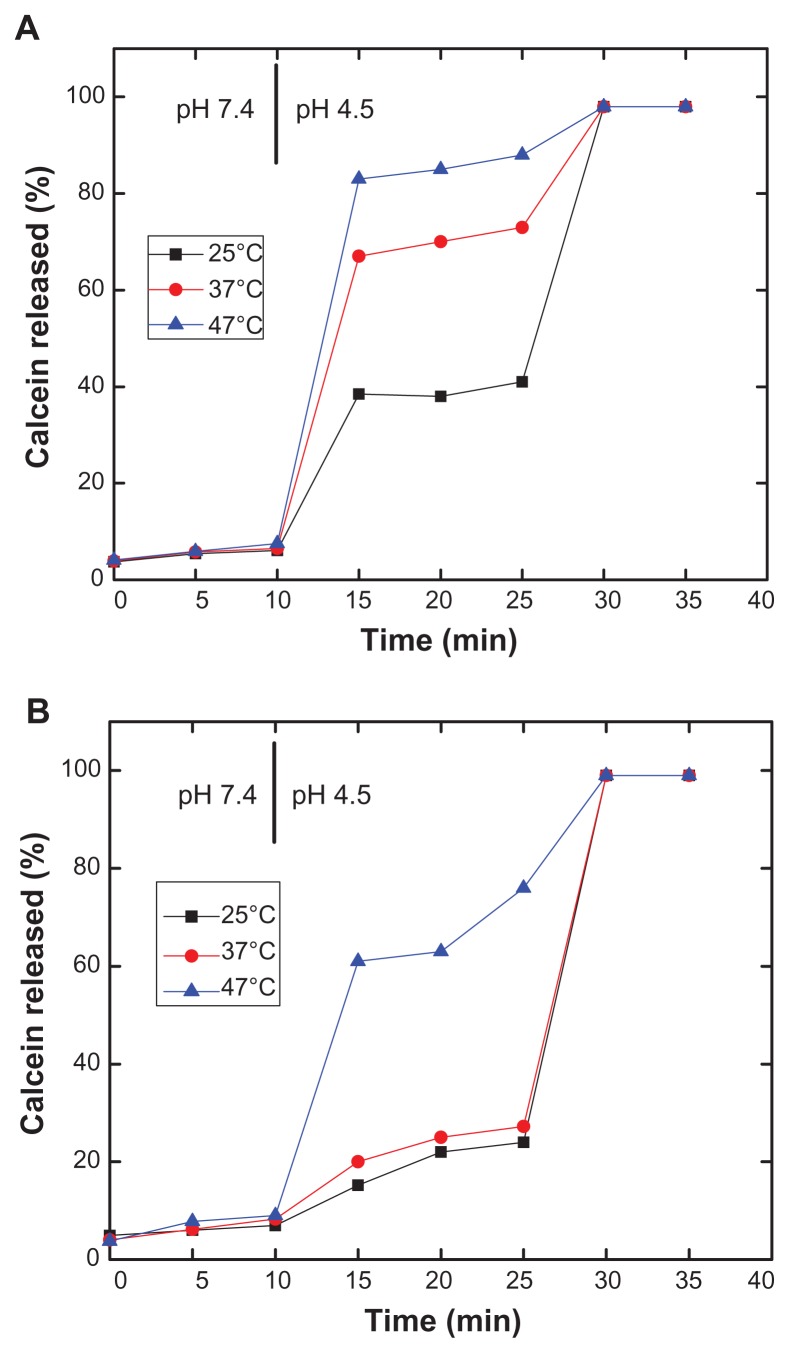
Effect of temperature on vesicle permeability
Given that release of vesicular contents from PEAA liposomes has already been demonstrated in our previous work and the potency of release has also been shown to be regulated by polymer size, lipid composition, and the surface charge at room temperature, it would be interesting to assess the permeability of PEAA vesicles at body temperature and under hyperthermic conditions. Hayashi et al constructed a thermosensitive liposome by using poly(N-isopropylacrylamide), a structurally related polymer.24 Temperature-sensitive liposomes for use with mild hyperthermia have also been reported by fine-tone of lipid composition to obtain good permeability for marching desired temperature.25 It was anticipated that PEAA in its acid form would be more hydrophobic and could also be responsible for the effects of temperature change, such that the efficiency of release could be further enhanced.
In our study, three different temperatures, ie, 25°C, 37°C, and 47°C, were used to test the effect of temperature on pH-induced calcein release from PEAA-LUVs. DSPC has the high transition temperature (55°C), so formulations containing DSPC were used to test the effect of temperature on vesicle permeability. As shown in Figure 6, calcein release increased as temperature increased. Enhancement of release by temperature was more efficient for DSPC formulations containing a lower amount of cholesterol (Figure 6A, 40 mol% cholesterol), with 50% more calcein being released at 37°C than at room temperature. When tested at 47°C, complete release of contents was observed. For liposomes with saturated cholesterol concentrations (Figure 6B, 45 mol% cholesterol), enhanced permeability was also seen, although the amount was much less than that shown in Figure 6A.
Figure 6.
Effect of temperature on pH-induced release of calcein from PEAA-LUVs. Preformed LUVs with (A) a DSPC to cholesterol ratio of 60:40 (mol/mol) and (B) a DSPC to cholesterol ratio of 55:45 (mol/mol) was prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-to-lipid ratio of 20 μg/mg. Calcein released from PEAA-LUVs was measured at different temperatures as described in the text.
Abbreviations: DSPC, 1,2 distearoyl-sn-glycero-3-phosphocholine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
Vesicles containing cholesterol 40 mol% (Figure 6A) showed more calcein release than those containing cholesterol 45 mol% (Figure 6B) at the same temperature and with the same incubation time, indicating high vesicle permeability with lower cholesterol content. Cholesterol acts as a fluid buffer in liposomes and reduces the freedom of motion of the liposome membrane. Cholesterol causes changes in the molecular order of the membrane and dynamics in the fluid and gel phases, as well as introducing a new thermodynamic phase when the cholesterol content is high.26 Thus, the higher transition temperature in Figure 6B with a high cholesterol content resulted in low vesicle permeability.
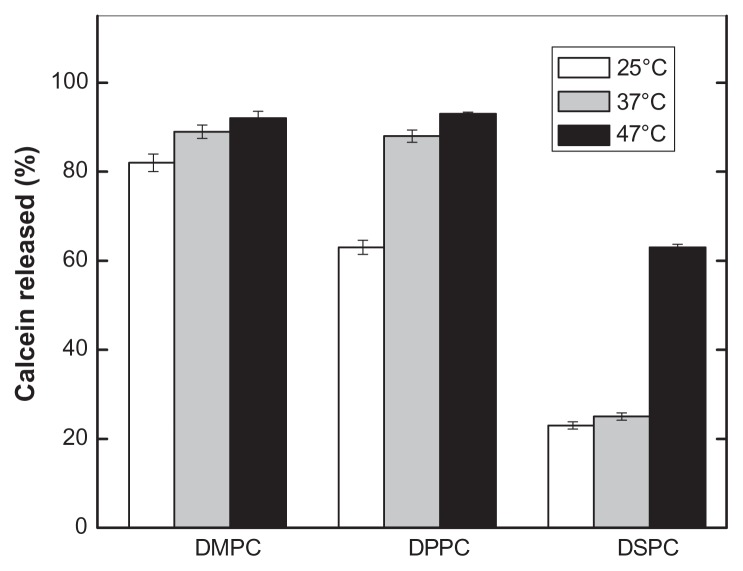
Figure 7 summarizes the effects of temperature on the permeability of PEAA liposomes comprised of different phosphocholines and 45 mol% cholesterol after 20 minutes of incubation in acid conditions (pH 4.5). Because DMPC has a lower transition temperature (23°C), calcein release was near maximal at room temperature, and the effect of increased temperature was not significant. Compared with calcein release at 25°C, there was a roughly 25% increase of calcein obtained at 37°C for DPPC with a transition temperature of 41°C. Calcein release was 22%, 25%, and 63% for DSPC at temperatures of 25°C, 37°C, and 47°C, respectively. The limited calcein release from the DSPC vesicle might be due to its high transition temperature (55°C).
Figure 7.
Effect of temperature on pH-induced calcein release of PEAA-LUVs containing different chain lengths of phosphocholine.
Notes: The preformed LUVs (45 mol% cholesterol) were prepared using an extrusion method with calcein 100 mM. PEAA-LUVs containing calcein were then obtained by post-insertion of PEAA-C10 at a final polymer concentration of 7 mol in PEAA-LUVs containing calcein at a polymer-to-lipid ratio of 20 μg/mg. The calcein released from PEAA-LUVs was measured after 20 minutes of incubation at differing temperatures as described in the text.
Abbreviations: DMPC, 1,2 dimyristoyl-sn-glycero-3-phosphocholine; DPPC, 1,2 dipalmitoyl-sn-glycero-3-phosphocholine; DSPC, 1,2 distearoyl-sn-glycero-3-phosphocholine; LUVs, large unilamellar vesicles; PEAA, poly(ethylacrylic acid).
Discussion
The development of drug carrier systems with an ability to deliver and release their therapeutic contents intracellularly is an important strategy and an interesting research project.27,28 As a continuation of our investigation of pH-sensitive liposomes containing the PEAA polymer, in the present study we have evaluated parameters influencing release of vesicular contents. We demonstrated that pH-sensitive vesicles comprised of different phosphatidylcholines, cholesterol, and negatively charged and protonable amino lipids can be constructed by insertion of lipid-anchored PEAA without loss of vesicular contents. The resulting formulations show a small and uniform particle size, a constant but control-lable amount of membrane-inserted polymer, good stability under neutral conditions, and efficient content release in a pH-dependent manner. Considering that a more complicated lipid composition (such as phosphocholine) with a different phase transition temperature, cholesterol, and charged lipids may be required for actual drug entrapment and application, the information acquired here may have an impact on further development of pH-sensitive vesicles with surface-bound PEAA.
The pH-induced release of liposomal contents by PEAA is dependent on the molecular weight of the polymer. Under acidic conditions, the PEAA conformation collapsed from an expanded hydrophilic form to a globular hydrophobic coil as a result of protonization of the carboxylic groups of PEAA.29 The hydrophobic portion of PEAA penetrates and disturbs the liposomal membrane bilayer, resulting in release of the liposomal contents. Previously, Chung et al found that the critical pH at which PEAA starts to induce release of liposomal contents decreases with decreasing molecular weight of the polymer.30 In the present study, we also observed dependence of liposomal content release on the molecular weight of PEAA. As shown in Figure 1, the potency of PEAA required to trigger release of the contents of the liposome, ie, membrane permeability, increases as the molecular weight of the PEAA increases. At molecular weights exceeding 8.4 kDa, the percentage release reaches a plateau. We selected a relative lower molecular weight of PEAA, which still induces efficient content release under acidic conditions. In practice, using reasonably sized polymers in a liposomal PEAA preparation reduces the risk of aggregation caused by larger molecular polymers and assures even distribution of the polymer on the surface of the liposome. A later experiment confirmed that the 8.4 kDa molecular size of PEAA is well suited to producing a well characterized liposomal PEAA formulation resulting in efficient release of its contents in a pH-dependent manner.
Because bilayer fluidity and rigidity can be an important determinant of the release of liposome-encapsulated compounds,31,32 the effects of fluidity (using phosphocholine with different phase transition temperatures) and rigidity (varying cholesterol content) on the characteristics of pH-sensitive PEAA liposomes were also studied. In a previous study, we found that neither natural nor synthetic phosphocholine is an ideal choice for construction of pH-sensitive liposomes with surface-bound PEAA because the PEAA polymers will still have some degree of interaction with the liposomal membrane, resulting in leakage of contents, even in neutral solution.12,33 In contrast, the stability of liposomes incorporating cholesterol enabled us to prepare PEAA vesicles which maintain their integrity but can still release their contents under acidic conditions. As in other liposomal release studies, pH-induced membrane permeability is also dependent on fluidity and rigidity. A trend of calcein release from PEAA vesicles under acidic conditions was observed in the order of DMPC DPPC DSPC, which reflects the fluidity of liposomes composed of DMPC, DPPC, and DSPC with a phase transition temperature of 23°C, 41°C, and 55°C, respectively. A similar conclusion can be drawn with regard to rigidity, ie, a higher cholesterol content results in greater membrane rigidity and lower permeability. More importantly, it indicates that the permeability of PEAA liposomes can be controlled by modifying the cholesterol content using different types of phosphocholine according to their intended applications.
When investigating pH-induced release from PEAA liposomes with different lipid compositions, the bilayer surface charge has also been found to be important for vesicle permeability.34,35 In general, it is not possible or at least very difficult to prepare PEAA vesicles with prominent positive charged preformed LUVs. Multiple positive-negative charge interactions cause premature release of liposomal contents and vesicle-vesicle fusion during the polymer insertion step. However, PEAA liposomes with preformed LUVs incorporating amine-containing lipids were successively prepared with desirable characteristics, ie, small size and retention of the encapsulated drug. Permeability of the liposomes caused by protonation of PEAA was similar for both neutral and negatively charged liposomes, but reduced for LUVs containing DODAP. Whether the reduction in permeability for liposomes containing DODAP was caused by partial ion-pairing of the amines in DODAP with the carboxylic groups in PEAA is not clear. However, we still obtained adequate release of the liposomal contents from PEAA-LUVs containing DODAP under acidic conditions.
Furthermore, pH-sensitive vesicles with the out-leaflet incorporated PEAA also show temperature-dependent release.36 The use of thermal-sensitive liposomal carriers for enhanced local release of drugs has been studied by several groups.37–40 Enhancement of therapeutic efficacy has been reported for many antitumor drugs.41–43 Temperature-sensitive vesicles containing a synthetic polyacrylic polymer have also been developed. Considering that there is a relatively higher temperature and lower local pH at most disease sites, the combination of pH and temperature sensitivity may be advantageous, and illustrates a new development in liposome research. Although far from optimized, the liposomal carrier developed in the current study has demonstrated an ability to respond to both pH and temperature changes by releasing its vesicular contents, and there may be a benefit to be derived from differences in pH and temperature between healthy tissues and sites of disease. In the future, more sensitive pH-responsive and temperature-responsive vesicles may be constructed by partially modifying the carboxylic groups of PEAA with diisopropylamine. It is well known that poly(diisopropylacrylamide) is a good thermal-sensitive polymer, and liposomes containing poly(diisopropylacrylamide) have shown temperature-dependent release of contents.44 PEAA has a chemical structure similar to that of poly(acrylic acid), with extra ethyl groups at the 2-position. Therefore, by carefully controlling the amount of diisopropylamide in the modified PEAA, the new PEAA liposomes would still be pH-sensitive and also thermal-sensitive.
Conclusion and further perspectives
In summary, this study has demonstrated that polymer size, liposome composition, surface charge, and temperature all influence the permeability of pH-sensitive liposomes containing lipid-anchored PEAA. Because the polymer size was kept at 8.4 kDa, the permeability of the PEAA liposome was dependent on its fluidity and rigidity, which could be modified by the cholesterol content and surface charge on the vesicles, and was varied using different types of phosphocholine. The PEAA liposome was also shown to be temperature-sensitive by careful control of the amount of diisopropylamide introduced into the modified PEAA. These new PEAA liposomes are both pH-sensitive and thermal-sensitive, suggesting possible applications in the treatment of tumor or localized infection. Moreover, the information obtained in this study should be useful for the design of liposomes that are highly sensitive to both ambient temperature and an acidic environment. We plan to explore these issues in our future in vivo experiments.
Acknowledgments
This paper was supported by the National Basic Research Program of China (973 Program, 2012CB619101) and the Natural Science Foundation of Shaanxi Province (2010JM2021). Ting Chen from the School of Life Science, Northwestern Polytechnical University, is acknowledged for her kind help in preparation of the figures accompanying this manuscript. We gratefully acknowledge Feng Xu from Harvard-MIT Health Science and Technology, Boston, MA, for his kind help with English language.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dhoot NO, Wheatley MA. Microencapsulated liposomes in controlled drug delivery: strategies to modulate drug release and eliminate the burst effect. J Pharm Sci. 2003;92:679–689. doi: 10.1002/jps.19104. [DOI] [PubMed] [Google Scholar]
- 2.Kunisawa J, Masuda T, Katayama K, et al. Fusogenic liposome delivers encapsulated nanoparticles for cytosolic controlled gene release. J Control Release. 2005;105:344–353. doi: 10.1016/j.jconrel.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DE, O’Brien DF. Photoactivated enhancement of liposome fusion. Biochemistry. 1995;34:3102–3113. doi: 10.1021/bi00009a042. [DOI] [PubMed] [Google Scholar]
- 4.Schneider H, Lemasters JJ, Hochli M, Hackenbrock CR. Fusion of liposomes with mitochondrial inner membranes. Proc Natl Acad Sci U S A. 1980;77:442–446. doi: 10.1073/pnas.77.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volodkin DV, Ball V, Voegel JC, Möhwald H, Dimova R, Marchi-Artzner V. Control of the interaction between membranes or vesicles: adhesion, fusion and release of dyes. Colloids Surf A Physicochem Eng Asp. 2007;303:89–96. [Google Scholar]
- 6.Drummond DC, Zignani M, Leroux JC. Current status of pH-sensitive liposomes in drug delivery. Prog Lipid Res. 2000;39:409–460. doi: 10.1016/s0163-7827(00)00011-4. [DOI] [PubMed] [Google Scholar]
- 7.Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Cytosolic drug delivery using pH- and light-sensitive liposomes. Adv Drug Deliv Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa Y, Kodaka M, Okuno H. Trigger lipids inducing pH-dependent liposome fusion. Chem Phys Lipids. 2002;119:51–68. doi: 10.1016/s0009-3084(02)00053-1. [DOI] [PubMed] [Google Scholar]
- 9.Simo–es S, Moreira JN, Fonseca C, Düzgünes N, Pedroso de Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv Drug Deliv Rev. 2004;56:947–965. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Chen T, Palmer LR, Fenske DB, Lam AMI, Wong KF, Cullis PR. Distal cationic poly(ethylene glycol) lipid conjugates in large unilamellar vesicles prepared by extrusion enhance liposomal cellular uptake. J Liposome Res. 2004;14:155–173. doi: 10.1081/lpr-200033437. [DOI] [PubMed] [Google Scholar]
- 11.Chen T, McIntosh D, He YH, et al. Alkylated derivatives of poly(ethylacrylic acid) can be inserted into preformed liposomes and trigger pH-dependent intracellular delivery of liposomal contents. Mol Membr Biol. 2004;21:385–393. doi: 10.1080/09687860400010516. [DOI] [PubMed] [Google Scholar]
- 12.Fenske DB, Palmer LR, Chen T, Wong KF, Cullis PR. Cationic poly(ethyleneglycol) lipids incorporated into pre-formed vesicles enhance binding and uptake to BHK cells. Biochim Biophys Acta. 2001;1512:259–272. doi: 10.1016/s0005-2736(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen T, Wong KF, Fenske DB, Palmer LR, Cullis PR. Fluorescently labeled poly(ethylene glycol) lipid conjugates with distal cationic headgroups. Bioconjug Chem. 2000;11:433–437. doi: 10.1021/bc990171x. [DOI] [PubMed] [Google Scholar]
- 14.Hope MJ, Bally MB, Webb G, Cullis PR. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim Biophys Acta. 1985;812:55–65. doi: 10.1016/0005-2736(85)90521-8. [DOI] [PubMed] [Google Scholar]
- 15.Thomas JL, Barton SW, Tirrell DA. Membrane solubilization by hydrophobic polyelectrolyte: surface activity and membrane binding. Biophys J. 1994;67:1101–1106. doi: 10.1016/S0006-3495(94)80575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas JL, Devlin BP, Tirrell DA. Kinetics of membrane micellization by hydrophobic polyelectrolyte poly(2-ethylacrylic acid) Biochim Biophys Acta. 1996;1278:73–78. doi: 10.1016/0005-2736(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang RT, Chen T, Wang Z, Hui MQ, Fu JG. Property of liposomal fusion induced by acid-sensitive polymer. Yao Xue Xue Bao. 2008;43:951–955. Chinese. [PubMed] [Google Scholar]
- 18.Düzgüneş N, Faneca H, Lima MC. Methods to monitor liposome fusion, permeability, and interaction with cells. Methods Mol Biol. 2010;606:209–232. doi: 10.1007/978-1-60761-447-0_16. [DOI] [PubMed] [Google Scholar]
- 19.Bailey AL, Cullis PR. Modulation of membrane fusion by asymmetric transbilayer distributions of amino lipids. Biochemistry. 1994;33:12573–12580. doi: 10.1021/bi00208a007. [DOI] [PubMed] [Google Scholar]
- 20.Chen T, Lu TL, He RH. A study of calcein as an index for evaluation of the pH-sensitivity fusion induces materials and liposomes. Chin J Pharm Anal. 2007;27:1068–1071. [Google Scholar]
- 21.Liang X, Mao G, Simon Ng KY. Mechanical properties and stability measurement of cholesterol-containing liposome on mica by atomic force microscopy. J Colloid Interface Sci. 2004;278:53–62. doi: 10.1016/j.jcis.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 22.Miller CR, Bennett DE, Chang DY, O’Brien DF. Effect of liposomal composition on the critical fusion temperature of photoactivated liposome fusion. Biochemistry. 1996;35:11782–11790. doi: 10.1021/bi960198t. [DOI] [PubMed] [Google Scholar]
- 23.Mills JK, Eichenbaum G, Needham D. Effect of bilayer cholesterol and surface grafted poly(ethylene glycol) on pH-induced release of contents from liposomes by poly(2-ethylacrylic acid) J Liposome Res. 1999;9:275–290. [Google Scholar]
- 24.Hayashi H, Kono K, Takagishi T. Temperature-controlled release property of phospholipid vesicles bearing a thermo-sensitive polymer. Biochim Biophys Acta. 1996;1280:127–134. doi: 10.1016/0005-2736(95)00273-1. [DOI] [PubMed] [Google Scholar]
- 25.Koning GA, Eggermont AMM, Lindner LH, ten Hagen TLM. Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm Res. 2010;27:1750–1754. doi: 10.1007/s11095-010-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atrouse OM. The effects of liposome composition and the temperature on the stability of liposomes and the interaction of liposomes with human neutrophils. Pak J Biol Sci. 2002;5:948–951. [Google Scholar]
- 27.Allon N, Saxena A, Chambers C, Doctor BP. A new liposome-based gene delivery system targeting lung epithelial cells using endothelin antagonist. J Control Release. 2012;160:217–224. doi: 10.1016/j.jconrel.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 28.Roux E, Francis M, Winnik FM, Leroux JC. Polymer based pH-sensitive carriers as a means to improve the cytoplasmic delivery of drugs. Int J Pharm. 2002;242:25–36. doi: 10.1016/s0378-5173(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 29.Linhardt JG, Tirrell DA. pH-induced fusion and lysis of phosphatidylcholine vesicles by the hydrophobic polyelectrolyte poly(2-ethylacrylic acid) Langmuir. 2000;16:122–127. [Google Scholar]
- 30.Chung JC, Gross DJ, Thomas JL, Tirrell DA, Opsahl-Ong LR. pH-sensitive, cation-selective channels formed by a simple synthetic polyelectrolyte in artificial bilayer membranes. Macromolecules. 1996;29:4636–4641. [Google Scholar]
- 31.Barenholz Y. Liposome application: problems and prospects. Curr Opin Colloid Interface Sci. 2001;6:66–77. [Google Scholar]
- 32.Peschka R, Dennehy C, Szoka FC., Jr A simple in vitro model to study the release kinetics of liposome encapsulated material. J Control Release. 1998;56:41–51. doi: 10.1016/s0168-3659(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 33.Chen T, Choi LS, Einstein S, Klippenstein MA, Scherrer P, Cullis PR. H+-induced permeability and fusion of large unilamellar vesicles by covalently conjugated poly(2-ethylacrylic acid) J Liposome Res. 1999;9:387–405. [Google Scholar]
- 34.Bakker-Woudenberg IA, Lokerse AF, Roerdink FH. Antibacterial activity of liposome-encapsulated ampicillin in vitro and in vivo in relation to the lipid composition. J Pharmacol Exp Ther. 1989;251:321–327. [PubMed] [Google Scholar]
- 35.Silvander M, Johnsson M, Edwards K. Effects of PEG-lipids on permeability of phosphatidylcholine/cholesterol liposomes in buffer and in human serum. Chem Phys Lipids. 1998;97:15–26. doi: 10.1016/s0009-3084(98)00088-7. [DOI] [PubMed] [Google Scholar]
- 36.Zhou W, An X, Wang J, Shen W, Chen Z, Wang X. Characteristics, phase behavior and control release for copolymer-liposome with both pH and temperature sensitivities. Colloids Surf A Physicochem Eng Asp. 2012;395:225–232. [Google Scholar]
- 37.Anyarambhatla GR, Needham D. Enhancement of the phase transition permeability of DPPC liposomes by incorporation of MPPC: a new temperature-sensitive liposome for use with mild hyperthermia. J Liposome Res. 1999;9:491–506. [Google Scholar]
- 38.Kono K, Yoshino K, Takagishi T. Effect of poly(ethylene glycol) grafts on temperature-sensitivity of thermosensitive polymer-modified liposomes. J Control Release. 2002;80:321–332. doi: 10.1016/s0168-3659(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 39.Kono K, Nakai R, Morimoto K, Takagishi T. Thermo-sensitive polymer-modifed liposomes that release contents around physiological temperature. Biochim Biophys Acta. 1999;1416:239–250. doi: 10.1016/s0005-2736(98)00226-0. [DOI] [PubMed] [Google Scholar]
- 40.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 41.Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, Papahadjopoulos D. Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks. Int J Radiat Oncol Biol Phys. 1996;36:177–187. doi: 10.1016/s0360-3016(96)00389-6. [DOI] [PubMed] [Google Scholar]
- 42.Kono K. Thermosensitive polymer-modified liposomes. Adv Drug Deliv Rev. 2001;53:307–319. doi: 10.1016/s0169-409x(01)00204-6. [DOI] [PubMed] [Google Scholar]
- 43.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 1999;60:1197–1201. [PubMed] [Google Scholar]
- 44.Ta T, Convertine AJ, Reyes CR, Stayton PS, Por ter TM. Thermosensitive liposomes modified with poly(n-isopropylacrylamide-co-propylacrylic acid) copolymers for triggered release of doxorubicin. Biomacromolecules. 2010;11:1915–1920. doi: 10.1021/bm1004993. [DOI] [PMC free article] [PubMed] [Google Scholar]