Abstract
Drug-induced liver injury (DILI) in humans is difficult to predict using classical in vitro cytotoxicity screening and regulatory animal studies. This explains why numerous compounds are stopped during clinical trials or withdrawn from the market due to hepatotoxicity. Thus, it is important to improve early prediction of DILI in human. In this study, we hypothesized that this goal could be achieved by investigating drug-induced mitochondrial dysfunction as this toxic effect is a major mechanism of DILI. To this end, we developed a high-throughput screening platform using isolated mouse liver mitochondria. Our broad spectrum multiparametric assay was designed to detect the global mitochondrial membrane permeabilization (swelling), inner membrane permeabilization (transmembrane potential), outer membrane permeabilization (cytochrome c release), and alteration of mitochondrial respiration driven by succinate or malate/glutamate. A pool of 124 chemicals (mainly drugs) was selected, including 87 with documented DILI and 37 without reported clinical hepatotoxicity. Our screening assay revealed an excellent sensitivity for clinical outcome of DILI (94 or 92% depending on cutoff) and a high positive predictive value (89 or 82%). A highly significant relationship between drug-induced mitochondrial toxicity and DILI occurrence in patients was calculated (p < 0.001). Moreover, this multiparametric assay allowed identifying several compounds for which mitochondrial toxicity had never been described before and even helped to clarify mechanisms with some drugs already known to be mitochondriotoxic. Investigation of drug-induced loss of mitochondrial integrity and function with this multiparametric assay should be considered for integration into basic screening processes at early stage to select drug candidates with lower risk of DILI in human. This assay is also a valuable tool for assessing the mitochondrial toxicity profile and investigating the mechanism of action of new compounds and marketed compounds.
Key Words: mitochondria, hepatotoxicity, DILI, drugs, chemicals, screening, prediction
Drug-induced liver injury (DILI) occurrence is a major concern for pharmaceutical companies because it can lead to drug withdrawal from the market being imposed by an agency (Food and Drug Administration [FDA] or European Medicines Agency [EMA]) after a careful risk-benefit assessment, or during phase II or III clinical trials by consensus or by company decision. Actually, the worst scenario involves a postmarketing recall combining serious patient health and company damage, a major loss of income, lawsuits stretching over years, loss of credibility, and deteriorated image in media and medical community. Over a thousand drugs described in the modern pharmacopoeia can induce liver damage with different clinical presentations (Biour et al., 2004; Larrey, 2000). Most cases of DILI are benign, accompanied by slight (or moderate) alterations of plasma parameters such as transaminases and bilirubin, and reversible upon treatment cessation. However, with some hepatotoxic drugs and in some patients, DILI may trigger acute liver failure requiring liver transplantation, or even leading to a fatal outcome (Björnsson, 2009).
DILI is classically considered as either intrinsic or idiosyncratic. Whereas intrinsic DILI is usually dose-related and generally discovered during animal toxicity studies, idiosyncratic DILI is less predictable. Indeed, idiosyncratic hepatotoxicity occurs in some individuals with different genetic and metabolic predispositions, or in individuals exposed to other environmental factors (Begriche et al., 2011). It was reported that idiosyncratic DILI represents around 13% of acute liver failure cases in the United States (Ostapowicz et al., 2002). However, because drugs inducing intrinsic hepatotoxicity during clinical development rarely reach the market, most cases of DILI can be considered as idiosyncratic (Pessayre et al., 2010; Stirnimann et al., 2010). Importantly, both types of DILI can result from mitochondrial toxicity.
Indeed, mitochondrial dysfunction is considered as a key mechanism of DILI (Begriche et al., 2011; Labbe et al., 2008; Pessayre et al., 2010; Russmann et al., 2009), although chemicals can cause hepatotoxicity through other pathways, such as the generation of reactive metabolites and specific immune reactions (Lee, 2003; Russmann et al., 2009). All these initial events can have different deleterious consequences for the hepatocytes, thus leading to hepatic cytolysis. Importantly, drug-induced mitochondrial dysfunction may be elicited by a parent drug and/or by reactive metabolites generated through cytochrome P450 (CYP)-mediated metabolism (Begriche et al., 2011; Labbe et al., 2008; Masubuchi et al., 2005). Moreover, it is noteworthy that mitochondrial dysfunction is a generic term including alteration of different metabolic pathways and damage to mitochondrial components. For instance, drugs can (1) impair mitochondrial fatty acid oxidation, electron transfer within the mitochondrial respiratory chain, and the oxidative phosphorylation (OXPHOS) process; (2) deplete the mitochondrial genome by inhibiting the mitochondrial DNA (mtDNA) polymerase γ and/or induce oxidative damage to the mtDNA; and (3) trigger mitochondrial membrane permeabilization, thus inducing the release of mitochondrial proapoptotic proteins into the cytoplasm (Begriche et al., 2011; Fromenty and Pessayre, 1995; Labbe et al., 2008; Lee, 2003; Pessayre et al., 2010; Russmann et al., 2009). In addition, drug-induced blockade of the mitochondrial respiratory chain results in overproduction of reactive oxygen species and lipid peroxidation (Begriche et al., 2011; Berson et al., 1998; Pessayre et al., 2010). Importantly, drug-induced mitochondrial dysfunction can be responsible for cytolytic hepatitis, microvesicular steatosis (Reye-like syndrome), steatohepatitis, liver failure, and even cirrhosis (Begriche et al., 2011; Labbe et al., 2008; Pessayre et al., 2010). Drugs that can induce idiosyncratic DILI through mitochondrial toxicity are, for instance, valproic acid, troglitazone, and antiretroviral drugs such as stavudine and zidovudine (Boelsterli and Lim, 2007; Labbe et al., 2008; Stewart et al., 2010). Finally, it should be stressed that drug-induced mitochondrial toxicity can also involve extrahepatic tissues such as muscles, heart, pancreas, neurons, or kidney, thus eliciting reports on myopathy, rhabdomyolysis, pancreatitis, peripheral neuropathy, or renal dysfunction among others (Dykens and Will, 2007; Gougeon et al., 2004; Igoudjil et al., 2006; Lebrecht et al., 2009; Scatena et al., 2007).
Bearing in mind the serious consequences for patients and pharmaceutical industry, drug-induced mitochondrial dysfunction should be detected early, ideally during screening of potential drug candidates. The development of high-throughput in vitro screening techniques could represent a major breakthrough for a rapid selection of safer compounds (Begriche et al., 2011; Berson et al., 1998; Dykens and Will, 2007; Gougeon et al., 2004; Igoudjil et al., 2006; Labbe et al., 2008; Masubuchi et al., 2005; Pessayre et al., 2010). Hence, the main objective of this study was to determine whether DILI could be predicted using a combination of high-throughput in vitro screening tests performed on isolated mouse liver mitochondria. To this end, we selected 87 compounds documented for inducing DILI in human and 37 compounds without known clinical hepatotoxicity based on the updated “Hepatox” database (http://hepatoweb.com/hepatox.php).
MATERIALS AND METHODS
Reagents and compounds. Oligomycin A, rotenone, m-chlorocarbonylcyanide phenylhydrazone (mClCCP), alamethicin, and other chemicals were purchased either from Sigma Aldrich (Saint-Quentin-Fallavier, France) or from Santa Cruz Biotechnology (Heidelberg, Germany). Cyclosporin A (CsA) was purchased from Tebu Bio SA (Le Perray-en-Yvelines, France).
Purification of mouse liver mitochondria. Liver mitochondria from 6-week-old BALB/cByf female mice (Charles River, Saint-Germain-sur-L’arbresle, France) were isolated and purified by isopycnic density-gradient centrifugation in Percoll, as previously described (Buron et al., 2010; Lecoeur et al., 2004), allowing pure and stable mitochondrial preparations. In previous experiments, we found that parameters measured from mitochondria isolated from female mice showed less interindividual variability than mitochondria from male mice, in particular in response to chemicals (Brenner and Borgne-Sanchez, unpublished data).
Assessment of large amplitude swelling and ΔΨ m. Mitochondrial swelling and mitochondrial transmembrane potential (ΔΨm) were evaluated as described previously (Buron et al., 2010) in presence of succinate and rotenone. Calcium (CaCl2; 50µM) and mCICCP (50µM) were used as the 100% baseline for swelling and loss of ΔΨm, respectively. Effective concentration at 20% of the maximal effect (EC20) for these parameters were the drug concentrations leading to 20% of the maximal swelling and 20% of the maximal loss of ΔΨm after a 30-min incubation, respectively.
Determination of cytochrome c release. Cytochrome c release was evaluated as described previously (Buron et al., 2010) using an ELISA kit (R&D Systems, France). Treatment with 20 µg/ml alamethicin, a peptide able to form channels in membranes, was used as the 100% baseline. EC20 for this parameter was the drug concentration inducing 20% of the maximal cytochrome c release after a 30-min incubation.
Measurement of oxygen consumption. Oxygen consumption was monitored as previously described (Will et al., 2006), with some modifications. Briefly, isolated mitochondria (100 µg proteins) were incubated with drug in buffer containing 250mM sucrose, 30mM K2HPO4, 1mM EGTA, 5mM MgCl2, 15mM KCl, and 1mg/ml bovine serum albumin supplemented with respiratory substrates and 50nM MitoXpress, an oxygen-sensitive phosphorescent dye (LUXCEL, Cork, Ireland). Mitochondrial respiration was measured in the presence of 1.65mM ADP (state 3 of mitochondrial respiration) and with substrates for complex I (5mM malate and 12.5mM glutamate) or complex II (25mM succinate). Rotenone (2µM), a specific inhibitor of complex I, was also added for the assessment of mitochondrial respiration with succinate. Oxygen consumption was then measured in real time for 60min at 37°C in 96-well plates using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) and oligomycin A (1µM) were used as 100% baseline for complex I and complex II inhibition, respectively. The areas under curve were used for calculations. To calculate the activation, the untreated mitochondria were taken as 0% activation. EC20 was the drug concentration causing 20% of the maximal inhibition or activation of oxygen consumption. For some fluorescent or colored compounds (n = 17) interfering with the MitoXpress probe, oxygen consumption through complex I and complex II was measured using a Clark electrode as described previously (Buron et al., 2010).
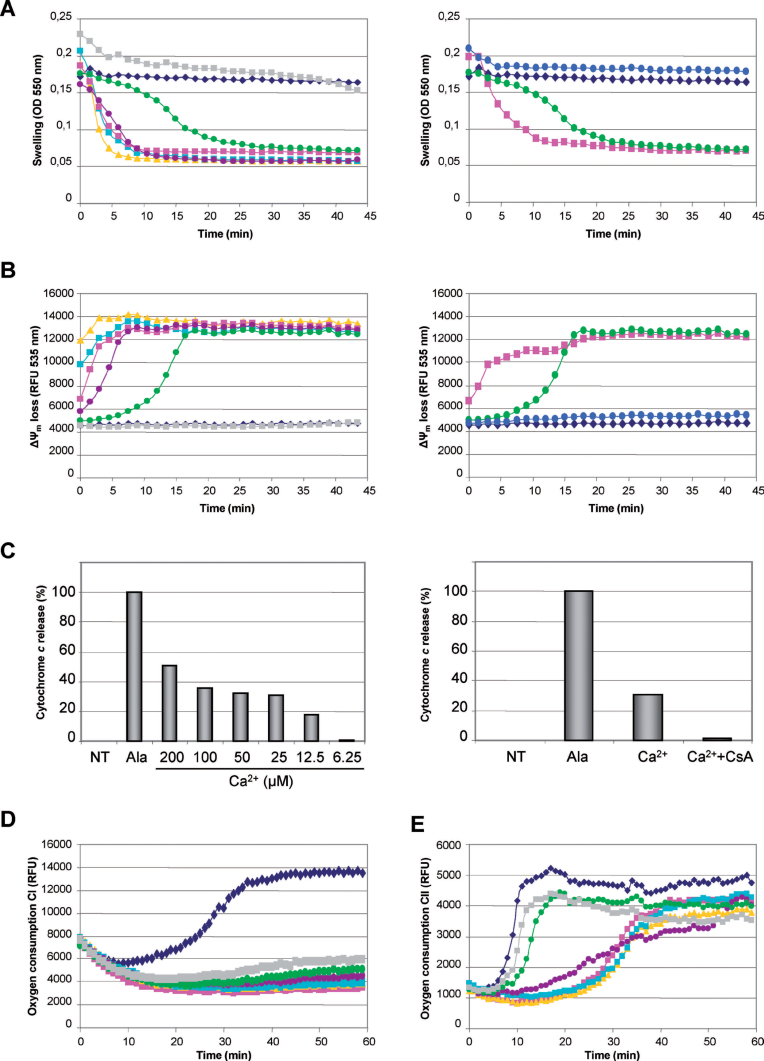
Selection of parameters to detect drug-induced mitochondrial toxicity. Drugs can induce mitochondrial toxicity by altering mitochondrial membrane permeability and/or inhibiting the respiratory chain (Begriche et al., 2011; Labbe et al., 2008; Pessayre et al., 2010). These events can be rapidly studied on isolated mouse liver mitochondria by assessing the mitochondrial swelling, loss of ΔΨm, cytochrome c release, and oxygen consumption (i.e., respiration). Swelling and loss of ΔΨm were comonitored by real-time spectrofluorimetry (Figs. 1A and 1B). Calcium (Ca2+) was used as positive control inducing a massive mitochondrial swelling (Fig. 1A, left) and loss of ΔΨm (Fig. 1B, left) through mitochondrial permeability transition pore (mPTP) opening with subsequent cytochrome c release (Fig. 1C, left). Importantly, the specific mPTP inhibitor CsA inhibited the Ca2+-induced swelling (Fig. 1A, right), loss of ΔΨm (Fig. 1B, right), and cytochrome c release (Fig. 1C, right). The OXPHOS uncoupler mClCCP was used to decrease the ΔΨm in a mPTP-independent manner (Fig. 1B, right). Alamethicin was chosen as a positive control inducing a massive cytochrome c release (Fig. 1C). Besides mitochondrial membrane integrity, we also assessed the oxygen consumption with respiratory substrates oxidized by complex I (malate + glutamate) (Fig. 1D) or complex II (succinate) (Fig. 1E). The oxygen sensitive probe MitoXpress was used for most compounds for a rapid screening by spectrofluorimetry (Will et al., 2006).
FIG. 1.
Selected parameters used to detect mitochondrial alterations. (A) Mitochondrial swelling. Left panel: mitochondria isolated from mouse liver were untreated (◆) or treated with increasing Ca2+ concentrations before evaluation of mitochondrial swelling (▲200, ◼100, ◼50, •25, •12.5, and ◼6.25µM). Right panel: massive mitochondrial swelling induced by 50µM Ca2+ (◼) was taken as 100% baseline. Preincubation of mitochondria with 10µM CsA (•) fully inhibited mitochondrial swelling induced by 12.5µM Ca2+ (•), thus giving a curve similar to untreated mitochondria (◆). (B) Loss of ΔΨm. Left panel: loss of ΔΨm was simultaneously measured in real time in presence of increasing Ca2+ concentrations (same symbols as left panel of Fig. 1A). Right panel: collapse of ΔΨm induced by 50µM mClCCP (◼) was taken as 100% baseline. Preincubation with 10µM CsA (•) fully inhibited the collapse of ΔΨm induced by 12.5µM Ca2+ (•), thus giving a curve similar to untreated mitochondria (◆). (C) Cytochrome c release. Left panel: supernatants of Ca2+- and alamethicin-treated mitochondria were subjected to ELISA assays to assess cytochrome c release. Massive cytochrome c release obtained with 20 µg/ml alamethicin was used as 100% baseline. Right panel: preincubation with 10µM CsA almost fully inhibited the cytochrome c release induced by 25µM Ca2+. (D) Mitochondrial respiration through complex I. Oxygen consumption in the presence of malate, glutamate, and ADP was measured without (◆) or with rotenone (◼2, ▲1, ◼0.5, •0.25, •0.125, and ◼0.062µM). The massive oxygen consumption inhibition caused by 2µM rotenone was taken as 100% baseline. (E) Mitochondrial respiration through complex II. Oxygen consumption in the presence of succinate, ADP, and rotenone was measured without (◆) or with oligomycin A (◼1, ▲0.5, ◼0.25, •0.125, •0.062, and ◼0.031µM). The massive oxygen consumption inhibition caused by 1µM oligomycin A was taken as 100% baseline.
Statistical analysis. In this study, data related to mitochondrial toxicity were only those obtained from our multiparametric assay, and thus data from the literature were not considered for statistical analysis. The relationship between mitochondriotoxicity (yes/no) and hepatotoxicity (yes/no) was assessed using a χ2-test. Sensitivity, specificity, and predictive values of mitochondriotoxicity in term of hepatotoxicity were calculated as below:
 |
RESULTS
Multiparametric Screening of 124 Compounds, Mainly Drugs, on Isolated Mouse Liver Mitochondria
We measured the ability of numerous hepatotoxic (n = 87) and nonhepatotoxic (n = 37) compounds to induce mitochondrial toxicity (Table 1). Compounds able to induce liver injury were selected within the library created by Biour et al. (2004) and the corresponding updated “Hepatox” database (http://hepatoweb.com/hepatox.php). These 124 compounds were all tested for their ability to induce swelling, loss of ΔΨm, cytochrome c release, or an inhibition of the succinate-driven state 3 oxygen consumption. Compounds not altering these four parameters were then evaluated for their ability to inhibit oxygen consumption in the presence of malate and glutamate. For all these parameters, EC20 were determined in comparison with the 100% baseline obtained with their respective positive controls. It is noteworthy that some compounds (e.g., carbamazepine, erlotinib, lidocaine, saquinavir, spectinomycin, and zidovudine) increased oxygen consumption with malate/glutamate and/or succinate. Nevertheless, these compounds were considered as toxic for mitochondria. Indeed, enhanced oxygen consumption often reflects an increased mitochondrial entry of protons, which can be elicited by OXPHOS uncoupling, or by a global loss of inner mitochondrial membrane integrity (Begriche et al., 2011; Fromenty and Pessayre, 1995; Labbe et al., 2008).
TABLE 1.
Effects of the Compounds on Each Parameter of Mitochondrial Toxicity
| Compound | Therapeutic class | Route of administration | Swelling | ΔΨm loss | Cyto c | O2 cons CII | O2 cons CI | C max µM |
|---|---|---|---|---|---|---|---|---|
| EC20 µM | EC20 µM | EC20 µM | EC20 µM | EC20 µM | ||||
| Acetaminophen | Analgesic | IV, PO, R | > 200 | > 200 | > 400 | 348.5 | > 400 | 130 |
| Acetylsalicylic acid | NSAID | PO | > 800 | 335.5 | > 200 | > 800 | 149.8 | 1650 |
| Alpidem | Anxiolitic | PO | ND | 83.7 | 394.7 | 25.6 | 29.6 | 0.3 |
| Amantadine | Antiviral | PO | > 800 | 261.2 | > 400 | > 400 | > 400 | 1.7 |
| Ambroxol | Expectorant | PO | > 400 | > 200 | > 400 | > 400 | > 200 | 0.48 |
| Amiodarone | Antiarythmic | IV, PO | ND | 2.6 | < 50 | 45.92 | ND | 0.81 |
| Amoxicillin | Antibiotic | IM, IV, PO | > 400 | > 400 | > 400 | 90.8* | 188.8* | 14.1 |
| Ampicillin | Antibiotic | IM, IV | > 400 | > 400 | > 400 | > 400 | 161.2* | 8.42 |
| Antipyrine | NSAID | A, PO | > 400 | 300.0 | > 400 | > 400 | > 400 | 92.5 |
| Arsenic trioxide | Anticancer | IV | > 200 | 237.9 | > 200 | < 50 | 0.9 | 0.17 |
| Atorvastatin | Hypolipidemic | PO | 5.6 | 4.3 | < 50 | 44.5 | ND | 0.06 |
| Biotin | Nutritive agent | IM, IV, PO | > 400 | > 400 | > 400 | > 400 | > 400 | < 1 |
| Bisacodyl | Antihypertensive | PO, R | > 400 | 312.4 | > 400 | 50.9 | 52.5 | 0.15 |
| β-Estradiol | Hormone | C, IM, PO, V | ND | > 200 | > 200 | > 200 | > 200 | 0.0006 |
| Bupivacaine | Local anesthetic | IS | > 800 | 258.2 | > 800 | 60.6 | > 800 | 0.7 |
| Busulfan | Anticancer | IV, PO | > 800 | 483.1 | > 400 | 169.8 | ND | 4.9 |
| Butein | Anticancer | IP | > 200 | > 200 | > 200 | 29.6 | ND | ND |
| Caffeine | Analgesic | PO | > 400 | > 400 | > 400 | > 400 | > 400 | 42 |
| Capsaicin | Topical analgesic | C | > 200 | 275.0 | > 200 | 15.0 | 15.7 | 0.06 |
| Carbamazepine | Anticonvulsivant | PO | > 200 | 66.9 | > 400 | 53.4 | 170.8* | 6.43 |
| Cefixime | Antibiotic | PO | > 400 | > 400 | > 400 | 41.8 | 216.8 | 7.29 |
| Chlorambucil | Anticancer | PO | > 200 | > 200 | > 200 | 138.7 | 140.9 | 1.97 |
| Ciprofloxacin | Antibiotic | IV, PO | > 400 | > 400 | > 400 | 195.0* | ND | 16 |
| Clodronate | Antihypercalcemic | IV, PO | > 400 | > 400 | > 400 | > 400 | 227.2 | 2.77 |
| Clotrimazole | Antifungal | V | ND | 23.9 | > 800 | 2.9 | ND | 1.02 |
| Coumarin | Anticoagulant | C, PO | > 200 | 0.9 | > 100 | ND | ND | 1.25 |
| Curcumin | Phytotherapy | PO | > 200 | > 200 | > 200 | > 200 | > 200 | 1.75 |
| Dapsone | Antibiotic | PO | > 400 | > 400 | > 400 | > 400 | 205.5 | 5.6 |
| Dasatinib | Anticancer | PO | 127.0 | 20.8 | 167.7 | 200.4 | 42.8* | 0.23 |
| Daunorubicin | Anticancer | IV | < 6.25 | ND | 47.2 | 12.8 | 10.9 | 78 |
| Dexamethasone | Glucocorticoid | A, O, PO | > 200 | > 200 | > 200 | > 200 | > 200 | 0.23 |
| Diazoxide | Antihypertensive | PO | > 200 | > 200 | > 200 | 4.9 | ND | 151.73 |
| Diclofenac | NSAID | C, IM, PO | > 800 | 137.9 | > 200 | 9.1 | 29.8 | 4.2 |
| Diflunisal | NSAID | IV, PO | > 200 | 17.9 | > 200 | 9.8 | ND | 495 |
| Dipyrone | Analgesic | IV, PO | > 200 | 354.4 | > 200 | 136.0 | 107.3 | 34.5 |
| Disulfiram | Antialcoholism | PO | > 400 | 12.6 | > 400 | < 100 | ND | 5.4 |
| Doxorubicin | Anticancer | PO | 85 | 8.9 | 23.3 | 15.9 | ND | 0.2 |
| D-penicillamine | Immunosuppressor | IV | > 400 | > 400 | > 400 | > 800 | > 800 | 53.62 |
| Econazole | Antifungal | C, V | 30.5 | 13.3 | 298.7 | 4.2 | ND | 540 |
| Erlotinib | Anticancer | PO | ND | 195.9 | ND | 328.8 | 17.4* | 13.79 |
| Erythromycin | Antibiotic | C, IV, PO | > 400 | > 400 | > 400 | 293.7 | 27.9 | 11 |
| Ethyl paraben | Preservative | C, PO | > 400 | 297.5 | > 400 | 293.1 | 47.8 | ND |
| Folic acid | Vitamin | IV, PO | > 800 | 276.2 | > 400 | > 400 | 213.0 | 3.4 |
| Fluconazole | Antifungal | PO | > 400 | 512.7 | > 400 | > 400 | 186.6 | 21.94 |
| Flufenamic acid | NSAID | PO | > 200 | 42.5 | > 200 | 1.7 | ND | 46 |
| Flutamide | Antiandrogen | PO | > 800 | 27.0 | > 400 | ND | ND | 6 |
| Gallic acid | Antioxidant | PO | > 200 | > 200 | > 200 | 103.7 | 153.9 | 2 |
| Gefitinib | Anticancer | PO | 63.6 | 9.6 | 50.5 | 269.6 | ND | 0.72 |
| Genistein | Anticancer | PO | > 200 | 150.6 | > 200 | 81.3 | ND | 1.84 |
| Gentamicin | Antibiotic | IM, IV, O | > 800 | 49.0 | > 200 | > 200 | ND | 13 |
| Glimepiride | Antidiabetic | PO | ND | 94.6 | > 800 | 16.6 | ND | 0.5 |
| Gossypol | Anticancer | PO | > 10 | 1.0 | > 10 | 30.3 | ND | 1.99 |
| Hyperforin | Antidepressant | PO | > 10 | 0.1 | > 10 | ND | ND | 0.23 |
| Ibuprofen | NSAID | C, PO | > 200 | 355.9 | > 200 | 170.1 | 132.1 | 250 |
| Imatinib | Anticancer | PO | ND | 25.7 | 163.6 | ND | ND | 2.71 |
| Imipramine | Antidepressant | PO | 274.4 | 74.8 | > 400 | 75.5* | 18.3* | 0.6 |
| Indomethacin | NSAID | O, PO, R | > 800 | 443.2 | > 200 | 25.2 | ND | 6 |
| Isoniazide | Antibiotic | PO | > 800 | 504.6 | > 400 | 59.8 | ND | 40 |
| Ketoconazole | Antifungal | C | > 400 | 243.0 | > 400 | > 400 | 2.9 | 7 |
| Lamivudine | Antiretroviral | PO | > 400 | 347.2 | ND | 160.7 | 317.4 | 17 |
| Lidocaine | Local anesthetic | A, B, O | > 800 | 339.5 | > 800 | > 400 | 188.2* | 36 |
| Lonidamine | Anticancer | IV, PO | 660.0 | 138.1 | > 800 | 18.9 | ND | 23.6 |
| Lovastatin | Hypolipidemic | PO | 71.5 | 43.6 | 118.2 | 4.4 | 6.2 | 0.01 |
| Lumiracoxib | NSAID | PO | > 200 | 148.8 | > 800 | 26.3 | 12.0 | 22.47 |
| Manganese chloride | Nutritive agent | IV, PO | > 800 | > 800 | > 800 | > 400 | > 400 | 0.053 |
| Mefenamic acid | NSAID | PO | > 400 | 49.7 | > 200 | 10.1 | ND | 15.74 |
| Mercaptopurine | Anticancer | PO | > 400 | 406.9 | > 400 | 130.8 | 131.8* | 1 |
| Metformin | Antidiabetic | PO | > 400 | 388.4 | > 800 | > 400 | 351.8 | 4.5 |
| Methimazole | Antithyroid | PO | > 400 | > 400 | ND | 193.8 | 368.6 | 9.2 |
| Methyldopa | Antihypertensive | PO | > 400 | > 400 | ND | > 800 | 63.1 | 11 |
| Methyl paraben | Preservative | C, PO | > 200 | > 200 | > 200 | > 200 | 94.8 | ND |
| Mitomycin C | Antineoplastic antibiotic | IV | ND | > 200 | > 200 | 4.9 | ND | 7.1 |
| Mitoxantrone | Anticancer | IV | ND | 7.00 | > 400 | ND | ND | 1.9 |
| Molsidomine | Antianginal | PO | > 400 | > 400 | > 400 | > 200 | > 200 | 0.31 |
| Naloxone | Analgesic | IV | > 400 | 298.7 | > 400 | 233* | > 400 | 0,00047 |
| Nelfinavir | Antiretroviral | PO | ND | 6.3 | > 200 | < 25 | ND | 6 |
| Nicergoline | Anti-ischemic | PO | 169.8 | 82.6 | > 200 | 57.0* | 42.7* | 0.0002 |
| Nicotine | Smoking deterrent | C, PO | > 400 | > 400 | > 400 | > 400 | 312 | 0.09 |
| Nifuroxazide | Antibiotic | PO | > 400 | 388.2 | > 400 | 61.5 | 3.7 | ND |
| Nilotinib | Anticancer | PO | ND | 11.5 | > 400 | ND | 71.4* | 3.6 |
| Nimesulide | NSAID | PO | > 200 | 10.1 | > 200 | < 25 | ND | 15 |
| Nitrofurantoin | Antibiotic | PO | > 800 | 442.5 | > 400 | 232.3 | 8.7 | 6 |
| Novobiocin | Antibiotic | PO | 289.5 | 351.6 | > 400 | 88.2 | 173.5 | 1 |
| Oxybutynine | Spasmolytic | PO | 302.8 | 178.3 | > 400 | 172.8 | 115.4 | 0.17 |
| Pargyline | Stimulant laxative | PO | > 400 | 243 | > 400 | > 400 | 206.8 | 0.3 |
| Perhexiline | Antianginal | PO | 14.8 | 3.2 | < 25 | 88.4 | 87.7 | 0.28 |
| Phenylbutazone | NSAID | C | > 400 | 140.2 | ND | 11.4 | ND | 438 |
| Phloroglucinol | Antispasmodic | IM, IV, PO | > 800 | > 800 | > 400 | > 400 | > 400 | ND |
| Piroxicam | NSAID | IM, PO | > 200 | 101.7 | > 200 | 224.5 | 6.6 | 5 |
| Pravastatin | Hypolipidemic | PO | > 200 | > 200 | > 200 | 5.0 | ND | 0.10 |
| Propyl paraben | Preservative | C, PO | > 800 | 162.8 | > 800 | 63.0 | 28.4 | ND |
| Propylthiouracil | Antithyroid | PO | > 800 | 391.5 | > 400 | 66.9* | 15.8* | 42 |
| Pyrazinamide | Antibiotic | PO | > 400 | > 400 | > 400 | 107.5 | 190.9 | 325 |
| Ranitidine | Antiulcer agent | IM, IV, PO | > 400 | 368.1 | ND | > 400 | 97.3 | 3.99 |
| Resveratrol | Antiaging | PO | > 200 | 395.4 | > 200 | 7.7 | ND | 0.18 |
| Riboflavin | Vitamin | IV, PO | 672.3 | ND | > 400 | 264.6 | 182.8 | 0.0039 |
| Rifampicin | Anti-infectious | IV, PO | > 800 | > 800 | > 200 | 124.1 | ND | 9 |
| Ritonavir | Antiretroviral | PO | ND | 24.8 | ND | 35.5 | ND | 7.07 |
| Roxithromycin | Antibiotic | PO | 310.0 | 244.8 | > 400 | 104.9 | ND | 13.14 |
| Saccharin | Sweetening agent | PO | > 400 | > 400 | > 400 | > 400 | 179.4 | 147.37 |
| Salicylic acid | NSAID | C, O | > 400 | 304.3 | > 200 | 354.4 | 120.9 | 604.63 |
| Saquinavir | Antiretroviral | PO | ND | 10.3 | > 400 | 21.1* | 30.4* | 0.37 |
| Simvastatin | Hypolipidemic | PO | 173.2 | 76.5 | > 200 | 1.6 | ND | 0.02 |
| Sorafenib | Anticancer | PO | ND | 0.5 | > 400 | 283.4 | ND | 5.60 |
| Spectinomycin | Antibiotic | IM | > 400 | > 400 | > 400 | 185.9* | 118.2* | 322.97 |
| Streptomycin | Antibiotic | IM, IV | 417.5 | 78.8 | ND | > 400 | ND | 52 |
| Sucrose | Antiasthenic | PO | > 800 | > 800 | > 800 | > 800 | > 800 | ND |
| Sulfamethoxazole | Antibiotic | IV, PO | > 400 | > 400 | > 400 | > 400 | > 400 | 159.9 |
| Sulindac | NSAID | PO | > 200 | > 200 | > 200 | > 400 | 35.6 | 19 |
| Sumatriptan | Antiheadaches | N, PO, SCI | > 400 | 248.3 | > 400 | > 400 | > 400 | 0.04 |
| Sunitinib | Anticancer | PO | ND | 23.2 | ND | 222.8 | ND | 70.8 |
| Tamoxifen | Anticancer | PO | 9.0 | 2.9 | 7.7 | ND | ND | 0.40 |
| Taurine | Antiasthenic | IV | > 800 | > 800 | > 800 | > 800 | > 800 | ND |
| Terbinafine | Antifungal | C, PO | 20.6 | 12.5 | > 50 | ND | ND | 4 |
| Ticlopidine | Antiplatelet | PO | 58.9 | 50.5 | ND | ND | ND | 7.06 |
| Tolcapone | Anti-Parkinson | PO | > 400 | 3.9 | > 400 | ND | ND | 27.81 |
| Tolfenamic acid | NSAID | PO | 335.3 | 7.1 | > 200 | 3.7 | ND | 4.16 |
| Tramadol | Analgesic | IV, PO | > 800 | 263.8 | > 400 | > 400 | 238.7* | 1.9 |
| Troglitazone | Antidiabetic | PO | > 200 | 3.4 | > 400 | 3.9 | 6.0 | 6.60 |
| Troxerutin | Antihemoroid | PO | > 400 | > 400 | > 400 | 39.3 | 170.5 | 0.004 |
| Valproic acid | Antiepileptic | IV | > 800 | 267.2 | > 800 | > 400 | 44.9 | 902 |
| Vinblastine | Anticancer | IV | 185.7 | 25.7 | > 400 | 5.6 | 102.3* | 0.13 |
| Ximelagatran | Anticoagulant | PO | > 400 | 535.7 | > 800 | 85.6 | 341.8* | 0.45 |
| Zidovudine | Antiretroviral | IV, PO | > 200 | 416.1 | > 200 | > 800 | 242.0* | 4 |
Note. Selected compounds (n = 124) were tested for their ability to induce swelling, loss of ΔΨm, cytochrome c release, and inhibition of mitochondrial respiration through complexes I and II. EC20 was calculated using GraphPad Prism 4. EC20 with an asterisk (*) indicates drug-induced acceleration of oxygen consumption. Results are means of two to three independent experiments (SD < 10%). The C max found in the literature (PubMed) or databases (Pharmapendium, RxList, and ToxNet) are indicated. For each compound the therapeutic class and the usual route of administration are indicated (data from the Vidal and DrugBank databases). A, auricular; B, buccal; C, cutaneous; IM, intramuscular; IP, intraperitoneal; IS, intraspinal; IV, intravenous; N, nasal; ND, not determined; O, ophthalmic; PO, per os; R, rectal; SCI, subcutaneous injection; V, vaginal.
Correlation Analysis Between Mitochondrial Toxicity and DILI in Human
Two different approaches were used to ascertain drug-induced mitochondrial toxicity (Table 2). In the first approach, a drug was considered as toxic for mitochondria if, for at least one of the five mitochondrial parameters, the EC20 was ≤ 100 × C max (maximal plasma concentration), a cutoff currently used for safety assessment in pharmaceutical industry (Dykens et al., 2008). Because C max was not available for several compounds selected in this study, we used a second approach for which a compound was considered as toxic for mitochondria if the EC20 was ≤ 200µM for at least one of the five mitochondrial parameters. We indicated for each hepatotoxicant (Table 2A) the proposed (or suspected) mechanisms of toxicity, if any, and whether DILI was detected in animals. For nonhepatotoxic compounds (Table 2B) the occurrence of other organ toxicities was reported.
TABLE 2A.
Mitochondrial Toxicity of the 124 Selected Compounds
| Compound | Mitotox | Known or suspected mechanism(s) | Animal hepatotox | ||
|---|---|---|---|---|---|
| 100 × C max | 200µM | ||||
| Acetaminophen | Y | N | RM, OS, M | Y | |
| Acetylsalicylic acid | Y | Y | OS, M, Apop | ||
| Alpidem | Y | Y | RM, OS, M | ||
| Amiodarone | Y | Y | OS, M, SL | N | |
| Amoxicillin | Y | Y | OS | N | |
| Ampicillin | Y | Y | M | Y | |
| Arsenic trioxide | Y | Y | M, Apop | N | |
| Atorvastatin | Y | Y | IM, M, Apop | N | |
| Bupivacaine | Y | Y | M, OS | N | |
| Busulfan | Y | Y | OS | Y | |
| Carbamazepine | Y | Y | RM, M | Y | |
| Cefixime | Y | Y | Y | ||
| Chlorambucil | Y | Y | OS | ||
| Ciprofloxacin | Y | Y | OS | N | |
| Clotrimazole | Y | Y | Y | ||
| Coumarin | Y | Y | RM, OS | ||
| Dapsone | Y | N | RM, OS | N | |
| Dasatinib | Y | Y | N | ||
| Daunorubicin | Y | Y | OS | Y | |
| Dexamethasone | N | N | SL, M | N | |
| Diclofenac | Y | Y | RM, OS, M, Apop, IM | Y | |
| Diflunisal | Y | Y | RM, M | N | |
| Dipyrone | Y | Y | |||
| Disulfiram | Y | Y | OS, M | N | |
| Doxorubicin | Y | Y | OS, Apop | N | |
| D-penicillamine | N | IM | N | ||
| Econazole | Y | Y | N | ||
| Erlotinib | Y | Y | RM | Y | |
| Erythromycin | Y | Y | IBST, RM | N | |
| Fluconazole | Y | Y | Y | ||
| Flufenamic acid | Y | Y | M | N | |
| Flutamide | Y | Y | RM, M | N | |
| Gefitinib | Y | Y | RM | Y | |
| Gentamicin | Y | Y | M, OS | N | |
| Glimepiride | Y | Y | N | ||
| Gossypol | Y | Y | M | ||
| Ibuprofen | Y | Y | M | N | |
| Imatinib | Y | Y | Y | ||
| Imipramine | Y | Y | RM, M | N | |
| Indomethacin | Y | Y | M | N | |
| Isoniazid | Y | Y | IM, RM, OS | N | |
| Ketoconazole | Y | Y | RM, OS, M | N | |
| Lamivudine | Y | Y | M | N | |
| Lidocaine | Y | Y | M | N | |
| Lonidamine | Y | Y | M, Apop | ||
| Lovastatin | N | Y | M | N | |
| Lumiracoxib | Y | Y | RM | ||
| Mefenamic acid | Y | Y | M | N | |
| Mercaptopurine | N | Y | OS, M, DNA Syn | N | |
| Metformin | Y | N | M | Y | |
| Methimazole | Y | Y | RM, OS | N | |
| Methyldopa | Y | Y | IM, RM | N | |
| Mitomycin C | Y | Y | RM, OS, DNA Syn | N | |
| Mitoxantrone | Y | Y | RM, DNA Syn | N | |
| Nelfinavir | Y | Y | SL, OS | Y | |
| Nimesulide | Y | Y | M, RM, OS | N | |
| Nitrofurantoin | Y | Y | IM, OS, M | N | |
| Novobiocin | Y | Y | DNA Syn, M | N | |
| Perhexiline | Y | Y | M | N | |
| Phenylbutazone | Y | Y | M | N | |
| Piroxicam | Y | Y | M | N | |
| Pravastatin | Y | Y | Y | ||
| Propylthiouracil | Y | Y | IM | N | |
| Pyrazinamide | Y | Y | OS | N | |
| Ranitidine | Y | Y | N | ||
| Rifampicin | Y | Y | IBST, OS | Y | |
| Ritonavir | Y | Y | Apop, SL | Y | |
| Roxithromycin | Y | Y | N | ||
| Saccharin | Y | Y | |||
| Saquinavir | Y | Y | SL | N | |
| Simvastatin | Y | Y | Apop, M | N | |
| Sorafenib | Y | Y | Apop | N | |
| Spectinomycin | Y | Y | |||
| Streptomycin | Y | Y | M | ||
| Sulindac | Y | Y | IBST, OS, M | N | |
| Sunitinib | Y | Y | Apop | N | |
| Tamoxifen | Y | Y | M, RM, OS, SL | N | |
| Terbinafine | Y | Y | RM | Y | |
| Ticlopidine | Y | Y | RM, IM | Y | |
| Tolcapone | Y | Y | M, RM | N | |
| Tolfenamic acid | Y | Y | M | ||
| Tramadol | N | N | N | ||
| Troglitazone | Y | Y | RM, M, OS, IBST, Apop | Y | |
| Valproic acid | Y | Y | M, RM | N | |
| Vinblastine | Y | Y | Apop | N | |
| Ximelagatran | N | Y | IM | N | |
| Zidovudine | Y | N | M | N | |
| Compared to human | concordance: 94% 92% error rate: 6% 8% |
||||
| Compared to animal | concordance: 100% 90% error rate: 0% 10% |
||||
TABLE 2B.
| Compound | Mitotox 100 × Cmax | Mitotox 200µM | Other organ toxicity |
|---|---|---|---|
| Amantadine | N | N | Heart |
| Ambroxol | N | N | |
| Antipyrine | Y | N | |
| Biotin | N | N | |
| Bisacodyl | N | Y | |
| β-Oestradiol | N | N | Pancreas |
| Butein | Y | ||
| Caffeine | N | Reproduction | |
| Capsaicin | N | Y | Skin |
| Clodronate | Y | N | Gastro intestinal |
| Curcumin | N | N | |
| Diazoxide | Y | Y | Kidney, pancreas (*) |
| Ethyl paraben | Y | Reproduction (*) | |
| Folic acid | Y | N | Gastro intestinal, CNS |
| Gallic acid | Y | Y | |
| Genistein | Y | Y | Reproduction |
| Hyperforin | Y | Y | |
| Manganese chloride | N | N | |
| Methyl paraben | Y | Reproduction (*) | |
| Molsidomine | N | N | |
| Naloxone | N | N | Heart |
| Nicergoline | N | Y | |
| Nicotine | N | N | |
| Nifuroxazide | Y | Pancreas | |
| Nilotinib | Y | Y | Heart (*) |
| Oxybutynine | N | Y | Brain |
| Pargyline | N | N | |
| Phloroglucinol | N | N | |
| Propyl paraben | Y | Reproduction (*) | |
| Resveratrol | Y | Y | |
| Riboflavin | N | Y | |
| Salicylic acid | Y | Y | Kidney |
| Sucrose | N | Kidney | |
| Sulfamethoxazole | N | Pancreas | |
| Sumatriptan | N | N | |
| Taurine | N | ||
| Troxerutin | N | Y | |
| Compared to human | concordance: 64% error rate: 36% |
51% 49% |
Note. The compounds are listed for their ability to induce (Y) or not (N) mitochondrial toxicity on isolated mouse liver mitochondria. Mitochondrial toxicity was ascertained by two approaches using a different cutoff (100 × Cmax or 200µM). Blanks mean that the mitochondrial toxicity related to Cmax could not have been determined because the Cmax value was not found in the literature or databases. (A) Hepatotoxicants in human according to Biour et al. (2004) and to the updated “Hepatox” database (http://hepatoweb.com/hepatox.php) with indication of known (or suspected) mechanisms of DILI and detection of hepatotoxicity in animals during preclinical studies. (B) Nonhepatotoxicants in human with indication of known toxicity to other organs where (*) indicates known (or suspected) mitochondrial toxicity. Apop, apoptosis; DNA Syn, DNA synthesis; IBST, inhibition of bile salt transport; IM, immune-mediated; OS, oxidative stress; M, mitochondrial; RM, reactive metabolites; SL, stimulation of lipogenesis. Blanks are data not found in the databases or literature. The concordance and error rate between mitochondrial toxicity in our assay and human hepatotoxicity, and/or animal hepatototoxicity are indicated for each cutoff.
Using the first definition of mitochondrial toxicity (100 × C max), only 114 compounds were taken into account (Table 3A). Interestingly, 81 of the 86 (94%) compounds able to induce DILI were found to be toxic for mitochondria, demonstrating a very high sensitivity. In contrast, 36% of the compounds not inducing DILI were found to be toxic for mitochondria. This difference was statistically significant and showed a significant relationship between mitochondrial toxicity and DILI (p < 0.001). With the second approach (EC20 ≤ 200µM), all the 124 compounds were included (Table 3B) and 80 of the 87 (92%) compounds known to induce DILI were found to be toxic for mitochondria. However, 49% of the compounds not inducing DILI were found to be toxic for mitochondria. This difference was also statistically significant (p < 0.001). Thus, by using two different definitions of mitochondrial liability, our study showed a highly significant relationship between toxicity on isolated mouse liver mitochondria and DILI in human.
TABLE 3.
Relationship Between Mitochondrial Toxicity and the Occurrence of DILI in Human
| A. | ||||
| Number (%) cutoff at 100 × C max | Hepatotoxic compound | p value | ||
| Yes n = 86 |
No n = 28 |
|||
| Mitochondriotoxic compound | Yes | 81 (94%) | 10 (36%) | < 0.001 (χ2) |
| No | 5 (6%) | 18 (64%) | ||
| Predictive positive value: 89% Predictive negative value: 78% |
||||
| B. | ||||
| Number (%) cutoff at 200µM | Hepatotoxic compound | p value | ||
| Yes n = 87 |
No n = 37 |
|||
| Mitochondriotoxic compound | Yes | 80 (92%) | 18 (49%) | < 0.001 (χ2) |
| No | 7 (8%) | 19 (51%) | ||
| Predictive positive value: 82% Predictive negative value: 73% |
||||
Note. Results of the χ2-test are reported with the two approaches used to establish mitochondrial toxicity induced by chemicals (A) ascertained with the 100 × C max cutoff and (B) ascertained with the 200µM cutoff. The p values are indicated for both conditions.
Our results indicated positive predictive values of 89 (81/91) and 82% (80/98) depending on the definition of mitochondrial toxicity (Table 3). Thus, any compound found to induce mitochondrial toxicity with our multiparametric assay would have a high probability for inducing DILI in human. We also calculated the negative predictive values that provided a reasonable threshold for correct clinical outcome with 78 (18/23) and 73% (19/26) depending on cutoff (Table 3). Specificity calculated as 64 or 51% depending on cutoff was modest, probably due to sample size and involvement of mitochondrial toxicity in other tissues. Interestingly, the false-negative group (i.e., nonmitochondriotoxic but hepatotoxic compounds) showed a low percentage (6 or 8%), which is encouraging in a screening assay contrary to the false-positive group (i.e., mitochondriotoxic but nonhepatotoxic compounds), which was much higher (36 or 49%).
Mitochondrial Toxicity of Compounds Already Known to Have Deleterious Effects on Mitochondria
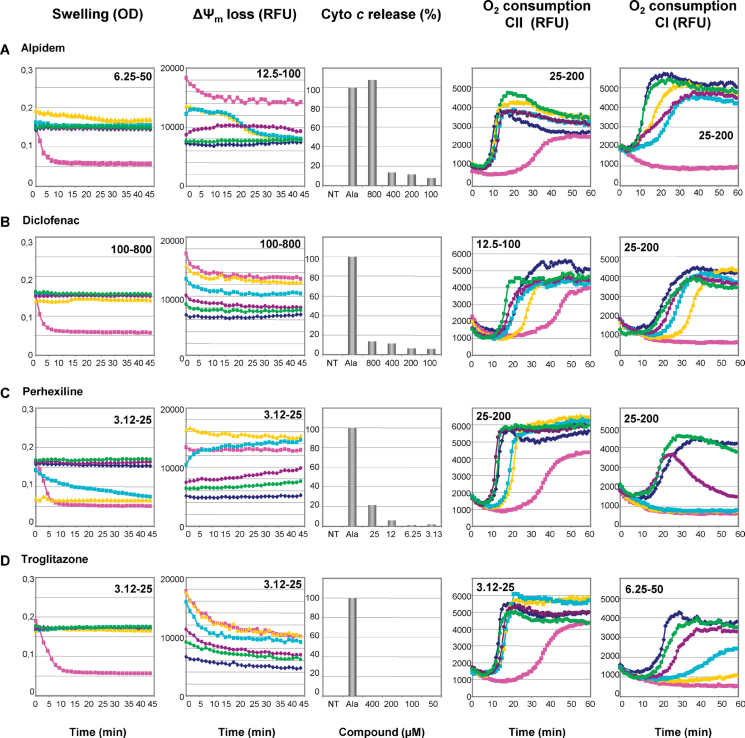
In this study, we confirmed the mitochondrial toxicity of several chemicals mainly compounds for which such deleterious effect had already been demonstrated in different experimental models including rodent liver, primary cultured hepatocytes, and isolated mitochondria. This is, for instance, the case for alpidem, diclofenac, perhexiline maleate, and the highly debated troglitazone (Table 1; Fig. 2).
FIG. 2.
Mitochondrial toxicity of compounds already described to cause mitochondrial dysfunction. Isolated mouse liver mitochondria were untreated (◆) or treated with increasing concentrations (•, •, ◼, and ▲; range indicated on the graphs) of alpidem, diclofenac, perhexiline maleate, and troglitazone, or with positive controls (◼). Mitochondrial swelling, loss of ΔΨm, cytochrome c release, and inhibition of oxygen consumption were thus assessed as described in Materials and Methods and Figure 1.
Alpidem was described to accelerate Ca2+-induced mPTP, uncouple OXPHOS, and selectively inhibit the respiratory complex I on isolated rat liver mitochondria (Berson et al., 2001). In this study, this anxiolytic drug induced ΔΨm loss (EC20 = 83.7µM) and inhibited mitochondrial respiration for concentrations lower than 30µM, thus confirming a significant mitochondrial toxicity (Table 1; Fig. 2A).
The nonsteroidal anti-inflammatory drug (NSAID), diclofenac, was shown to uncouple OXPHOS and induce mPTP opening on isolated rat mitochondria (Masubuchi et al., 2002). However, it is noteworthy that in this study, the assessment of mPTP opening was performed in the presence of Ca2+. Interestingly, we found that diclofenac was unable to induce mitochondrial swelling and cytochrome c release (Table 1; Fig. 2B). Because our assay was carried out without Ca2+ pulse, these data suggest that diclofenac-induced mPTP opening occurs only when mitochondria are loaded with Ca2+. We demonstrated a clear inhibition of mitochondrial respiration through complex I (EC20 = 29.8µM) and complex II (EC20 = 9.1µM) with subsequent ΔΨm loss (Table 1; Fig. 2B).
Several studies demonstrated that perhexiline maleate can induce mitochondrial dysfunction by different mechanisms, including OXPHOS uncoupling and inhibition of different enzymes involved in the mitochondrial respiratory chain and fatty acid oxidation (Deschamps et al., 1994; Kennedy et al., 1996). In our study, we confirmed that perhexiline maleate inhibited mitochondrial respiration when substrates gave their electrons to complexes I and II (Table 1; Fig. 2C). Moreover, we discovered that perhexiline maleate induced mitochondrial swelling and cytochrome c release when very low concentrations were used (Table 1; Fig. 2C), indicating that this antianginal drug can seriously disturb the mitochondrial membrane integrity.
The antidiabetic troglitazone has been reported to impair mitochondrial function by different mechanisms including mPTP opening and mtDNA damage (Masubuchi et al., 2006; Okuda et al., 2010; Rachek et al., 2009). However, as already mentioned for diclofenac, troglitazone-induced mPTP opening occurred in conditions of Ca2+ pulse (Masubuchi et al., 2006; Okuda et al., 2010). In this study, troglitazone was unable to trigger mitochondrial swelling and cytochrome c release (Table 1; Fig. 2D), thus indicating that Ca2+ preloading is mandatory for troglitazone-induced mPTP opening. Nevertheless, we demonstrated that troglitazone inhibited mitochondrial respiration and induced ΔΨm loss when relatively low concentrations were used.
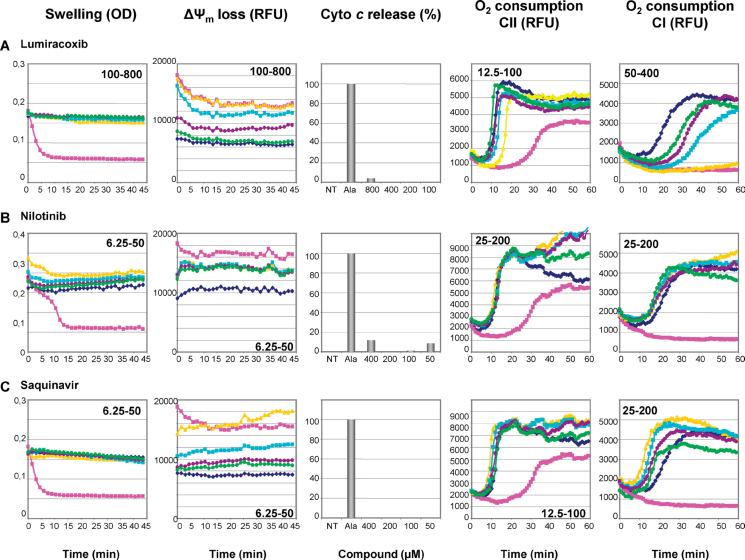
Mitochondrial Toxicity of Compounds Not Already Described to Induce Mitochondrial Dysfunction
This study allowed disclosing for the first time the mitochondrial toxicity of several drugs, such as lumiracoxib, nilotinib, and saquinavir (Table 1, Fig. 3). Lumiracoxib and saquinavir were reported to cause liver injury (http://hepatoweb.com/hepatox.php), but the mechanisms underlying this hepatotoxicity had not been elucidated. Further investigations will be required to determine whether lumiracoxib- and saquinavir-induced impairment of the respiratory chain (Table 1, Fig. 3) is indeed a key mechanism leading to liver injury. Although nilotinib hepatotoxicity has not been reported so far, this anticancer drug can induce moderate to severe cardiotoxicity (Brauchli et al., 2010; Orphanos et al., 2009).
FIG. 3.
Mitochondrial toxicity of drugs with no previously reported mitochondrial liability. Isolated mouse liver mitochondria were untreated (◆) or treated with increasing concentrations (•, •, ◼, and ▲; range indicated on the graphs) of lumiracoxib, nilotinib, and saquinavir, or with positive controls (◼). Mitochondrial swelling, loss of ΔΨm, cytochrome c release, and inhibition of oxygen consumption were thus assessed as described in Materials and Methods and Figure 1.
DISCUSSION
Drug-induced mitochondrial dysfunction can cause several types of hepatotoxicity including cytolytic hepatitis, microvesicular steatosis, steatohepatitis, liver failure, and even cirrhosis (Begriche et al., 2011; Labbe et al., 2008; Pessayre et al., 2010). Furthermore, by inducing mitochondrial liability, compounds can also damage other tissues, such as skeletal muscles, heart, and pancreas. Thus, mitochondrial toxicity should be detected as early as possible during drug development, and ideally during the steps of hit selection and lead optimization. In this context, we showed that a combination of high-throughput in vitro screening tests performed on isolated mouse liver mitochondria allowed predicting whether a drug can be potentially harmful for the human liver. Indeed, we found a strong correlation between mitochondrial dysfunction as assessed with our selected tests and hepatotoxicity in humans independently of the type of liver damage. Furthermore, our study unveiled mitochondrial dysfunction with some hepatotoxic compounds for which such a liability had not been previously reported. Finally, these multiparametric assays clarified the mechanisms of mitochondrial toxicity for some compounds already known to induce mitochondrial dysfunction.
Preclinical toxicological tests should have high positive predictive value and sensitivity. In this study, as far as hepatotoxicity was concerned, the positive predictive value of our high-throughput in vitro screening tests was over 82%, whereas sensitivity was excellent (> 92%). This indicates that the risk of DILI is expected to be high with new chemical entities affecting one (or several) of our selected mitochondrial parameters. From Table 2A, it appears that about 90% of human hepatotoxicants for which DILI was not detected during preclinical animal studies were found to be mitochondriotoxic in our assays. This was, for instance, the case for amiodarone, ketoconazole, and perhexiline, which showed mitochondrial toxicities using our testing platform. The concordance between mitochondrial toxicity measured in our assays and animal hepatotoxicity was 100% for the cutoff related to C max and 90% for the second cutoff. This study indicates that our platform could allow a better predictivity compared with classical animal studies used during preclinical development. This is in keeping with studies reporting that the concordance between hepatotoxicity detected in animal studies and that observed in clinical practice is around 50% (Greaves et al., 2004; Olson et al., 2000). However, such valuable information on mitochondrial toxicity could not be used by itself as a decision-making go–no go strategy, but rather as a tool for ranking and prioritizing compounds in order to select safer candidates for subsequent in vivo preclinical safety investigations. Moreover, detection of mitochondrial toxicity with compounds of high pharmacological interest should prompt pharmaceutical companies to perform additional investigations using other in vitro studies or appropriate animal models (Boelsterli and Hsiao, 2008; Labbe et al., 2008). Hence, in order to select drug candidates, each pharmaceutical company should consider the potential medical benefits of the selected compounds and their toxicological profiles determined during preclinical safety studies. For instance, some nucleoside analogs, such as stavudine (d4T) and zidovudine (AZT), are currently key components of the highly active antiretroviral therapy despite the occurrence of mitochondrial toxicity in a significant number of treated patients (Begriche et al., 2011; Fromenty and Pessayre, 1995; Labbe et al., 2008).
The negative predictive value of our screening tests ranged between 73 and 78%. Accordingly, the lack of mitochondrial dysfunction observed with a new chemical entity cannot exclude the possible occurrence of subsequent liver injury in human. A probable explanation is that mechanisms other than mitochondrial dysfunction can be involved in DILI, such as specific immune reactions and impairment of mitochondria-independent lipid homeostasis (e.g., reduced VLDL secretion or enhanced de novo lipogenesis) (Begriche et al., 2011; Lee, 2003; Russmann et al., 2009).
If the false-negative group was very low (6 or 8%), the false-positive group was much higher (36 or 49%). This rather high level of false-positive results can be explained by the fact that drugs can be toxic to other organs through mitochondrial toxicity (Table 2B). Other reasons for detecting false positives could be attributed to the selected cutoff concentrations (which can be adjusted to the pharmacological efficient concentrations), or to the detection of transient and reversible mitochondrial dysfunction that will have no detrimental effect on liver integrity.
Because our screening tests are performed on isolated mitochondria, it can be argued that they cannot detect mitochondrial toxicity induced by CYP-generated reactive metabolite(s). However, it is noteworthy that Kamel et al. (2012) recently showed that a short incubation of isolated mitochondria with sumatriptan was sufficient to metabolize 30% of the compound. This is most probably due to the presence of some CYPs within mitochondria such as CYP2E1 (Robin et al., 2002), CYP1A2, and CYP2D6 (Buron and Borgne-Sanchez, unpublished data). Thus, it is possible that reactive metabolite(s) could be generated with some drugs during the assay and that the observed mitochondrial dysfunction resulted from the parent drug or from a CYP-generated toxic metabolite. It is noteworthy that, in this study, one-third of the investigated chemicals (with identified mechanism of action) are metabolized into reactive metabolites (Table 2A). Interestingly, all these compounds were found mitochondriotoxic in our assay. Although further investigations will be needed, these data suggest that for some drugs mitochondrial toxicity could occur via the generation of reactive metabolite(s). It is noteworthy that besides parent drugs, metabolites can be also tested for their mitochondrial liability with our assay provided they can be synthesized in stable forms. Finally, in contrast to CYP-generated reactive metabolites, our screening tests on isolated mitochondria will be unable to detect some mitochondrial alterations requiring repeated and long-term exposure, such as mtDNA depletion (Begriche et al., 2011; Fromenty and Pessayre, 1995; Lebrecht et al., 2009). Although this mechanism of mitochondrial toxicity could be uncommon, this issue underscores the need to use other in vitro models, such as hepatoma cell lines, whenever long-term toxicity should be investigated.
Mitochondrial toxicity had already been described for numerous hepatotoxic compounds selected in our research program. However, our study allowed disclosing mitochondrial toxicity with some compounds for which such a detrimental effect had not been previously reported. For instance, we showed that lumiracoxib (a NSAID removed from the market for hepatotoxicity) strongly inhibited the oxygen consumption through respiratory chain complexes I and II (Table 1, Fig. 3A). We also found that the antiretroviral protease inhibitors nelfinavir, ritonavir, and saquinavir collapsed the ΔΨm and altered the mitochondrial respiration for concentrations lower than 50μM (Table 1). Because the reduced mitochondrial respiration and ΔΨm have been reported in cells treated with indinavir (Jiang et al., 2007; Viengchareun et al., 2007), our investigations suggest that mitochondrial toxicity could be common to the whole class of protease inhibitors. Moreover, we showed that low concentrations of the tyrosine kinase inhibitors gefitinib, imatinib, nilotinib, and sunitinib reduced the ΔΨm (Table 1), although mitochondrial dysfunction has been reported only for gefitinib and sunitinib (Höpfner et al., 2004; Kerkela et al., 2009; Will et al., 2008). In addition to the three antiretroviral protease inhibitors and the six tyrosine kinase inhibitors that are all hepatotoxic but one (nilotinib), we carried out investigations with other therapeutic classes that included more compounds. For instance, 16 antibiotics and 15 NSAIDs were studied, but only two drugs were not hepatotoxic in each of these classes. Thus, the low number of drugs not inducing DILI in these therapeutic classes precluded any conclusion regarding the performance of our assay for drugs belonging to the same therapeutic class but differing in their potential hepatotoxicity. Much more drugs should be studied in order to address this important issue.
Our study also permitted to clarify the mechanisms of mitochondrial toxicity for a few compounds already known to induce mitochondrial dysfunction. For instance, we established that low concentrations of flufenamic acid and tolfenamic acid inhibited the succinate-driven mitochondrial respiration and lowered the ΔΨm (Table 1). However, although the latter effect is most probably due to OXPHOS uncoupling (Jordani et al., 2000; Li et al., 2009; McDougall et al., 1983), inhibition of the respiratory chain has not been described so far with these anti-inflammatory agents. Further investigations will be needed in order to determine whether this novel mechanism plays a major role in flufenamic- and tolfenamic acid-induced hepatotoxicity. We also demonstrated that several paraben derivatives impaired the mitochondrial respiration, especially by inhibiting the glutamate/malate-driven respiration (Table 1). Previous investigations have only reported paraben-induced mPTP opening in the presence of Ca2+ (Nakagawa and Moore, 1999). Interestingly, paraben-induced liver abnormalities have recently been reported in rodents (Vo et al., 2010). Thus, long-term exposure to parabens may cause liver toxicity in human and may represent a major health problem, regarding their widely spread use.
Several parameters were selected in this work in order to assess mitochondrial function because chemicals can be toxic for liver mitochondria by different mechanisms. However, it is currently not possible to tell which parameters could be considered as a primary marker because any significant disturbance of each of the selected parameters could have severe consequences on mitochondrial function and hepatocyte viability. For instance, whereas cytochrome c release can induce apoptosis, the loss of transmembrane potential can impair ATP production and lead to necrosis (Labbe et al., 2008; Pessayre et al., 2010). Moreover, from the current knowledge, it is also impossible to tell whether drugs inducing more than one parameter would induce DILI more frequently. Finally, it is important to underline that the different selected parameters can be interdependent. For instance, drugs impairing oxygen consumption can also disturb the transmembrane potential. Thus, more investigations are required to determine whether one particular mitochondrial parameter is better than the others in order to predict DILI.
In this study, hepatotoxicity was determined using the “Hepatox” database (Biour et al., 2004; http://hepatoweb.com/hepatox.php), which includes compounds inducing different types of injury, such as cytolysis, cholestasis, and steatosis. Even if numerous investigations showed that mitochondrial dysfunction plays a predominant role in hepatic cytolysis and steatosis (Begriche et al., 2011; Fromenty and Pessayre, 1995; Labbe et al., 2008; Lee, 2003; Pessayre et al., 2010; Russmann et al., 2009), no subclasses were done in this study. Indeed, the limited number of compounds within each DILI subgroup precluded a valid statistical analysis. In contrast, mitochondrial toxicity may not be a major mechanism involved in drug-induced cholestasis, although this issue will deserve further investigations because many bile salt transporters function in an ATP-dependent manner (Pellicoro and Faber, 2007). We plan to study additional compounds to determine whether our screening tests could provide a significant positive predictive value for one or several types of DILI.
In conclusion, our multiparametric assay performed on isolated mouse liver mitochondria may be an interesting tool for screening drug candidates and helpful to select safer compounds for further development. Indeed, our tests for screening drug-induced mitochondrial toxicity can provide rapid and valuable information that can be used to determine the potential ability of drug candidates to induce DILI. Moreover, this multiparametric assay can represent an appealing tool to decipher the mechanisms whereby marketed drugs induce dire side effects related to mitochondrial dysfunction.
Funding
French Minister of Education and Research (A0907034 Q); OSEO Innovation (A1011019 Q).
Acknowledgments
We thank Dr Françoise Brunner-Ferber for critical reading of the manuscript. We are grateful to Dr Pierre Rustin (Inserm U676, Robert Debré Hospital, Paris) for giving us access to its Clark electrodes and for helpful discussions.
References
- Begriche K.,, Massart J.,, Robin M. A.,, Borgne-Sanchez A.,, Fromenty B.(2011)Drug-induced toxicity on mitochondria and lipid metabolism: Mechanistic diversity and deleterious consequences for the liver.J. Hepatol. 54 773–794 [DOI] [PubMed] [Google Scholar]
- Berson A.,, De Beco V.,, Lettéron P.,, Robin M. A.,, Moreau C.,, El Kahwaji J.,, Verthier N.,, Feldmann G.,, Fromenty B.,, Pessayre D.(1998)Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes.Gastroenterology 114 764–774 [DOI] [PubMed] [Google Scholar]
- Berson A.,, Descatoire V.,, Sutton A.,, Fau D.,, Maulny B.,, Vadrot N.,, Feldmann G.,, Berthon B.,, Tordjmann T.,, Pessayre D.(2001)Toxicity of alpidem, a peripheral benzodiazepine receptor ligand, but not zolpidem, in rat hepatocytes: Role of mitochondrial permeability transition and metabolic activation.J. Pharmacol. Exp. Ther. 299 793–800 [PubMed] [Google Scholar]
- Biour M.,, Ben Salem C.,, Chazouillères O.,, Grangé J. D.,, Serfaty L.,, Poupon R.(2004)Drug-induced liver injury; fourteenth updated edition of the bibliographic database of liver injuries and related drugs.Gastroenterol. Clin. Biol. 28 720–759 [DOI] [PubMed] [Google Scholar]
- Björnsson E.(2009)The natural history of drug-induced liver injury.Semin. Liver Dis. 29 357–363 [DOI] [PubMed] [Google Scholar]
- Boelsterli U. A.,, Lim P. L.(2007)Mitochondrial abnormalities-a link to idiosyncratic drug hepatotoxicity? Toxicol. Appl. Pharmacol. 220 92–107 [DOI] [PubMed] [Google Scholar]
- Boelsterli U. A.,, Hsiao C. J.(2008)The heterozygous Sod2(+/-) mouse: Modeling the mitochondrial role in drug toxicity.Drug Discov. Today 13 982–988 [DOI] [PubMed] [Google Scholar]
- Brauchli Y. B.,, Wais T.,, Gratwohl A.,, Heim D.,, Schipf A.,, Diebold J.,, Krähenbühl S.(2010)Fatal myocardial infarction during nilotinib treatment in a 60-year-old male patient.Acta Oncol. 49 523–525 [DOI] [PubMed] [Google Scholar]
- Buron N.,, Porceddu M.,, Brabant M.,, Desgué D.,, Racoeur C.,, Lassalle M.,, Péchoux C.,, Rustin P.,, Jacotot E.,, Borgne-Sanchez A.(2010)Use of human cancer cell lines mitochondria to explore the mechanisms of BH3 peptides and ABT-737-induced mitochondrial membrane permeabilization.PLOS ONE 5 e9924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps D.,, DeBeco V.,, Fisch C.,, Fromenty B.,, Guillouzo A.,, Pessayre D.(1994)Inhibition by perhexiline of oxidative phosphorylation and the beta-oxidation of fatty acids: Possible role in pseudoalcoholic liver lesions.Hepatology 19 948–961 [PubMed] [Google Scholar]
- Dykens J. A.,, Jamieson J. D.,, Marroquin L. D.,, Nadanaciva S.,, Xu J. J.,, Dunn M. C.,, Smith A. R.,, Will Y.(2008)In vitro assessment of mitochondrial dysfunction and cytotoxicity of nefazodone, trazodone, and buspirone.Toxicol. Sci. 103 335–345 [DOI] [PubMed] [Google Scholar]
- Dykens J. A.,, Will Y.(2007)The significance of mitochondrial toxicity testing in drug development.Drug Discov. Today 12 777–785 [DOI] [PubMed] [Google Scholar]
- Fromenty B.,, Pessayre D.(1995)Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity.Pharmacol. Ther. 67 101–154 [DOI] [PubMed] [Google Scholar]
- Gougeon M. L.,, Pénicaud L.,, Fromenty B.,, Leclercq P.,, Viard J. P.,, Capeau J.(2004)Adipocytes targets and actors in the pathogenesis of HIV-associated lipodystrophy and metabolic alterations.Antivir. Ther. (Lond.) 9 161–177 [PubMed] [Google Scholar]
- Greaves P.,, Williams A.,, Eve M.(2004)First dose of potential new medicines to humans: How animals help.Nat. Rev. Drug Discov. 3 226–236 [DOI] [PubMed] [Google Scholar]
- Höpfner M.,, Sutter A. P.,, Huether A.,, Schuppan D.,, Zeitz M.,, Scherübl H.(2004)Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma.J. Hepatol. 41 1008–1016 [DOI] [PubMed] [Google Scholar]
- Igoudjil A. B. K., Pessayre D., Fromenty B.(2006)Mitochondrial, metabolic and genotoxic effects of antiretroviral nucleoside reverse-transcriptase inhibitors Anti-Infective Agents Med. Chem. 5 273–292 [Google Scholar]
- Jiang B.,, Hebert V. Y.,, Li Y.,, Mathis J. M.,, Alexander J. S.,, Dugas T. R.(2007)HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production, but not apoptosis.Toxicol. Appl. Pharmacol. 224 60–71 [DOI] [PubMed] [Google Scholar]
- Jordani M. C.,, Santos A. C.,, Prado I. M.,, Uyemura S. A.,, Curti C.(2000)Flufenamic acid as an inducer of mitochondrial permeability transition.Mol. Cell Biochem. 210 153–158 [DOI] [PubMed] [Google Scholar]
- Kamel A.,, Colizza K.,, Gunduz M.,, Harriman S.,, Obach R. S.(2012)In vitro-in vivo correlation for intrinsic clearance for CP-409,092 and sumatriptan: A case study to predict the in vivo clearance for compounds metabolized by monoamine oxidase.Xenobiotica 42 355–362 [DOI] [PubMed] [Google Scholar]
- Kennedy J. A.,, Unger S. A.,, Horowitz J. D.(1996)Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone.Biochem. Pharmacol. 52 273–280 [DOI] [PubMed] [Google Scholar]
- Kerkela R.,, Woulfe K. C.,, Durand J. B.,, Vagnozzi R.,, Kramer D.,, Chu T. F.,, Beahm C.,, Chen M. H.,, Force T.(2009)Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase.Clin. Transl. Sci. 2 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe G.,, Pessayre D.,, Fromenty B.(2008)Drug-induced liver injury through mitochondrial dysfunction: Mechanisms and detection during preclinical safety studies.Fundam. Clin. Pharmacol. 22 335–353 [DOI] [PubMed] [Google Scholar]
- Larrey D.(2000)Drug-induced liver diseases.J. Hepatol. 32 77–88 [DOI] [PubMed] [Google Scholar]
- Lebrecht D.,, Venhoff A. C.,, Kirschner J.,, Wiech T.,, Venhoff N.,, Walker U. A.(2009)Mitochondrial tubulopathy in tenofovir disoproxil fumarate-treated rats.J. Acquir. Immune Defic. Syndr. 51 258–263 [DOI] [PubMed] [Google Scholar]
- Lecoeur H.,, Langonné A.,, Baux L.,, Rebouillat D.,, Rustin P.,, Prévost M. C.,, Brenner C.,, Edelman L.,, Jacotot E.(2004)Real-time flow cytometry analysis of permeability transition in isolated mitochondria.Exp. Cell Res. 294 106–117 [DOI] [PubMed] [Google Scholar]
- Lee W. M.(2003)Drug-induced hepatotoxicity.N. Engl. J. Med. 349 474–485 [DOI] [PubMed] [Google Scholar]
- Li Y.,, Qi X. M.,, Xue X.,, Wu X. F.,, Wu Y. F.,, Chen M.,, Xing G. Z.,, Luan Y.,, Ren J.(2009)The relationship between diphenylamine structure and NSAIDs-induced hepatocytes injury.Toxicol. Lett. 186 111–114 [DOI] [PubMed] [Google Scholar]
- Masubuchi Y.,, Kano S.,, Horie T.(2006)Mitochondrial permeability transition as a potential determinant of hepatotoxicity of antidiabetic thiazolidinediones.Toxicology 222 233–239 [DOI] [PubMed] [Google Scholar]
- Masubuchi Y.,, Nakayama S.,, Horie T.(2002)Role of mitochondrial permeability transition in diclofenac-induced hepatocyte injury in rats.Hepatology 35 544–551 [DOI] [PubMed] [Google Scholar]
- Masubuchi Y.,, Suda C.,, Horie T.(2005)Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice.J. Hepatol. 42 110–116 [DOI] [PubMed] [Google Scholar]
- McDougall P.,, Markham A.,, Cameron I.,, Sweetman A. J.(1983)The mechanism of inhibition of mitochondrial oxidative phosphorylation by the nonsteroidal anti-inflammatory agent diflunisal.Biochem. Pharmacol. 32 2595–2598 [DOI] [PubMed] [Google Scholar]
- Nakagawa Y.,, Moore G.(1999)Role of mitochondrial membrane permeability transition in p-hydroxybenzoate ester-induced cytotoxicity in rat hepatocytes.Biochem. Pharmacol. 58 811–816 [DOI] [PubMed] [Google Scholar]
- Okuda T.,, Norioka M.,, Shitara Y.,, Horie T.(2010)Multiple mechanisms underlying troglitazone-induced mitochondrial permeability transition.Toxicol. Appl. Pharmacol. 248 242–248 [DOI] [PubMed] [Google Scholar]
- Olson H.,, Betton G.,, Robinson D.,, Thomas K.,, Monro A.,, Kolaja G.,, Lilly P.,, Sanders J.,, Sipes G.,, Bracken W.,, et al. (2000)Concordance of the toxicity of pharmaceuticals in humans and in animals.Regul. Toxicol. Pharmacol. 32 56–67 [DOI] [PubMed] [Google Scholar]
- Orphanos G. S.,, Ioannidis G. N.,, Ardavanis A. G.(2009)Cardiotoxicity induced by tyrosine kinase inhibitors.Acta Oncol. 48 964–970 [DOI] [PubMed] [Google Scholar]
- Ostapowicz G.,, Fontana R. J.,, Schiødt F. V.,, Larson A.,, Davern T. J.,, Han S. H.,, McCashland T. M.,, Shakil A. O.,, Hay J. E.,, Hynan L.,, et al. (2002)Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States.Ann. Intern. Med. 137 947–954 [DOI] [PubMed] [Google Scholar]
- Pellicoro A.,, Faber K. N.(2007)Review article: The function and regulation of proteins involved in bile salt biosynthesis and transport.Aliment. Pharmacol. Ther. 26(Suppl. 2)149–160 [DOI] [PubMed] [Google Scholar]
- Pessayre D.,, Mansouri A.,, Berson A.,, Fromenty B.(2010)Mito chondrial involvement in drug-induced liver injury.Handb. Exp. Pharmacol. 196, 311–365 [DOI] [PubMed] [Google Scholar]
- Rachek L. I.,, Yuzefovych L. V.,, Ledoux S. P.,, Julie N. L.,, Wilson G. L.(2009)Troglitazone, but not rosiglitazone, damages mitochondrial DNA and induces mitochondrial dysfunction and cell death in human hepatocytes.Toxicol. Appl. Pharmacol. 240 348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin M. A.,, Anandatheerthavarada H. K.,, Biswas G.,, Sepuri N. B.,, Gordon D. M.,, Pain D.,, Avadhani N. G.(2002)Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation.J. Biol. Chem. 277 40583–40593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russmann S.,, Kullak-Ublick G. A.,, Grattagliano I.(2009)Current concepts of mechanisms in drug-induced hepatotoxicity.Curr. Med. Chem. 16 3041–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnimann G., Kessebohm K., Lauterburg B.(2010)Liver injury caused by drugs: An update Swiss Med. Wkly. 140 w13080 [DOI] [PubMed] [Google Scholar]
- Scatena R.,, Bottoni P.,, Botta G.,, Martorana G. E.,, Giardina B.(2007)The role of mitochondria in pharmacotoxicology: A reevaluation of an old, newly emerging topic.Am. J. Physiol. 293 C12–C21 [DOI] [PubMed] [Google Scholar]
- Stewart J. D.,, Horvath R.,, Baruffini E.,, Ferrero I.,, Bulst S.,, Watkins P. B.,, Fontana R. J.,, Day C. P.,, Chinnery P. F.(2010)Polymerase γ gene POLG determines the risk of sodium valproate-induced liver toxicity.Hepatology 52 1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viengchareun S.,, Caron M.,, Auclair M.,, Kim M. J.,, Frachon P.,, Capeau J.,, Lombès M.,, Lombès A.(2007)Mitochondrial toxicity of indinavir, stavudine and zidovudine involves multiple cellular targets in white and brown adipocytes.Antivir. Ther. (Lond.) 12 919–929 [PubMed] [Google Scholar]
- Vo T. T.,, Yoo Y. M.,, Choi K. C.,, Jeung E. B.(2010)Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model.Reprod. Toxicol. 29 306–316 [DOI] [PubMed] [Google Scholar]
- Will Y., Dykens J. A., Nadanaciva S., Hirakawa B., Jamieson J., Marroquin L. D., Hynes J., Patyna S., Jessen B. A.(2008).Effect of the multi-targeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells.Toxicol. Sci. 106, 153–161 [DOI] [PubMed] [Google Scholar]
- Will Y.,, Hynes J.,, Ogurtsov V. I.,, Papkovsky D. B.(2006)Analysis of mitochondrial function using phosphorescent oxygen-sensitive probes.Nat. Protoc. 1 2563–2572 [DOI] [PubMed] [Google Scholar]